Abstract
Upon B-cell antigen receptor (BCR) activation, the protein tyrosine kinase Syk phosphorylates the adaptor protein SH2 domain-containing leukocyte protein of 65 kDa (SLP-65), thus coupling the BCR to diverse signalling pathways. Here, we report that SLP-65 is not only a downstream target and substrate of Syk but also a direct binding-partner and activator of this kinase. This positive feedback is mediated by the binding of the SH2 domain of SLP-65 to an autophosphorylated tyrosine of Syk. The mutant B cells that cannot form the Syk/SLP-65 complex are defective in BCR-induced extracellular signal-regulated kinase, nuclear factor κ B and nuclear factor of activated T cells, but not Akt activation, and are blocked in B-cell development. Furthermore, we show that formation of the Syk/SLP-65 complex is required for sustained Ca2+ responses in activated B cells. We suggest that after activation and internalization of the BCR, Syk remains active as part of a membrane-bound Syk/SLP-65 complex controlling sustained signalling and calcium influx.
Keywords: adaptors, B-cell antigen receptor signalling, calcium, lymphocyte development, tyrosine kinase
Introduction
The B-cell antigen receptor (BCR) is a multisubunit complex comprising the membrane-bound immunoglobulin molecule, and the signal transducing Ig-α/Ig-β heterodimer (Schamel and Reth, 2000). Upon BCR activation, the two immunoreceptor tyrosine-based activation motif (ITAM) tyrosines present in Ig-α and Ig-β are phosphorylated by the protein tyrosine kinases Lyn and Syk (Johnson et al, 1995; Sada et al, 2001; Xu et al, 2005). The Src-family kinase Lyn predominantly phosphorylates the first ITAM tyrosine, whereas Syk phosphorylates and binds to both ITAM tyrosines (Rolli et al, 2002). The adaptor protein SH2 domain-containing leukocyte protein of 65 kDa (SLP-65) (also known as BLNK or BASH) is then recruited to the BCR complex, where it is phosphorylated by Syk and then activates downstream signalling cascades (Fu et al, 1998; Goitsuka et al, 1998; Wienands et al, 1998).
The expression of Syk is required for normal B-cell development. Mice carrying a deletion of the Syk gene are embryonic lethal, as expression of Syk is required for maintenance of vascular integrity (Cheng et al, 1995; Turner et al, 1995; Saijo et al, 2003). Syk is a multidomain protein containing two N-terminal SH2 domains followed by the interdomain B region, the kinase domain and a short C-terminal tail. The 120-amino-acid-long interdomain B region contains a number of tyrosines that, upon phosporylation, can recruit other adaptor/signalling molecules such as Cbl, Vav-1 and phospholipase C-γ2 (PLC-γ2) (Simon et al, 2005; Groesch et al, 2006). The kinase activity of Syk is regulated by its N-terminal region carrying the two tandem SH2 domains that presumably form an autoinhibitory complex with interdomain B and the kinase domain, in a manner similar to what has recently been described for the Syk-family kinase ZAP-70 (Deindl et al, 2007). The binding of the tandem SH2 domains to the phosphorylated ITAM relieves Syk from autoinhibition and strongly activates the kinase.
SLP-65, the major substrate of Syk, limits pre-B-cell proliferation and promotes B-cell differentiation, thus acting as a tumour suppressor in mouse and human pre-B cells (Jumaa et al, 2005; Yamamoto et al, 2006). SLP-65 has an N-terminal leucine zipper that mediates membrane association of the protein (Kohler et al, 2005) and a C-terminal SH2 domain that can bind to a non-ITAM tyrosine (Y204) in the tail of Ig-α (Engels et al, 2001; Kabak et al, 2002; Patterson et al, 2006). Activated Syk phosphorylates SLP-65 on multiple tyrosine residues, which serve as docking sites for the SH2 domains of Nck, Btk and PLC-γ2 (Chiu et al, 2002; Koretzky et al, 2006). Once activated, PLC-γ2 catalyses the generation of the second messengers diacylglycerol and inositol-1,4,5-trisphosphate (IP3) resulting in PKC activation and Ca2+ release, respectively.
BCR-mediated Ca2+ mobilization occurs in two phases. In the first phase, the binding of IP3 to its receptors in the endoplasmic reticulum (ER) membrane results in release of Ca2+ from ER stores. The second phase involves a sustained influx of extracellular Ca2+ across the plasma membrane by Ca2+ release-activated Ca2+ (CRAC) channels. This process is activated upon depletion of the ER Ca2+ stores and is termed capacitative Ca2+ entry or store-operated Ca2+ entry (SOCE). The molecular components that mediate this process in T cells were only recently identified as STIM1, which functions as a Ca2+ sensor in the ER and Orai, which forms a Ca2+ channel at the plasma membrane (Liou et al, 2005; Roos et al, 2005; Feske et al, 2006; Vig et al, 2006).
