Abstract
The European Centre for Disease Prevention and Control was founded in response to newly emerging infections such as severe acute respiratory syndrome and avian influenza. However, Europe faces other communicable disease challenges that have proven to be remarkably resilient to public health interventions.
We present examples of communicable diseases with inequitable distribution among those with poor educational attainment, low income, or other socioeconomic factors in every European country. Because these findings are incompatible with social justice and fairness, we examine strategic interventions targeting upstream causes of communicable disease transmission keeping in mind 10 indispensable public health functions essential to reach marginalized groups.
These interventions have to be tailored to the socio-political context and rely on community-based decision-making and intersectorial collaboration.
CHRONIC DISEASE MORTALITY has taken on epidemic proportions in Europe, with 86% of deaths attributed to chronic conditions, despite the fact that these deaths are largely preventable.1,2
These staggering mortality statistics drive public health policy aimed at reducing death rates from noncommunicable diseases in Europe; in fact, effective interventions exist for which changes in lifestyles can eliminate 80% of heart disease, stroke, and type 2 diabetes, as well as 40% of cancer—a clear call to action.
However, this chronic disease predicament masks the dramatic public health triumphs of effective public health measures and medical interventions that rolled back communicable disease mortality in Europe during the 20th century.
Thus, proportional mortality rates shifted to different categories such as chronic disease mortality, according to the epidemiological transition theory.3
Successful communicable disease control in Europe, an ongoing and concerted effort by public health and government officials, is thus indirectly responsible for high chronic disease mortality statistics.
The quandary of public health lies in the fact that successful prevention undermines the reason for its own existence.4 Nevertheless, Europe faces a number of challenges related to communicable diseases that might not manifest in a crude death and disease rate analysis that masks elevated disease burden in sub-populations. These subpopulations may be characterized by low socioeconomic status, poor educational attainment, low occupational class, or recent migration status. Low vaccine coverage or certain infection-related conditions (e.g., infertility) in marginalized groups might be overlooked in overall country statistics; certain subpopulations may suffer from elevated exposures (e.g., crowding) and display high-risk health behaviors. These characteristics and limited access to health care can determine predisposition for disease or low-grade morbidity not detected in health statistics.5
However, disease burden is only one of the criteria for public health priority setting. Other criteria include the possibility for outbreaks, potential spread in the general population, the severity and preventability of disease, socioeconomic burden (e.g., loss of work), potential to drive public health policy, risk perception,6 trends over time, and health care utilization. Furthermore, opportunistic nosocomial infections, microbial resistance, Crimean Congo hemorrhagic fever virus, Hantavirus, Toscana virus, tick-borne encephalitis virus, West Nile virus, and other infectious disease threats are looming on the horizon,7 neck to neck with their celebrity counterparts such as avian influenza or severe acute respiratory syndrome (SARS).
Both emerging and established infections tend to propagate in inequitable health systems and can threaten the capacity of existing public health infrastructures. Tuberculosis, HIV, and sexually transmitted infections are now endemic in certain populations and have proven to be remarkably resilient to public health interventions. It has become apparent that without renewed efforts and concerted coordination between countries, communicable diseases will remain on the forefront of public health.
The European Centre for Disease Prevention and Control (ECDC) is a new European Union (EU) agency. It was founded in the aftermath of the SARS pandemic and at the dawn of the recurrent avian influenza outbreaks in 2003 with the mandate to prevent and control communicable disease transmission in Europe. In a free-market economy, the open movement of goods, services, capital, animals, and people requires an overarching public health agency for coordinated response across borders. In contrast to the World Health Organization Regional Office for Europe, with its 53 member states, ECDC has 27 member states and a mandate to coordinate the public health response to communicable diseases between countries. ECDC has been operational since May 2005, and although still relatively small (151 staff members as of February 2008), the organization is rapidly growing. According to its mission, “the Centre shall identify, assess and communicate current and emerging threats to human health from communicable diseases.”8(p1) Thus, the center has focused on developing a comprehensive surveillance system for the European Union, setting up a system for rapid response to outbreaks and epidemics, and establishing a unit for health communication and scientific advice.
