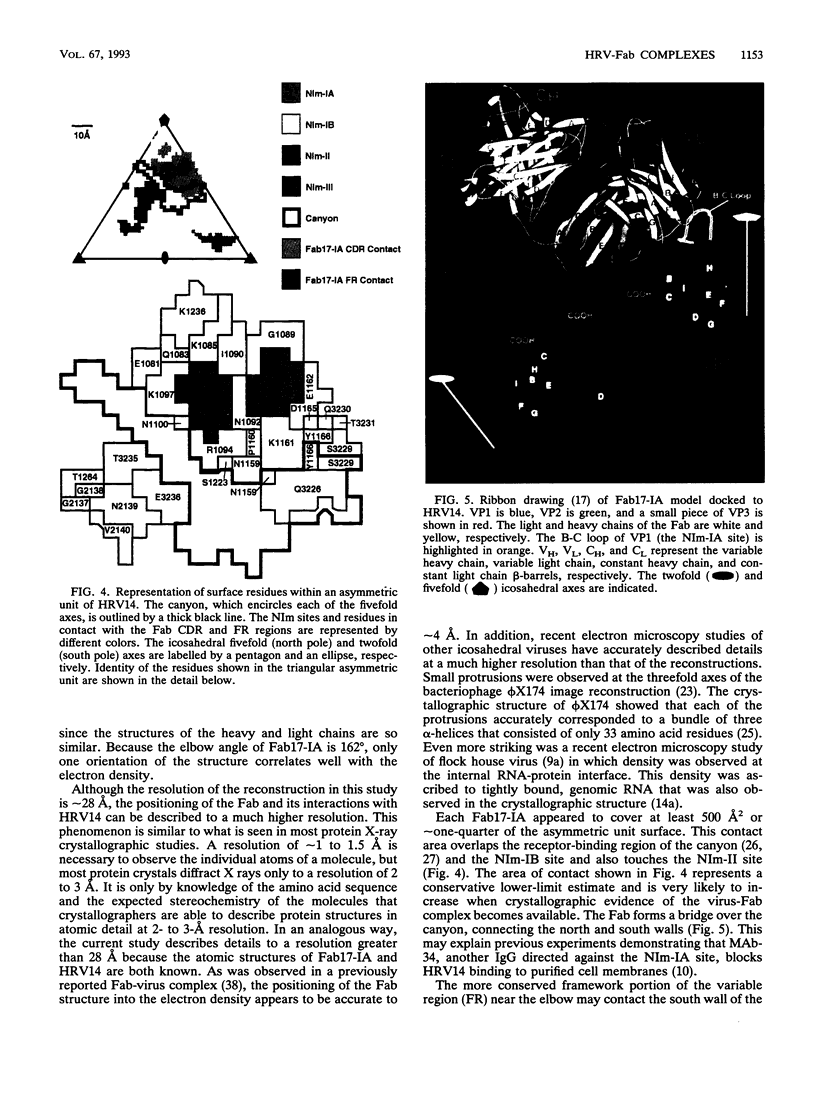
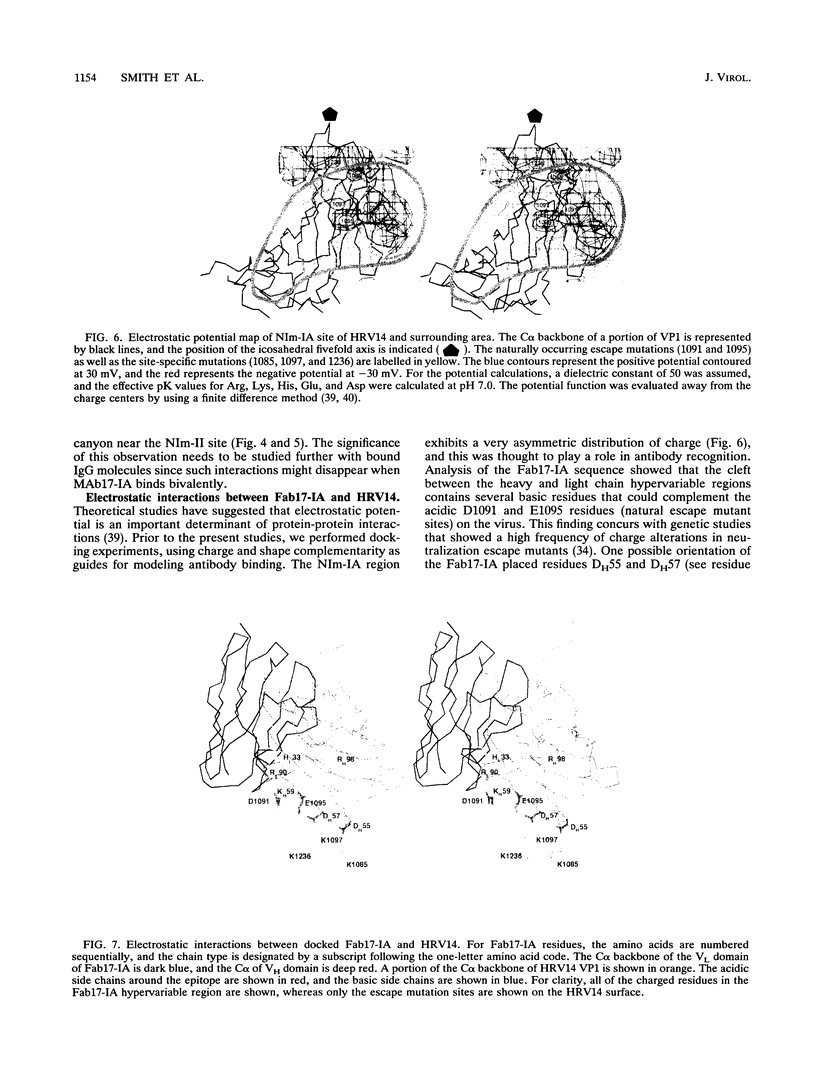
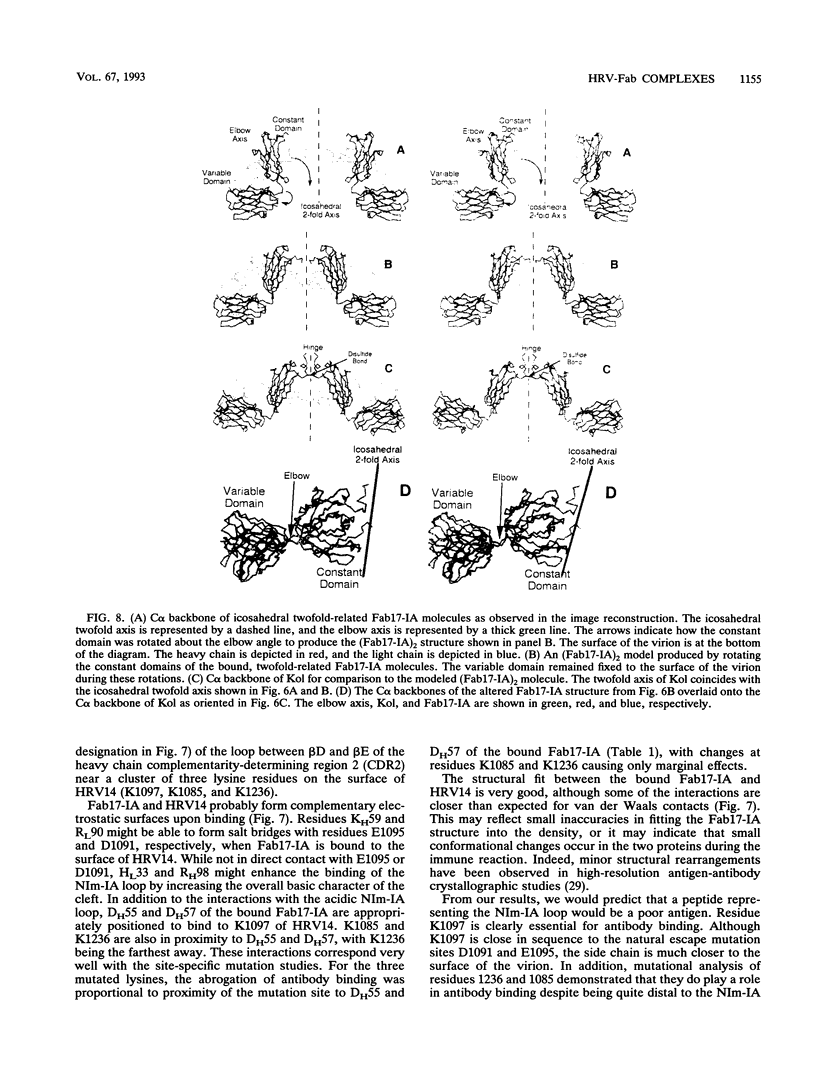
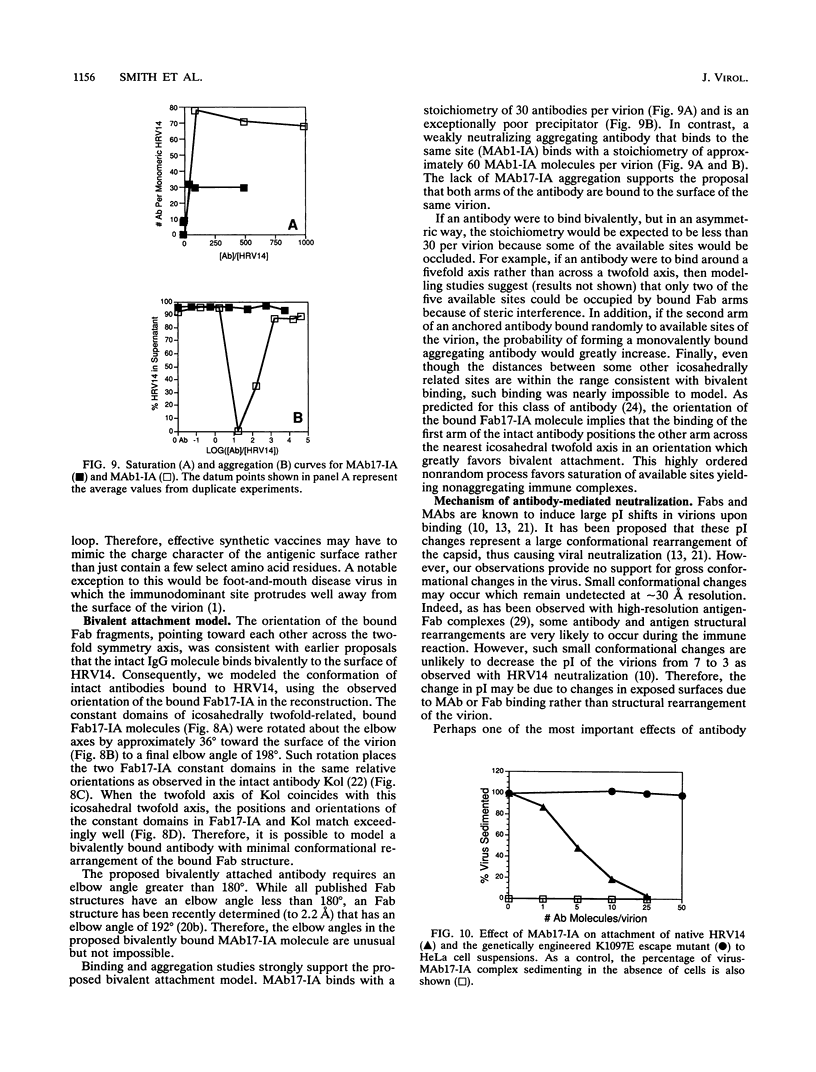
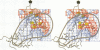Abstract
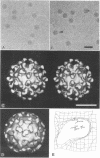
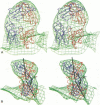
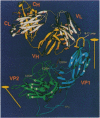
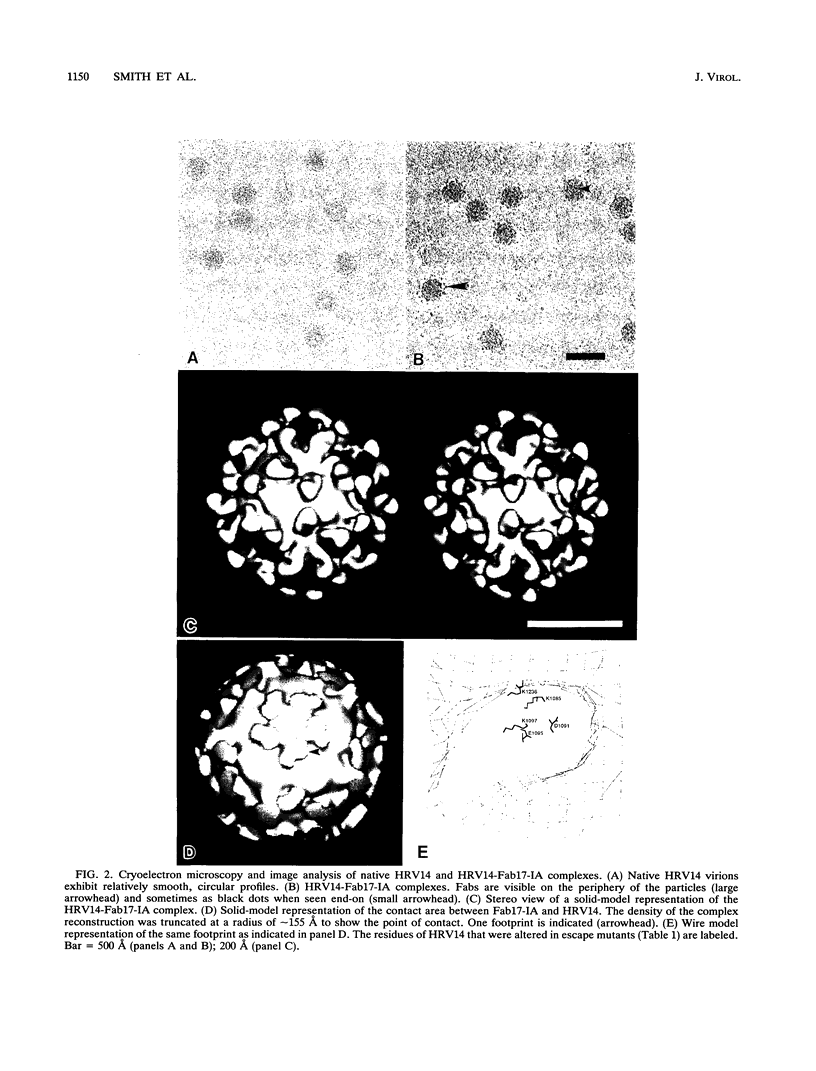
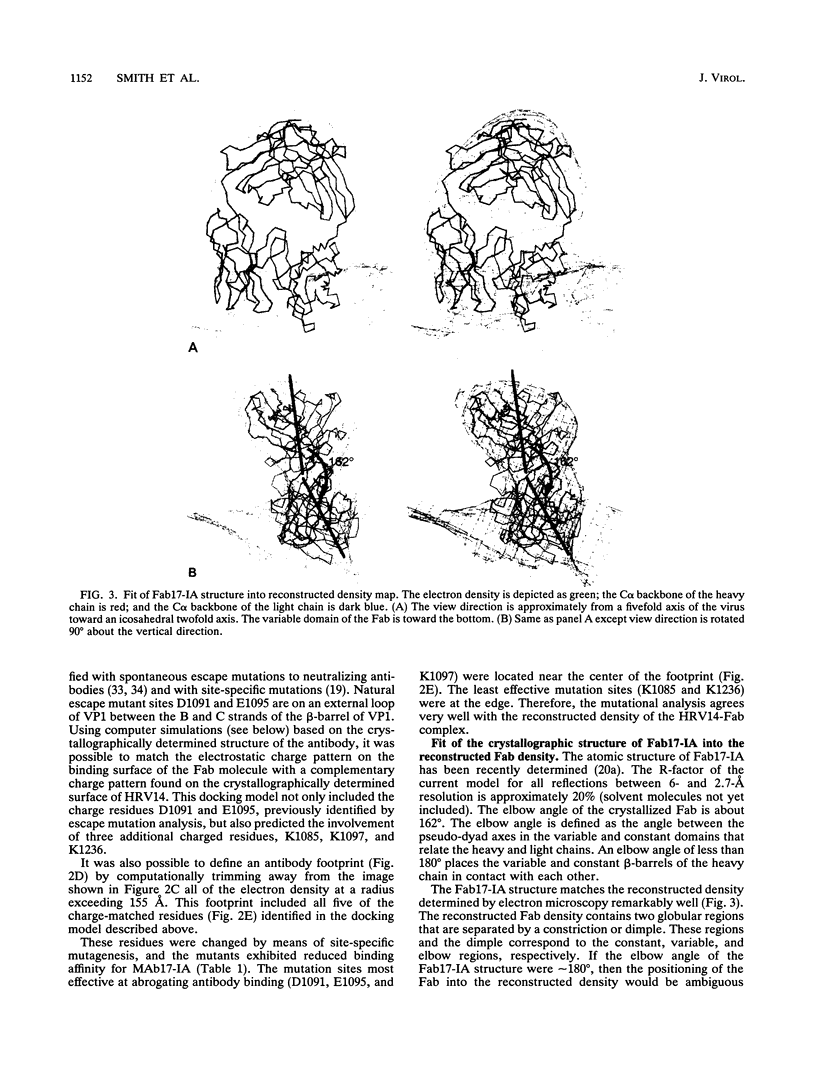
We have determined the structure of a human rhinovirus (HRV)-Fab complex by using cryoelectron microscopy and image reconstruction techniques. This is the first view of an intact human virus complexed with a monoclonal Fab (Fab17-IA) for which both atomic structures are known. The surface area on HRV type 14 (HRV14) in contact with Fab17-IA was approximately 500 A2 (5 nm2), which is much larger than the area that constitutes the NIm-IA epitope (on viral protein VP1) defined by natural escape mutants. From modeling studies and electrostatic potential calculations, charged residues outside the neutralizing immunogenic site IA (NIm-IA) were also predicted to be involved in antibody recognition. These predictions were confirmed by site-specific mutations and analysis of the Fab17-IA-HRV14 complex, along with knowledge of the crystallographic structures of HRV14 and Fab17-IA. The bound Fab17-IA reaches across a surface depression (the canyon) and meets a related Fab at the nearest icosahedral twofold axis. By adjusting the elbow angles of the bound Fab fragments from 162 degrees to 198 degrees, an intact antibody molecule can be easily modeled. This, along with aggregation and binding stoichiometry results, supports the earlier proposal that this antibody binds bivalently to the surface of HRV14 across icosahedral twofold axes. One prediction of this model, that the intact canyon-spanning immunoglobulin G molecule would block attachment of the virus to HeLa cells, was confirmed experimentally.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature. 1989 Feb 23;337(6209):709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Newcomb W. W., Olson N. H., Cowsert L. M., Olson C., Brown J. C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991 Dec;60(6):1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy F. P., Newcomb W. W., Trus B. L., Brown J. C., Baker T. S., Steven A. C. Liquid-crystalline, phage-like packing of encapsidated DNA in herpes simplex virus. Cell. 1991 Mar 8;64(5):1007–1015. doi: 10.1016/0092-8674(91)90324-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioen P., Dekegel D., Boeyé A. Neutralization of poliovirus by antibody-mediated polymerization. Virology. 1983 Jun;127(2):463–468. doi: 10.1016/0042-6822(83)90159-9. [DOI] [PubMed] [Google Scholar]
- Brioen P., Rombaut B., Boeyé A. Hit-and-run neutralization of poliovirus. J Gen Virol. 1985 Nov;66(Pt 11):2495–2499. doi: 10.1099/0022-1317-66-11-2495. [DOI] [PubMed] [Google Scholar]
- Brioen P., Thomas A. A., Boeyé A. Lack of quantitative correlation between the neutralization of poliovirus and the antibody-mediated pI shift of the virions. J Gen Virol. 1985 Mar;66(Pt 3):609–613. doi: 10.1099/0022-1317-66-3-609. [DOI] [PubMed] [Google Scholar]
- Cheng R. H., Olson N. H., Baker T. S. Cauliflower mosaic virus: a 420 subunit (T = 7), multilayer structure. Virology. 1992 Feb;186(2):655–668. doi: 10.1016/0042-6822(92)90032-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Callahan P. L., Leippe D. M., Rueckert R. R., Tomassini J. E. Inhibition of rhinovirus attachment by neutralizing monoclonal antibodies and their Fab fragments. J Virol. 1989 Jan;63(1):36–42. doi: 10.1128/jvi.63.1.36-42.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Diana G. D., McKinlay M. A., Otto M. J., Akullian V., Oglesby C. [[(4,5-Dihydro-2-oxazolyl)phenoxy]alkyl]isoxazoles. Inhibitors of picornavirus uncoating. J Med Chem. 1985 Dec;28(12):1906–1910. doi: 10.1021/jm00150a025. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Ostapchuk P., Wimmer E. Bivalent attachment of antibody onto poliovirus leads to conformational alteration and neutralization. J Virol. 