Elevation of intracellular Ca2+ levels regulates a number of downstream molecules including transcription factors such as nuclear factor κ B (NF-κB) and nuclear factor of activated T cells (NFAT) (Dolmetsch et al, 1997). In resting cells, NFAT is heavily phosphorylated and is located in the cytosol. Ca2+ signalling activates the Ca2+-dependent phosphatase calcineurin, which dephosphorylates NFAT and facilitates its translocation to the nucleus (Timmerman et al, 1996; Gallo et al, 2006). The activation of NFAT in particular requires a sustained Ca2+ flux that typically lasts for several hours (Feske et al, 2001). Here, we show that the adaptor SLP-65 binds to its upstream kinase Syk and this association promotes Syk activity. The Syk/SLP-65 complex is also required for sustained Ca2+ influx, efficient NF-κB, mitogen-activated protein kinase (MAPK) and NFAT activation and normal B-cell development.
Results
SLP-65 binds to Syk and activates the kinase
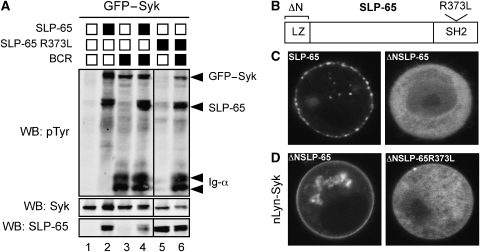
When Syk and SLP-65 are coexpressed in the S2 Schneider cell reconstitution system, Syk is not active and does not phosphorylate its substrate SLP-65 (Rolli et al, 2002). Release from autoinhibition and activation of Syk requires coexpression of the BCR (Supplementary Figure S1). In the presence of the BCR, the tandem SH2 domains of Syk can bind to the phosphorylated ITAM tyrosines of Ig-α and Ig-β, thus shifting the dynamic equilibrium from the closed autoinhibited to the open active conformation of Syk. Interestingly, a green fluorescent protein (GFP)–Syk fusion protein behaves differently from wild-type (wt) Syk in this aspect. GFP–Syk is inactive when it is expressed alone in S2 cells, but it becomes active when coexpressed with the adaptor SLP-65, resulting in GFP–Syk and SLP-65 phosphorylation (Figure 1A, lanes 1 and 2). This activation of GFP–Syk does not require coexpression of the BCR, but it is dependent on the SH2 domain of SLP-65. A SLP-65 mutant that can no longer bind to phosphotyrosines because of a point mutation in its SH2 domain (SLP-65R373L) can no longer efficiently activate GFP–Syk (Figure 1A, lane 5). In the presence of the BCR, the GFP–Syk fusion protein is active, independent of the adaptor and can also phosphorylate the SLP-65R373L mutant, albeit less efficiently than the wild-type adaptor (Figure 1A, lanes 3, 4 and 6). These results indicate that in addition to being a substrate of Syk, SLP-65 also mediates feedback activation of this kinase.
Figure 1.
The SH2 domain of SLP-65 mediates interaction between Syk and SLP-65. (A) Western blot analysis of tyrosine phosphorylation in S2 cell lysates expressing GFP–Syk (lane 1), in combination with SLP-65 (lane 2), the BCR (lane 3) and SLP-65 and BCR (lane 4) or along with the SH2 domain mutant of SLP-65 (SLP-65R373L) (lane 5), SLP-65R373L and the BCR (lane 6). The bottom panel shows expression levels of Syk and SLP-65 in the total cell lysates. Protein expression was induced for 24 h by incubation of cells with 0.1 mM CuSO4 and lysates separated by 10% reducing SDS–PAGE and western blotted for total protein tyrosine phosphorylation, Syk and SLP-65. Western blots were analysed using the Odyssey infrared imager (LI-COR Biosciences). (B) Structural organization of the adaptor protein SLP-65 showing the location of the leucine zipper at the N terminus and the conserved SH2 domain at the C-terminal end of the protein. Also indicated is the position of the arginine residue (R373) in the FLVR motif of the SH2 domain required for binding to phosphorylated tyrosines. (C) Confocal images of GFP fusions of SLP-65 and N terminus deletion of SLP-65 (ΔNSLP-65) in transiently transfected S2 cells after induction of expression for 12 h followed by a chase of 8 h. (D) Representative confocal image of a cell expressing nLyn-Syk (a fusion of the first 24 amino acids of Lyn and Syk) in combination with ΔNSLP-65–GFP (left) or the N-terminal deleted SLP-65 fusion protein with a mutation of the SH2 domain, ΔNSLP-65R373L–GFP (right).
To determine whether there is an interaction between Syk and SLP-65, we established a translocation assay in S2 cells testing the binding of SLP-65–GFP fusion proteins to a membrane-bound Syk protein. The N-terminal leucine zipper of SLP-65 (Figure 1B) mediates binding of the adaptor to membranes (Kohler et al, 2005). When expressed in S2 cells, SLP-65–GFP is localized at a vesicular, membrane-proximal compartment, whereas ΔNSLP-65–GFP, which lacks the leucine zipper, is cytosolic (Figure 1C). As described previously, constitutive membrane attachment can activate Syk or ZAP-70 (Kolanus et al, 1993). Indeed, a fusion protein of Syk with the myristoylation and palmitoylation anchors of the src-family kinase Lyn (nLyn-Syk) is constitutively attached to the membrane and is strongly phosphorylated in S2 cells, even in the absence of the BCR (Supplementary Figure S1). The coexpression of ΔNSLP-65–GFP with nLyn-Syk results in a translocation of ΔNSLP-65–GFP from the cytoplasm to the membrane, suggesting that the two proteins directly bind to each other (Figure 1D, left panel). The formation of the Syk/SLP-65 complex requires the SH2 domain of SLP-65, as the R373L mutation of the SH2 domain abolished the binding of ΔNSLP-65–GFP to nLyn-Syk (Figure 1D, right panel). We conclude from these experiments that SLP-65 is not only an activator but also a binding partner of Syk.