INEQUALITIES AND INFECTIONS IN EUROPE
No variation in the health of the states of Europe is the result of chance; it is the direct result of physical and political conditions in which nations live.
—William Farr (1807–1883)9
True throughout history, and still true today, communicable diseases disproportionately affect poor and vulnerable groups. For today’s public health practitioners, it is second nature to know that hazardous environmental and social conditions of the past caused major epidemics. However, it is less intuitive that today’s major health disparities in Europe are driven by inequitable distribution of environmental and socioeconomic factors.
Mortality and morbidity rates differ systematically by socioeconomic status throughout Europe; higher education, income, and social class are associated with longer and healthier lives.10 Comprehensive and systematic reviews have documented mortality and morbidity inequalities for chronic diseases for all European countries with increasing trends.10 Inequality impels infections, but the small relative contribution of communicable diseases to overall mortality and morbidity makes an analysis by socioeconomic status difficult. A comprehensive assessment is further complicated by the fact that national and international reports on morbidity and mortality are often based on health statistics using codes for diseases and conditions from The International Classification of Diseases, 10th Edition,11 (ICD-10) which does not systematically group all infectious diseases under 1 heading; the majority are grouped under the organ system they mostly affect. For example, chapter 1, “Infectious and Parasitic Diseases,” includes only a subset of all infections; influenza and all the pneumonias are found under “Diseases of the Respiratory System.”
Across the European Union, there have been recent studies of communicable disease inequalities in all 27 member states (selected examples are given in Table 1 ▶). A systematic Medline review of more than 200 articles on socioeconomic factors and infections revealed a number of high-risk populations in Europe, including those with a low level of education, low occupational class, or low income level; other marginalized groups include migrants or people engaged in high-risk activities. These subpopulations suffer disproportionally from a range of infections, including Helicobacter pylori, respiratory infections, sexually transmitted diseases, and nosocomial infections. It is evident that not all communicable infections are associated with inequalities, but some, such as tuberculosis, HIV, or vaccine-preventable infections, are more implicated than others. For many communicable diseases, social and ethnic groups within European countries differ in incidence and prevalence rates, treatment and cure rates, and access to health services (Table 1 ▶).
TABLE 1—
Selected Examples of Infections and Inequalities in the European Union, by Member State: 1998–2007
| Member State | Study Findings |
| Austria | Children from large and socially deprived families have the lowest rates of vaccination coverage against tick-borne encephalitis.25 |
| Belgium | In 2 hospitals, heterosexual route of infection, Black African race, African origin of the virus, and year of diagnosis were predictors for infection with HIV-1.26 |
| Bulgaria | Roma communities, characterized by pervasive social health problems, widespread poverty, limited educational opportunities, and discrimination, held from misconceptions about HIV transmission and other sexually transmitted infections.27 |
| Cyprus | Murine typhus, a zoonosis transmitted by the rat flea, was detected in 21 children younger than 15 years, 71% of whom were living in rural areas with agricultural activity.28 |
| Czech Republic | A random sample of the general population indicated that Helicobacter pylori infection was strongly influenced by socioeconomic conditions and childhood poverty.29 |
| Denmark | The highest risk of hospitalization for infectious diseases was found in children of mothers with only basic schooling, particularly among children coming from single-parent homes with a low income.30 |
| Estonia | An analysis of syphilis incidence and selected sociodemographic factors during the economic transition revealed changes of syphilis incidence that correlated significantly with concurrent changes in unemployment rates and tuberculosis incidence.31 |
| France | At hospitals and prevention units serving pediatric patients in Seine-Saint-Denis, a low-income Paris suburb, tuberculosis remained a serious public health problem.32 |
| Finland | The risk of common cold and respiratory diseases in children was related to high combined parental smoking during infancy.33 |