1983 Nov;48(2):547–550. doi: 10.1128/jvi.48.2.547-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. W., Frankenberger E. A., Rossmann M. G., Fout G. S., Medappa K. C., Rueckert R. R. Crystallization of a common cold virus, human rhinovirus 14: "isomorphism" with poliovirus crystals. Proc Natl Acad Sci U S A. 1983 Feb;80(4):931–934. doi: 10.1073/pnas.80.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Larson S. B., Hasel K. W., Day J., Greenwood A., McPherson A. The three-dimensional structure of an intact monoclonal antibody for canine lymphoma. Nature. 1992 Nov 26;360(6402):369–372. doi: 10.1038/360369a0. [DOI] [PubMed] [Google Scholar]
- Icenogle J., Shiwen H., Duke G., Gilbert S., Rueckert R., Anderegg J. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology. 1983 Jun;127(2):412–425. doi: 10.1016/0042-6822(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mandel B. Neutralization of poliovirus: a hypothesis to explain the mechanism and the one-hit character of the neutralization reaction. Virology. 1976 Feb;69(2):500–510. doi: 10.1016/0042-6822(76)90480-3. [DOI] [PubMed] [Google Scholar]
- Marquart M., Deisenhofer J., Huber R., Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980 Aug 25;141(4):369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- McKenna R., Xia D., Willingmann P., Ilag L. L., Krishnaswamy S., Rossmann M. G., Olson N. H., Baker T. S., Incardona N. L. Atomic structure of single-stranded DNA bacteriophage phi X174 and its functional implications. Nature. 1992 Jan 9;355(6356):137–143. doi: 10.1038/355137a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson N. H., Baker T. S., Willingmann P., Incardona N. L. The three-dimensional structure of frozen-hydrated bacteriophage phi X174. J Struct Biol. 1992 Mar-Apr;108(2):168–175. doi: 10.1016/1047-8477(92)90016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Fancher M. J., Felock P. J., Rossmann M. G., Miller M. S., Diana G., Treasurywala A. M., McKinlay M. A., Dutko F. J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989 May;63(5):2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini J. M., Schulze-Gahmen U., Wilson I. A. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science. 1992 Feb 21;255(5047):959–965. doi: 10.1126/science.1546293. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Johnson J. E. Icosahedral RNA virus structure. Annu Rev Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]
- Sherry B., Mosser A. G., Colonno R. J., Rueckert R. R. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J Virol. 1986 Jan;57(1):246–257. doi: 10.1128/jvi.57.1.246-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. A., Brioen P., Boeyé A. A monoclonal antibody that neutralizes poliovirus by cross-linking virions. J Virol. 1985 Apr;54(1):7–13. doi: 10.1128/jvi.54.1.7-13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima C., Unwin N. Contrast transfer for frozen-hydrated specimens: determination from pairs of defocused images. Ultramicroscopy. 1988;25(4):279–291. doi: 10.1016/0304-3991(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Wang G. J., Porta C., Chen Z. G., Baker T. S., Johnson J. E. Identification of a Fab interaction footprint site on an icosahedral virus by cryoelectron microscopy and X-ray crystallography. Nature. 1992 Jan 16;355(6357):275–278. doi: 10.1038/355275a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwicker J. Investigating protein-protein interaction surfaces using a reduced stereochemical and electrostatic model. J Mol Biol. 1989 Mar 20;206(2):381–395. doi: 10.1016/0022-2836(89)90487-7. [DOI] [PubMed] [Google Scholar]
- Warwicker J., Watson H. C. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982 Jun 5;157(4):671–679. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Baker T. S., Rayment I. Three-dimensional structure of myosin subfragment-1 from electron microscopy of sectioned crystals. J Cell Biol. 1991 Aug;114(4):701–713. doi: 10.1083/jcb.114.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]