A conserved tyrosine residue in Syk mediates interaction with SLP-65
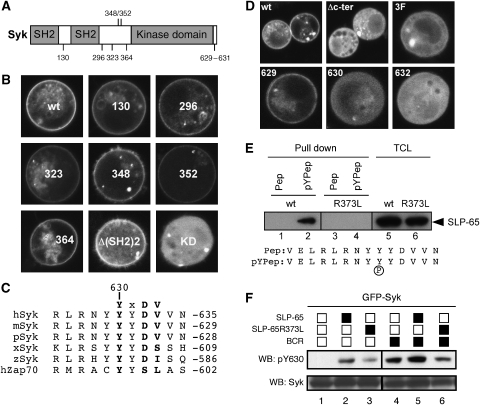
We next aimed to identify the tyrosine residue in Syk that is bound by the SH2 domain of SLP-65. Syk carries several tyrosines that upon Syk activation either become phosphorylated by Syk itself or by another protein tyrosine kinase (Furlong et al, 1997). One of these tyrosines (Y130) is located in the interdomain A between the two tandem SH2 domains, whereas five other tyrosines (Y296–Y364) are present in the interdomain B situated between the SH2 domains and the kinase domain of Syk (Figure 2A). Several signalling proteins including Vav, Cbl and PLCγ2 have been shown to bind to these tyrosines (Simon et al, 2005). In the S2 cell recruitment system described above, ΔNSLP-65–GFP was still able to bind to nLyn-Syk with point mutations of each of the interdomain B tyrosines to phenylalanine. It could also bind to an nLyn-Syk mutant with a deletion of the two tandem SH2 domains of Syk and interdomain A (Figure 2B). However, a K402A mutation of the ATP-binding site in the Syk kinase domain that renders the kinase inactive (nLyn-Syk-KD) abolishes recruitment of ΔNSLP-65–GFP to the membrane (Figure 2B), indicating that autophosphorylation of Syk is required for its binding by SLP-65.
Figure 2.
The SH2 domain of SLP-65 binds to a conserved tyrosine residue in the C terminus of activated Syk. (A) Schematic protein domain structure of Syk showing the two SH2 domains at the N terminus separated from the catalytic domain by a flexible linker called interdomain B. Also indicated are the positions of the tyrosine residues mutated in (B). (B) Translocation assay to test interaction between ΔNSLP-65–GFP and mutants of membrane-localized nLyn-Syk. Confocal images of S2 cells showing localization of ΔNSLP-65–GFP coexpressed with either nLyn-Syk (wt) or different tyrosine mutants in nLyn Syk: Y130F, Y296F, Y323F, Y348F, Y352F and Y364F. Localization of ΔNSLP-65–GFP expressed in combination with nLyn Syk with a deletion of its tandem SH2 domains, nLyn-Δ(SH2)2Syk, or a catalytically inactive form of Syk, nLyn-Syk K402A (KD), is shown in the bottom panel. (C) Sequence alignment of the C-terminus of Syk and its homologue ZAP-70, from human, mouse, pig, frog and zebra fish is shown. The conserved tyrosine Y630 and the motif YXDV are shown in bold. (D) Confocal images of S2 cells showing localization of ΔNSLP-65–GFP coexpressed with nLyn-Syk (wt) or a form lacking the seven most C-terminal amino acids (Δc-ter) or mutation of tyrosines 629–631 to phenylalanine (3F). Localization of ΔNSLP-65–GFP expressed in combination with nLyn-Syk carrying single tyrosine mutants Y629F, Y630F or mutation of D632 to alanine (D632A) is shown in the bottom panel. (E) Whole-cells lysates of S2 cells expressing SLP-65–GFP (wt) or the SH2 domain mutant SLP-65R373L–GFP were used in a peptide pull-down assay. Biotinylated peptides corresponding to the 14 C-terminal amino acids of Syk (sequence shown) in which Y630 is present either non-phosphorylated (Pep) or phosphorylated (pYPep) were bound to streptavidin-agarose beads and used to pull down SLP-65 from lysates of S2 transfectants. Reduced samples were separated by 10% SDS–PAGE and immunoblotted. Total cell lysates showing similar expression levels of SLP-65 are shown in the right panel. (F) Western blot analysis of Syk phosphorylation at Y630 in S2 cells. An antiserum recognizing phosphorylated Y630 was used to monitor Y630 phosphorylation when GFP–Syk was coexpressed alone (lane 1) or with wild-type SLP-65 (lane2) or the SH2 domain mutant SLP-65R373L (lane 3) or in combination with the BCR (lanes 4–6).