| Germany | H. pylori seroprevalence rates were higher in less-developed countries: age-adjusted overall seroprevalence rates were 13.1% among Germans in Germany, 30.4% among Turks in Germany, and 44.5% among Turks in Turkey (P < .001).34 |
| Greece | A hospital outpatient-based study from Athens found increased relative incidences of gonorrhea, syphilis, and chancroids among immigrants and that low educational and socioeconomic level were significant incidence predictors.35 |
| Hungary | A hospital-based study indicated that patients testing positive for Epstein-Barr virus were living in poor socioeconomic conditions.36 |
| Ireland | In the inner city of Dublin, clusters of tuberculosis were found to persist in younger, native populations.37 |
| Italy | A survey of public and private hospitals in 6 regions revealed that being foreign born (Asia, Africa, South America, and Eastern Europe) was an independent predictor of lack of adherence to perinatal hepatitis B screening.38 |
| Latvia | The prevalence of gonorrhea, active syphilis, bacterial vaginosis, trichomoniasis, and ectoparasites among street and sex club female prostitutes from the capital city of Riga was significantly elevated.39 |
| Lithuania | Data from a prospective cohort study in urban (Kaunas) and rural (Marijampole) regions of the country indicated that younger and less educated women were the groups most exposed to human papillomavirus (HPV).40 |
| Luxembourg | A prospective seroepidemiological survey of hepatitis A virus seroprevalence showed it was age dependent and highest in adult immigrants.41 |
| Malta | Overcrowding and overall levels of bed occupancy within hospitals, even in non–intensive care settings, was associated with incidence of methicillin-resistant staphylococcus aureus.42 |
| Netherlands | Rubella outbreak in an unvaccinated religious community led to cases of congenital rubella syndrome.43 |
| Poland | A survey of preschool children in Poznaniu revealed that exposure to unfavorable environmental factors such as active smokers in the family and low standard of living were risk factors for HPV infections.44 |
| Portugal | Indicators of poverty (crowding index and level of maternal education) were independent predictors of hepatitis A infection among students aged 6 to 19 years attending public and private schools.45 |
| Romania | Children with HIV infections whose mothers had a high school education or greater in Constanta County were more likely to obtain antiretroviral therapy and to not progress to AIDS.46 |
| Slovakia | Commercial sex workers and intravenous drug users in the streets of Bratislava suffered from sexually transmitted diseases and displayed high-risk health behaviors.47 |
| Slovenia | Deteriorated tuberculosis control was caused by socioeconomic crisis, health system weaknesses, HIV pandemic, multidrug-resistant tuberculosis, and failure to control tuberculosis in prisons and in other risk groups.48 |
| Spain | Infectious disease mortality for men and women with elementary school or lower education was 2.82 and 2.73 times higher than that of men and women with higher levels of education, respectively.49 |
| Sweden | In the Dalby primary health care district in southern Sweden, participants with combined positive serology for H. pylori and Chlamydia pneumoniae were characterized by greater age, lower social class, and higher body mass index, as well as higher fasting levels of insulin than those of seronegative participants.50 |
| United Kingdom | With geographical information system at the census ward level, a study from Hertfordshire found that socioeconomic deprivation as a risk factor for meningococcal disease was most pronounced in young children.51 |
These avoidable differences can and should be addressed; however, unless the fundamental causes of disease, namely the social determinants of health, are not improved, disparity in health outcomes will not be ameliorated.12 In fact, communicable disease outbreaks will prove to be disturbingly persistent within a permanent pool of people who are living disadvantaged lives and who are susceptible to emerging and reemerging communicable agents. The fundamental cause of these outbreaks is lack of access to resources and opportunities; thus, poverty is the “carrier status” of disease.
Treating infected people and releasing them into a noxious environment is both fruitless and unethical. According to Lee Jong-Wook, the late WHO director general, “Control of TB, HIV/ AIDS and malaria depend largely on social action based on clear knowledge.”13(p1006) Although poverty in Europe does not carry the same tragic face of the past, class, racial/ethnic, and gender differences, and migration persist to this day in every member state of the European Union. How, then, can the seeds of recurrent communicable disease epidemics be tackled with interventions and policies, and more important, how can they be prevented from germinating?