As none of the tyrosines in interdomain A and B are required for this binding, we shifted our attention to the C-terminal part of Syk, which contains tyrosines autophosphorylated by Syk (Furlong et al, 1997). The C-terminal sequence of Syk is highly conserved and contains a tyrosine (Y630) as part of an YXDV motif. This sequence is preferentially bound by the SH2 domains of SLP family adaptor proteins (Koretzky et al, 2006) and is evolutionarily conserved in Syk but not present in ZAP-70 (Figure 2C). Deletion of the last seven amino acids of the Syk tail (Δc-ter) that contains this binding motif abrogates ΔNSLP-65–GFP recruitment to the membrane and the same is true for the mutant nLyn-Syk3F (Y629-631F), in which the three tyrosines in the tail sequence were substituted to phenylalanine (Figure 2D, upper panels). Loss of binding upon mutation of either Y630 or D632, but not Y629, in the Syk tail identified the YXDV motif of Y630 as the binding target of the SH2 domain of SLP-65 (Figure 2D, lower panels).
To confirm the results of the S2 translocation assay, we used 14-amino-acid-long peptides of the C terminus of Syk with or without Y630 phosphorylation to pull down either wild type or SH2 mutant SLP-65 from total cellular lysates of transfected S2 cells (Figure 2E, lanes 5 and 6). In agreement with our earlier observations, SLP-65 only binds to the phosphorylated peptide, and this association is dependent on a functional SH2 domain (Figure 2E, lanes 1–4). Using a specific anti-pY630 antibody to monitor Y630 phosphorylation in an S2 cell reconstitution assay, we show that in the presence of SLP-65 or the BCR, the GFP–Syk fusion protein becomes phosphorylated on Y630 (Figure 2F, lanes 1, 2 and 4). The Y630 phosphorylation is increased upon coexpression of wild-type SLP-65, but not the SLP-65R373L mutant (Figure 2F, lanes 3, 5 and 6).
Syk/SLP-65 complex formation is required for pre-B-cell differentiation
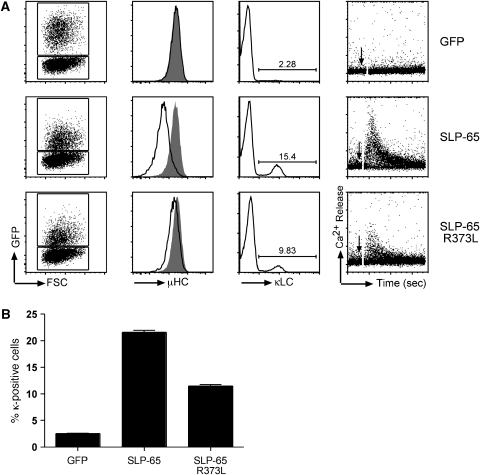
Our data suggest that the SH2 domain of the adaptor SLP-65 can specifically bind to a phosphotyrosine at the C terminus of its upstream kinase Syk. To test whether the SH2 domain of SLP-65 is required for the normal function of the adaptor, we transduced a murine SLP-65-deficient pre-B cell line with bicistronic internal ribosome entry site (IRES)–GFP containing retroviral vectors encoding GFP alone, SLP-65wt or SLP-65R373L. GFP was used as a marker for transfected cells. As shown previously (Flemming et al, 2003), the re-expression of SLP-65 in these cells results in reduced expression of the pre-BCR on the cell surface, increased differentiation to κ light chain expressing B cells and restoration of the calcium response (Figure 3A, second row). These responses are not observed in the GFP transfectant (Figure 3A, first row) and are markedly reduced in the SLP-65R373L transfectant (Figure 3A, third row; Figure 3B), indicating that the SH2 domain of the adaptor SLP-65 is required for efficient calcium responses and differentiation of pre-B cells.
Figure 3.
Mutation of the SH2 domain of SLP-65 abolishes its function. (A) SLP-65−/− pre-B cells were transduced with the indicated constructs. The first panel shows forward scatter versus GFP flow cytometry profiles. In the second panel, pre-BCR (μ surface expression of transduced cells (GFP+ve; black curves) were compared to untransduced cells (GFP-ve; grey curves) within the same culture. Expression of κ light chain (κLC) on the surface of transduced cells measured 3 days after withdrawal of IL-7 from culture is shown in the panel 3. In the panel 4, reconstituted SLP-65−/− cells were loaded with Indo-1 and stimulated with 20 μg/ml of anti-IgM to induce Ca2+ responses. Pre-BCR-induced intracellular Ca2+ mobilization as indicated by the Indo-1 ratio as a function of time is shown. The stimulus was added after 1 min (indicated by arrow) and measured for 5 min. (B) Statistical analysis of differentiation assay as measured by κ light chain (κLC) expression on the surface of the cells 3 days after IL-7 withdrawal. Error bars indicate s.e.m., n=3.
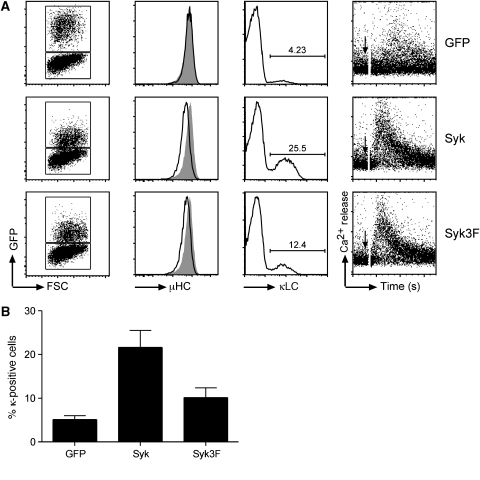
To test for the biological role of the C-terminal tail of Syk, we generated a Syk-deficient pre-B cell line. This line was derived from the bone marrow of a Sykfl/f × mb-1/Cre mouse, in which both Syk alleles are deleted in the B-cell lineage starting from the pro/pre-B-cell stage (Saijo et al, 2003; Hobeika et al, 2006). The Syk-deficient pre-B cells were expanded in culture with interleukin (IL)-7 and then transduced with IRES–GFP-containing retroviral vectors coding for GFP alone, Sykwt or the Syk3F mutant. Cells transfected with GFP show no pre-BCR downregulation and minor differentiation. These cells are also defective in Ca2+ mobilization after pre-BCR crosslinking (Figure 4A, first row). The weak Ca2+ response observed is probably mediated by the Syk-family kinase ZAP-70, which is expressed in pre-B cells (Schweighoffer et al, 2003). The re-expression of Sykwt results in a downregulation of pre-BCR expression, increased differentiation after withdrawal of IL-7 from culture and a robust Ca2+ response upon pre-BCR crosslinking (Figure 4A, second row). In comparison to the Syk wt transfectants, pre-B cells reconstituted with Syk3F mutant show slightly impaired pre-BCR downregulation and Ca2+ responses and differentiate less efficiently to κ-expressing cells (Figure 4A, third row; Figure 4B). Reduced differentiation is also observed in pre-B cells expressing Syk with the point mutations Y630F and D632A (Supplementary Figure S2). Together, these data demonstrate that the Syk/SLP-65 complex promotes pre-B-cell differentiation.
Figure 4.
C-terminal tyrosines of Syk are required for pre-B-cell responses. (A) Syk−/− pre-B cells were transduced with the constructs indicated on the right. The data are represented in the same way as in Figure 3, namely forward scatter versus GFP (left panel), pre-BCR (μ surface expression (panel 2), surface expression of κ light chain (κLC) 3 days after IL-7 withdrawal (panel 3) and Ca2+ flux after pre-BCR activation with anti-IgM antibodies (panel 4). Data are representative of at least three independent experiments. (B) Quantitation of pre-B-cell differentiation as in Figure 3B. Error bars indicate s.e.m.
SLP-65-binding motif in Syk is required for B-cell development
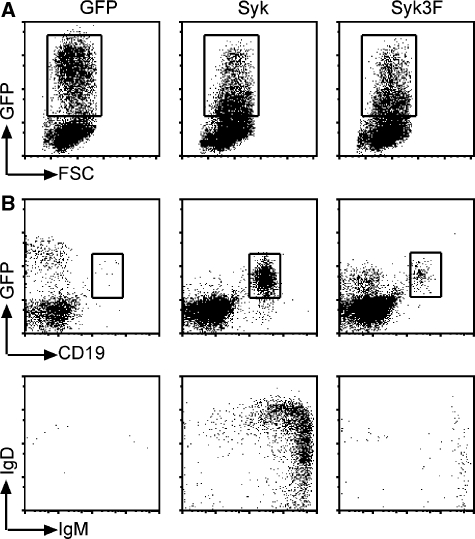
In the Sykfl/f × mb-1/Cre mice, B-cell development is arrested at the pro-B-cell stage and only a few cells escape the block to become pre-B cells or immature B cells (data not shown). To study the function of the Syk3F mutant in vivo, we performed reconstitution experiments with haematopoietic stem cells (HSC) isolated from the Sykfl/f × mb-1/Cre mice. These HSCs were transduced with retroviral vectors carrying IRES–GFP, Sykwt-IRES–GFP or Syk3F-IRES–GFP inserts (Figure 5A). Equal numbers of a mixed population of GFP-positive and GFP-negative cells were injected into sub-lethally irradiated Rag−/−/γC−/− mice and blood was analysed for B cells 4 weeks after injection. Mice reconstituted with only GFP-expressing HSCs did not produce any B cells. Mature B cells are found in mice blood reconstituted with Sykwt-expressing HSCs, whereas only immature B cells are found in mice reconstituted with Syk3F-expressing HSCs (Figure 5). Analysis of B cells in spleens of reconstituted mice 7 weeks after reconstitution confirmed that B-cell development was blocked in mice reconstituted with HSCs expressing Syk3F (Supplementary Figure S3). Reconstitution experiments performed using Syk−/− pre-B cells also showed a similar block (Supplementary Figure S4). Thus, these results show that although Syk3F can signal to some extent in pre-B cells, it does not support normal B-cell development.
Figure 5.