INTERVENING ON INEQUALITIES IN INFECTIONS IN EUROPE
Medicine is a social science and politics is nothing else but medicine on a large scale. Medicine as a social science, as the science of human beings, has the obligation to point out problems and to attempt their theoretical solution; the politician, the practical anthropologist, must find the means for their actual solution.
—Rudolf Virchow (1821–1902)14
There is a preponderance of studies of individual health behavior changes such as AIDS prevention interventions, smoking cessation programs, or weight loss plans, despite lower efficacy of these interventions in populations with a lower socioeconomic status.15 Because not only incidence and prevalence rates are higher in these populations but also response rates to health promotion, interventions should target the macrosocial environment rather than focusing solely on behavior change (microlevel interventions).16,17
Addressing health equity has proven to be multifactorial, complex, and slow at best, but Europe has a long record of research and practice in this area. The European Commission has funded “Closing the Gap,” a collection of strategies for action to reduce health inequalities.18 This extensive database of best practices includes 22 European countries and lists main policies, actors, and tools developed to deal with health inequalities on a national level. Because mortality rates in Europe are driven predominantly by chronic disease risk factors, the emphasis of this effort lies on noncommunicable diseases. Nevertheless, interventions on social determinants of communicable diseases should remain a top public health priority based on the inequitable distribution among socioeconomic groups presented in Table 1 ▶.
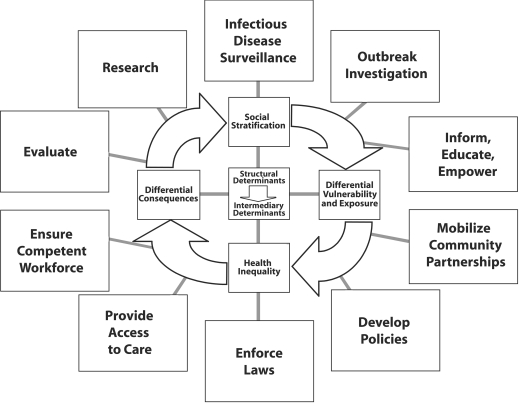
The societal, political, and economic contexts are the structural determinants of health, which in turn give rise to the distribution of income, education, professional prospects, and the like among certain societal groups as defined by specific cultural, gender, or race/ethnicity norms16 (Figure 1 ▶). This process drives social stratification, which gives rise to intermediary determinants of health (e.g., living and working conditions), and behavioral factors (e.g., high-risk health behaviors, drug use) that can generate potentially harmful exposures. A self-perpetuating cycle leads to adverse health effects in marginalized groups with differential consequences because of poor access to care (Figure 1 ▶). The “vicious cycle” can result in a descent down the socioeconomic ladder, because health care costs and loss of work disproportionately affect disadvantaged groups.
FIGURE 1—
Comprehensive approach to intervening on inequalities in infections.
Note. Structural determinants (e.g., political context, income, education) frame intermediary determinants (e.g., housing, occupational conditions) and give rise to social stratification. This process leads to different vulnerabilities and exposures between the better- or worse-off socioeconomic groups, which manifest as health inequities. These inequalities in turn have differential consequences and exacerbate social stratification. Interventions are designed to target the 10 essential public health functions that are fundamental and indispensable to public health.
This downward spiral can be disrupted by considering the 10 essential public health functions that are indispensable for advancing the health of marginalized groups.19,20 All 10 functions listed in Figure 1 ▶ are intended to disrupt the propagation of the cycle, which lies at the heart of inequalities in infections. Although the 10 functions may not be sufficient to advance public health in these subpopulations, they are certainly necessary. Thus, public health action directed toward marginalized groups needs to consider these indispensable functions:
- Conduct infectious disease surveillance:
- Monitor health indicators in subpopulations
- Collect sociodemographic variables from disadvantaged groups
- Analyze subgroup strata
- Investigate outbreaks:
- Diagnose and investigate health problems in subpopulations
- Respond effectively and rapidly, including contact tracing
- Inform, educate, and empower:
- Provide culturally sensitive health education and health promotion
- Mobilize community partnerships:
- Engage community leaders
- Reach out to stakeholders
- Connect different sectors and agencies
- Develop policies:
- Create guidelines and plans to advance health in marginalized groups
- Enforce laws:
- Implement regulations to minimize differential vulnerability and harmful exposures
- Protect health and ensure safety
- Provide access to care:
- Link marginalized groups to health services irrespective of social standing
- Ensure provision of health care when otherwise unavailable (e.g., infectious disease screening should not be linked to migration status because high-risk groups will be lost to follow-up)
- Ensure a competent workforce:
- Hire minority public health practitioners
- Train health care workforce
- Conduct outreach and sensitivity training to overcome cultural barriers
- Evaluate:
- Assess interventions in marginalized groups
- Evaluate effectiveness, accessibility, and quality of public health services for subpopulations
- Research:
- Promote studies of subpopulations
- Develop innovative solutions to health problems of disadvantaged groups
Ultimately, intervening at the level of these 10 essential public health functions should decrease social stratification—by reducing exposure to harmful factors, by lessening the vulnerability of disadvantaged people, and by increasing access to health care to prevent adverse consequences of disease (Figure 1 ▶). A public health response also can be seen through the lens of the policy principles proposed by the World Health Organization Commission on Social Determinants of Health.21 First, interventions ought to integrate a variety of different sectors, besides the health sector, to ensure a comprehensive approach by drawing from civil engineering, urban planning, education, nongovernmental organizations, and other stakeholders.
Second, interventions should consider the sociopolitical context of each situation and alter project goals accordingly. Each European country has specific sociopolitical circumstances requiring special attention and adjustment, and there is no approach that fits all circumstances.
Third, effective interventions should be evidence based and prioritized according to strategies with a high probability of success. Clear, measurable goals should be defined before project implementation and should be monitored for efficacy.
Fourth, there should be community participation in the decisionmaking process so as to build civic capacity. A participatory process by all stakeholders is important to obtain the best possible buy-in. All 4 policy principles must be simultaneously considered before implementation of these upstream public health interventions.22
A striking example of a national policy designed to ameliorate health inequalities, and thus the infectious disease burden, are the 11 Swedish general objectives for public health.17 The first National Public Health Report in Sweden in 1987 documented large socioeconomic differences in health between groups of people.17 In 1990, the government decided that equality was the overall goal of public health practice in Sweden. National objectives for public health were set in 1995 and eventually approved by the government as
Objectives for public health (Government bill 2002/03:35) with the mandate to create social conditions that will ensure good health on equal terms for the entire population.23(p11)
This innovative public health strategy was based on the social determinants model and included 11 objectives for the most important determinants of health, comprised of 6 structural determinants and 5 intermediary determinants. Furthermore, through an inclusive policy process, all major political parties, relevant sectors, trade unions, researchers, and health care providers were included with extensive community participation. The 11 objectives are as follows:
Participation and influence in society
Economic and social security
Secure and favorable conditions during childhood and adolescence
Healthier working life
Healthy and safe environments and products
Health and medical care that more actively promote good health
Effective protection against communicable diseases
Safe sexuality and good reproductive health
Increased physical activity
Good eating habits and safe food
Reduced use of tobacco and alcohol, a society free from illicit drugs and doping, and a reduction in harmful effects of excessive gambling
The remarkable feature of Sweden’s macrosocial approach is the idea of carrying out the majority of public health work outside of the narrow confines of traditional medical care services and placing it instead in the broader social and political realm (objectives 1–5). International comparisons of health indicators attest to the fact that Sweden’s public health approach has worked well there. The burden of infectious diseases in Sweden has been reduced to all-time lows through this comprehensive public health approach in combination with environmental interventions, high vaccination coverage, systematic testing, and contact tracing.24
Intervening on inequalities in infections entails a multifaceted approach with 10 crucial public health functions tailored at marginalized groups. Furthermore, a comprehensive approach should consider the sociopolitical context, intersectorial action, community participation and evidence-based interventions. Interventions of individual behavioral change work best if societal conditions support it through an integrated approach, including the personal, social, cultural, and physical environment. Ultimately, discrepancies between social strata are incompatible with the ideals of social justice and equity and ought to be attenuated, if not abolished; thus, innovative and unconventional interventions should be devised to lessen health inequalities of infections.