Loss of Syk/SLP-65 interaction leads to a block in B-cell maturation. (A) HSCs were isolated from mice with a conditional deletion of the Syk gene (Sykfl/f × mb-1/Cre), and then retrovirally transduced with the indicated constructs and expanded in culture. Equal numbers of cells were used for reconstitution in sublethally irradiated Rag2−/−γC−/− mice. (B) B-cell development was analysed 30 days after reconstitution. Blood from reconstituted mice was analysed by flow cytometry for CD19 and GFP expression (left panel). Gated CD19+GFP+ lymphocytes were analysed for IgM and IgD surface expression. Data shown are representative of at least three independent experiments.
Signalling defects in B cell lines without Syk/SLP-65 complex formation
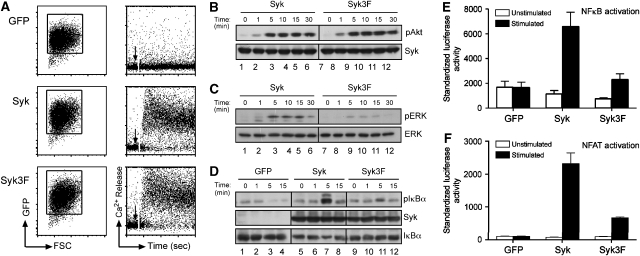
To learn more about the signalling function of the SLP-65-binding site in Syk, we used Syk-deficient DT40 cells. DT40 is a versatile model to study BCR signalling, as many loss-of-function mutants of kinases and adaptors have been characterized in this system (Takata et al, 1994; Kurosaki, 1999). DT40 Syk−/− cells are defective in BCR signalling and cannot generate a calcium response (Takata et al, 1994). These Syk-deficient DT40 cells were transduced with retroviral vectors containing IRES–GFP, Syk–IRES–GFP or Syk3F–IRES–GFP (Figure 6). The transduced DT40 cells were sorted for GFP-positive cells and tested for calcium mobilization upon BCR engagement (Figure 6A). DT40 Syk−/− cells expressing only GFP are still defective in calcium flux, whereas it is restored in cells reconstituted with either Sykwt or the Syk3F mutant (Figure 6A). It has previously been shown that DT40 Syk−/− cells are also defective in NF-κB, extracellular signal-regulated kinase (ERK) and Akt activation upon BCR stimulation (Supplementary Figure S5C) (Jiang et al, 1998; Gold et al, 1999; Brummer et al, 2003). There is no discernible difference in Akt activation between Syk- and Syk3F-expressing cells in response to BCR crosslinking (Figure 6B). However, cells expressing Syk3F show impaired ERK activation and IκBα phosphorylation as compared with Sykwt (Figure 6C and D). As phosphorylation of IκBα is defective, we also measured NF-κB activation using an NF-κB luciferase assay, which is defective in cells expressing mutations in the Syk tail (Figure 6E; Supplementary Figure S3B).
Figure 6.
Pleiotropic defects in signalling responses mediated by Syk3F in Syk−/− DT40 cells. (A) Syk−/− DT40 cells were transfected with the mouse ecotropic receptor and then retrovirally transduced with the indicated constructs. Transduced cells were enriched for GFP expression as shown in the forward scatter versus GFP profiles (left panel). Intracellular Ca2+ flux was measured after stimulation of the BCR with 10 μg/ml of anti-IgM (M4) (right panel). (B) Western blotting of whole-cell lysates from Syk−/− DT40 cells reconstituted with either Sykwt or Syk3F and stimulated with 5 μg anti-IgM for the indicated time points. Reduced protein lysates were separated by 10% SDS–PAGE and analysed for pAkt (S473) and Syk expression. (C) ERK activation of cells stimulated with anti-IgM (M4) for the indicated times and western blotted for phosphorylated ERK and total ERK. (D) Whole-cell lysates from Syk−/− DT40 cells reconstituted with either GFP or Sykwt or Syk3F and stimulated with 5 μg anti-IgM for the indicated time points were western blotted and analysed for phosphorylation of S32 of IκBα using a phospho-specific antibody. Expression levels of Syk and IκBα are shown in the lower panels. (E) An NF-κB luciferase assay was used to measure NF-κB activation in reconstituted Syk−/− DT40 cells. Luciferase activity was measured 6 h after stimulation of the cells with anti-IgM and Renilla luciferase; under the control of thymidine kinase promoter was used to normalize for transfection and mean values of triplicates are shown. Error bars indicate s.e.m. (F) Reconstituted Syk−/− DT40 cells were transfected with an NFAT luciferase reporter plasmid and luciferase activity was measured 6 h after stimulation of the cells with anti-IgM. Renilla luciferase was used to normalize for transfection and mean values of duplicate stimulations are shown. Error bars indicate s.e.m. Data shown are representative of three to five independent experiments.
BCR triggering is accompanied by an influx of calcium that activates components of the NFAT pathway resulting in the dephosphorylation and translocation of the transcription factor NFAT into the nucleus. We tested for NFAT activation using an NFAT-driven luciferase assay. In accordance with their defective calcium response and ERK activation (Figure 6A), DT40Syk−/− cells expressing only GFP do not activate the reporter gene upon BCR engagement (Figure 6E). The re-expression of Sykwt restores both responses; however, Syk3F-expressing cells are defective in NFAT activation (Figure 6F), although their calcium response is similar to that of Sykwt-expressing cells (Figure 6A).