Acknowledgments
The authors are grateful for critical feedback by 3 anonymous reviewers and for comments on an earlier draft of the article by Jo Asvall, David Buckley, Justin Denny, Christina Duran, Francoise Hamers, Zsuzsanna Jakab, Karl Ekdahl, Pierluigi Lopalco, Davide Manissero, Arun Nanda, Angus Nicoll, Amanda Ozin, Giorgio Semenza, and Lisa Weasel.
Human Participant Protection No institutional review board approval was required for this study.
Peer Reviewed
Contributors J. C. Semenza originated and designed the study and led the writing. J. Giesecke contributed to the content of the study and reviewed drafts of the commentary.
References
- 1.World Health Organization (WHO), Regional Office for Europe. Gaining Health. The European Strategy for the Prevention and Control of Noncommunicable Diseases. Copenhagen, Denmark: WHO, Regional Office for Europe; 2006. Available at: http://www.euro.who.int/Document/RC56/edoc08.pdf. Accessed December 21, 2007.
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006; 367(9524):1747–1757. [DOI] [PubMed] [Google Scholar]
- 3.Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49:509–538. [PubMed] [Google Scholar]
- 4.Giesecke J. Modern Infectious Disease Epidemiology. 2nd ed. London, England: Arnold; 2002.
- 5.Menke R, Streich W, Rossler G, Brand H. Report on Socio-Economic Differences in Health Indicators in Europe. Report No. 16. Bielefeld, Germany: Institute of Public Health; 2003.
- 6.Doherty JA. Establishing priorities for national communicable disease surveillance. Can J Infect Dis. 2000;11: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vorou RM, Papavassiliou VG, Tsiodras S. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol Infect. 2007; 135:1231–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Official Journal L 142, 30/04/ 2004 P. Eur Parl Regulation 851, 21 April 2004. 1.7: 0001–0011.
- 9.Dupaquier M. William Farr. In: Heyde CC, Seneta E, eds. Statisticians of the Centuries. New York, NY: Springer; 2001:163–166.
- 10.Mackenbach JP. Health Inequalities: Europe in Profile. London: UK Presidency of the EU; 2005.
- 11.International Classification of Diseases, 10th Revision. Geneva, Switzerland: World Health Organization; 2007. Available at http://www.who.int/classifications/apps/icd/icd10online/. Accessed January 10, 2008.
- 12.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;35:80–94. [PubMed] [Google Scholar]
- 13.Lee JW. Public health is a social issue. Lancet. 2005;365:1005–1006. [DOI] [PubMed] [Google Scholar]
- 14.Ackerknecht EH. Rudolf Virchow: Doctor, Statesman, Anthropolist. Madison: University of Wisconsin Press; 1953.
- 15.Beaglehole R. International trends in coronary heart disease mortality, morbidity, and risk factors. Epidemiol Rev. 1990;12:1–15. [DOI] [PubMed] [Google Scholar]
- 16.Semenza JC, Maty S. Acting upon the macrosocial environment to improve health: a framework for intervention. In: Galea S, ed. Macrosocial Determinants of Population Health. New York, NY: Springer Media Publishing; 2007: 443–461.
- 17.Semenza JC. Case studies: improving the macrosocial environment. In: Galea S, ed. Macrosocial Determinants of Population Health. New York, NY: Springer Media Publishing; 2007: 463–484.
- 18.European Portal for Action on Health Equity. Available at: http://www.health-inequalities.org. Accessed February 14, 2007.
- 19.Public Health Functions Steering Committee, Members. Public Health in America. 1994. Available at: http://www.health.gov/phfunctions/public.htm. Accessed December 19, 2007.
- 20.Faculty of public health medicine. Strengthening Public Health Function in the UAE, and EMR of the WHO. London Faculty of Public Health Medicine of the Royal College of Physicians of the United Kingdom; 2001.
- 21.World Health Organization. Commission on Social Determinants of Health. Available at: http://www.who.int/social_determinants/en. Accessed February 14, 2007.
- 22.World Health Organization Commission on Social Determinants of Health. Towards a Conceptual Framework for Analysis and Action on the Social Determinants of Health. Discussion paper for the Commission on Social Determinants of Health. Geneva, Switzerland: World Health Organization; 2005.
- 23.Persson G. The National Public Health Report 2005. Scand J Public Health Suppl. 2006;67:11–18. [DOI] [PubMed] [Google Scholar]
- 24.Carlson J. Chapter 5.9: major public health problems—infectious disease. Scand J Public Health Suppl. 2006;67: 132–138. [DOI] [PubMed] [Google Scholar]
- 25.Stronegger WJ, Freidl W, Rasky E, Berghold A. Educational status and resources for child care as predictors of TBE vaccination coverage in schoolchildren of an endemic area in Austria. Zentralbl Hyg Umweltmed. 1998;201 (4–5):437–445. [PubMed] [Google Scholar]
- 26.Snoeck J, Van Laethem K, Hermans P, et al. Rising prevalence of HIV-1 non-B subtypes in Belgium: 1983–2001. J Acquir Immune Defic Syndr. 2004;35:279–285. [DOI] [PubMed] [Google Scholar]
- 27.Kelly JA, Amirkhanian YA, Kabakchieva E, et al. Gender roles and HIV sexual risk vulnerability of Roma (Gypsies) men and women in Bulgaria and Hungary: an ethnographic study. AIDS Care. 2004;16:231–245. [DOI] [PubMed] [Google Scholar]
- 28.Koliou M, Psaroulaki A, Georgiou C, Ioannou I, Tselentis Y, Gikas A. Murine typhus in Cyprus: 21 paediatric cases. Eur J Clin Microbiol Infect Dis. 2007;26: 491–493. [DOI] [PubMed] [Google Scholar]
- 29.Bures J, Kopacova M, Koupil I, et al. European Society for Primary Care Gastroenterology. Epidemiology of Helicobacter pylori infection in the Czech Republic. Helicobacter. 2006; 11(1):56–65. [DOI] [PubMed] [Google Scholar]
- 30.Thrane N, Sondergaard C, Schonheyder HC, Sorensen HT. Socioeconomic factors and risk of hospitalization with infectious diseases in 0- to 2-year-old Danish children. Eur J Epidemiol. 2005;20:467–474. [DOI] [PubMed] [Google Scholar]
- 31.Uuskula A, Nygard JF, Kibur-Nygard M. Syphilis as a social disease: experience from the post-communist transition period in Estonia. Int J STD AIDS. 2004;15:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Pontual L, Hollebecque V, Bessa Z, et al. Childhood tuberculosis in a low-income Paris suburb: lessons from a resurgence brought under control. Int J Tuberc Lung Dis. 2004;8:976–981. [PubMed] [Google Scholar]
- 33.Hugg TT, Jaakkola MS, Ruotsalainen RO, Pushkarev VJ, Jaakkola JJ. Parental smoking behaviour and effects of tobacco smoke on children’s health in Finland and Russia. Eur J Public Health. 2008;18(1):55–62. PMID: 17569700. [DOI] [PubMed] [Google Scholar]
- 34.Porsch-Ozcurumez M, Doppl W, Hardt PD, et al. Impact of migration on Helicobacter pylori seroprevalence in the offspring of Turkish immigrants in Germany. Turk J Pediatr. 2003;45: 203–208. [PubMed] [Google Scholar]
- 35.Kyriakis KP, Hadjivassiliou M, Paparizos VA, Flemetakis A, Stavrianeas N, Katsambas A. Incidence determinants of gonorrhea, chlamydial genital infection, syphilis and chancroid in attendees at a sexually transmitted disease clinic in Athens, Greece. Int J Dermatol. 2003; 42:876–881. [DOI] [PubMed] [Google Scholar]