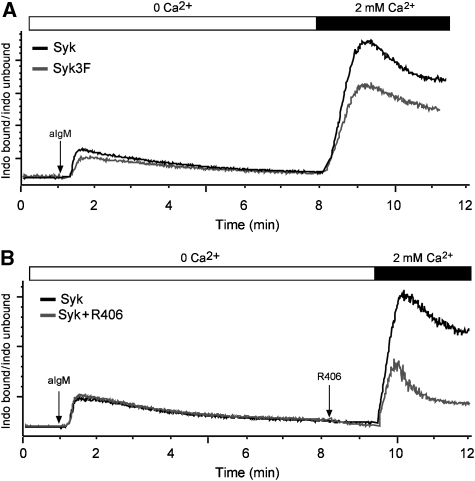
To explain the discrepancy between the apparently normal calcium levels and the defective NFAT activity in the DT40Syk3F mutant, we analysed the calcium increase in Syk- and Syk3F-expressing DT40 cells in more detail (Figure 7). The sustained increase in intracellular Ca2+ levels in activated B cells is composed of two distinct phases: a transient release of Ca2+ from intracellular stores such as the ER and the influx of Ca2+ from the outside through CRAC channels that are activated upon ER store depletion. To distinguish between the two phases, we first monitored the Ca2+ flux of Syk- and Syk3F-expressing DT40 cells in the absence of free extracellular Ca2+. Both transfectants responded to BCR stimulation with a transient rise in Ca2+ levels, which returned to base line levels within 7 min (Figure 7A). The addition of 2 mM Ca2+ at this point triggers Ca2+ entry into the cell and a strong increase in intracellular Ca2+ levels (Figure 7A). Interestingly, this SOCE response was more pronounced in the Syk- than in the Syk3F-expressing DT40 cells. Furthermore, this calcium entry is also inhibited by the specific Syk tyrosine kinase inhibitor, R406 (Cha et al, 2006; Figure 7B). These results indicate that Syk kinase activity and the adaptor function of Syk are required for Ca2+ entry and sustained signalling.
Figure 7.
Syk regulates BCR-mediated Ca2+ entry in DT40 cells. (A) Measurement of Ca2+ flux of Syk−/− DT40 cells reconstituted with Syk (black) or Syk3F (grey) after BCR crosslinking with 10 μg/ml of M4 antibody. Cells were placed in medium where extracellular Ca2+ was chelated by EGTA, and after store depletion, 2 mM of Ca2+ was added at the indicated time point. (B) Ca2+ measurement performed as in (A) with Syk−/− DT40 cells reconstituted with Syk (grey and black curve). In the grey curve, Syk-specific inhibitor R406 was added after store depletion and 1 min before addition of 2 mM Ca2+ (grey curve).
SH2 domain of SLP-65 connects the BCR to distinct signalling pathways
A stronger signalling defect is observed in the SLP-65R373L-expressing pre-B cells as opposed to Syk3F-expressing pre-B cells, suggesting that the SH2 domain of SLP-65 has other functions besides binding to phosphorylated Y630 of Syk. Indeed, it has been shown that this SH2 domain also binds to phosphorylated Y204 in Ig-α (Engels et al, 2001; Kabak et al, 2002). To learn more about these differences, we compared the signalling function of Syk3F and SLP-65R373L–GFP with that of their wt counterparts in the S2 reconstitution system (Supplementary Figure S6). Sykwt and Syk3F phosphorylate Ig-α and SLP-65 to similar levels, showing that Syk3F can be activated at the BCR. The SLP-65R373L–GFP mutant, on the other hand, is less well phosphorylated than SLP-65wt, suggesting an early defect in signalling at the BCR.
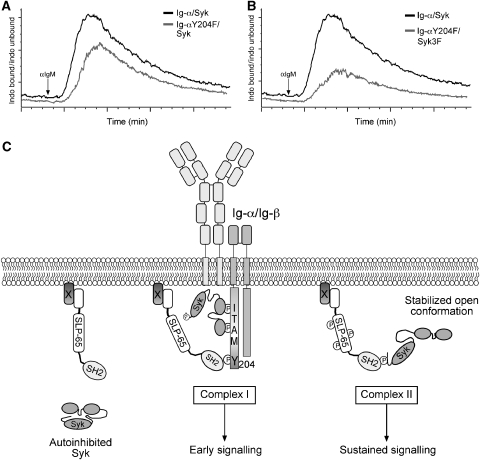
To understand the regulation of signalling by binding of the SH2 domain of SLP-65 to either Y204 of Ig-α or to Y630 of Syk, we reconstituted Ig-α/Syk double deficient pro-B cells with a BCR containing either Ig-α wt or Ig-αY204F and coexpressed either Sykwt or Syk3F. Comparison of Ca2+ responses of these transfectants shows a delay in induction of peak Ca2+ mobilization when SLP-65 cannot bind to Y204, suggesting a requirement of this interaction in early signalling (Figure 8A). The Ig-αY204F and Syk3F double transfectants, in which SLP-65 can no longer bind to both Ig-α and Syk, show a drastic impairment of Ca2+ influx after BCR crosslinking (Figure 8B). Taken together, these results reveal a complex role of the adaptor SLP-65 in both allosteric regulation of an upstream kinase and in connecting it to specific downstream signalling pathways.