- 36.Keresztes K, Bessenyei B, Szollosi Z, et al. Association of Hodgkin lymphoma with Epstein-Barr virus in Hungary. Orv Hetil. 2005;24(146):1575–1582. [PubMed] [Google Scholar]
- 37.Fair E, O’Meara M, Corbally N, Keogh B, Hannan M. Molecular epidemiologic investigation of tuberculosis in an area of increasing incidence in inner-city Dublin. Ir Med J. 2006;99(3): 87–90. [PubMed] [Google Scholar]
- 38.Stroffolini T, Bianco E, Szklo A, et al. Factors affecting the compliance of the antenatal hepatitis B screening programme in Italy. Vaccine. 2003; 21(11–12):1246–1249. [DOI] [PubMed] [Google Scholar]
- 39.Kurova T, Shoubnikova M, Malceva A, Mardh PA. Prostitution in Riga, Latvia—a socio-medical matter of concern. Acta Obstet Gynecol Scand. 1998;77:83–86. [DOI] [PubMed] [Google Scholar]
- 40.Kliucinskas M, Nadisauskiene RJ, Minkauskiene M. Prevalence and risk factors of HPV Infection among high-risk rural and urban Lithuanian women. Gynecol Obstet Invest. 2006;62: 173–180. [DOI] [PubMed] [Google Scholar]
- 41.Mossong J, Putz L, Patiny S, Schneider F. Seroepidemiology of hepatitis A and hepatitis B virus in Luxembourg. Epidemiol Infect. 2006;134: 573–578. PMID: 16316492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borg MA. Bed occupancy and overcrowding as determinant factors in the incidence of MRSA infections within general ward settings. J Hosp Infect. 2003;54:316–318. [DOI] [PubMed] [Google Scholar]
- 43.van der Veen Y, Hahné S, Ruijs H, et al. Rubella outbreak in an unvaccinated religious community in the Netherlands leads to cases of congenital rubella syndrome. Euro Surveill. 2005; 10(11):E051124.3. [DOI] [PubMed] [Google Scholar]
- 44.Szydlowski J, Myga M, Grzegorowski M, Gozdzicka-Jozefiak A. The role of environmental factors in HPV infections of the upper respiratory tract of healthy children. Przegl Lek. 2004;61: 1043–1045. [PubMed] [Google Scholar]
- 45.Barros H, Oliveira F, Miranda H. A survey on hepatitis A in Portuguese children and adolescents. J Viral Hepat. 1999;6:249–253. [DOI] [PubMed] [Google Scholar]
- 46.Kozinetz CA, Matusa R, Cazacu A. The burden of pediatric HIV/AIDS in Constanta, Romania: a cross-sectional study. BMC Infect Dis. 2001;1:7. PMID: 11495632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staneková D, Jiresová K, Habeková M, et al. HIV infection and risk behaviour of commercial sex workers and intravenous drug users in Slovakia. Cent Eur J Public Health. 2004;12:197–200. [PubMed] [Google Scholar]
- 48.Migliori GB, Centis R. Problems to control TB in eastern Europe and consequences in low incidence countries. Monaldi Arch Chest Dis. 2002;57: 285–290. [PubMed] [Google Scholar]
- 49.Regidor E, De Mateo S, Calle ME, Dominguez V. Educational level and mortality from infectious diseases. J Epidemiol Community Health. 2002;56: 682–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekesbo R, Nilsson PM, Lindholm LH, Persson K, Wadstrom T. Combined seropositivity for H. pylori and C. pneumoniae is associated with age, obesity and social factors. J Cardiovasc Risk. 2000;7:191–195. [DOI] [PubMed] [Google Scholar]
- 51.Williams CJ, Willocks LJ, Lake IR, Hunter PR. Geographic correlation between deprivation and risk of meningococcal disease: an ecological study. BMC Public Health. 2004;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]