Figure 8.
Model for activation of BCR signalling. (A) Ig-α/Syk double-deficient cells were reconstituted to express Syk along with a BCR containing either Ig-αwt or Ig-αY204F, and Ca2+ flux was measured after crosslinking of the BCR with 10 μg/ml of anti-IgM. Baseline measurement was performed for 1 min before addition of anti-IgM and Ca2+ influx was measured for 5 min. (B) Ig-α/Syk double-deficient cells expressing a BCR containing Ig-αY204F along with either Sykwt, or Syk3F were compared for Ca2+ influx after BCR crosslinking as described above. (C) In resting cells, Syk exists in a closed autoinhibited conformation in a manner analogous to ZAP-70. Upon activation of the BCR, Syk binds to phosphorylated ITAMs and becomes activated. Activated Syk phosphorylates other neighbouring ITAM tyrosines and also the non-ITAM tyrosine of Ig-α. Phosphorylated Y204 serves as a docking site for the SH2 domain of SLP-65. Binding of SLP-65 to Ig-α brings it into close proximity of activated Syk (Complex I), which can then phosphorylate SLP-65 on multiple tyrosines leading to activation of many early signalling events. Activation of Syk leads to a conformation change in Syk, leading to exposure of its C terminus unmasking a phosphorylated tyrosine to which the SH2 domain of SLP-65 can bind. Binding of SLP-65 to Syk stabilizes Syk in an open active conformation (Complex II) and nucleates specific signalling complexes that activate many B-cell signalling pathways. Alternatively, the SLP-65/Syk complex could localize to specific locations in the cell and then mediate sustained signalling.
Discussion
The tyrosine kinase Syk is a central signalling element for many ITAM-containing receptors. Our study shows that Syk is not only activated via binding to the phosphorylated ITAM tyrosines, but also by its immediate downstream target, the adaptor protein SLP-65. This unusual kinase–substrate interaction involves binding of the SH2 domain of SLP-65 to the autophosphorylated Y630 at the C-terminal tail of Syk. We show that the tyrosines in the tail of Syk are required for sustained Ca2+ signalling in activated B cells and for normal B-cell development.
Key to understanding our data is the allosteric regulation of Syk. Similar to many signalling proteins, Syk seems to adopt different conformations inside a cell ranging from a closed autoinhibited to a more open active structure (Cheetham, 2004; Wossning and Reth, 2004). In the absence of an activating signal, the equilibrium between autoinhibition and activation is shifted predominantly towards the autoinhibited state. Indeed, the expression of Syk alone in S2 cells does not result in kinase activity (Supplementary Figure S1). In ZAP-70, the intramolecular inhibitory interactions mostly involve amino acids in the interdomain A, the region bridging the two SH2 domains (Deindl et al, 2007). This region forms contacts with two tyrosines in the interdomain B and two tyrosines in the distal end of the kinase domain of ZAP-70, one of which is homologous to Y630 of Syk, to form an inhibitory linker-kinase sandwich. Most amino acids involved in autoinhibition are conserved between ZAP-70 and Syk, suggesting that both kinases may have a similar autoinhibitory structure.
Our finding that the Syk3F mutant phosphorylates Ig-α more efficiently than its wild-type counterpart (Supplementary Figure S6) supports the notion that the tyrosines in the C-terminal tail of Syk are involved in autoinhibition. Furthermore, an antibody directed against the C-terminal tail of Syk can detect its epitope only in activated Syk, suggesting that the C terminus is buried and not available for binding in the autoinhibited state (Kimura et al, 1996). It has also been shown that tyrosine to phenylalanine mutations at the C-terminal tail of ZAP-70 generate a gain-of-function phenotype in Jurkat T cells (Zeitlmann et al, 1998). The expression of a kinase-negative Syk3F mutant (Syk DP) even has a dominant-positive effect on B-cell signalling (Hsueh et al, 2002). Apparently, SykDP exists in a more open structure, thus activating the adaptor function of Syk involving interdomain B tyrosines even in the absence of kinase activity. Binding of the SH2 domain of SLP-65 to the C-terminal tail of Syk could serve to stabilize the kinase in an open active conformation. Interestingly, a similar mechanism has been proposed for the Tec family kinase Itk, in which binding of its SH2 domain to a phosphorylated tyrosine in SLP-76 is required for maintenance of Itk activation (Bogin et al, 2007). Binding of the SLP-65 SH2 domain to Syk may also protect Y630 from dephosphorylation, thereby keeping Syk in an active conformation.
In the solved structure of ZAP-70, the inhibitory linker-kinase sandwich is located in close proximity to the N terminus of the kinase. Fusion of a GFP domain to the N terminus of Syk probably interferes with autoinhibition. The reduced autoinhibition of GFP–Syk may explain why this fusion protein is more readily trapped by the SH2 domain of SLP-65 in an active conformation (see Figure 1A). The interference with autoinhibition may also be one of the reasons why nLyn-Syk (Supplementary Figure S1) or other membrane-bound forms of Syk are constitutively active (Kolanus et al, 1993).
In the open active conformation, Syk not only functions as a kinase but also as an adaptor mediating interactions with c-Cbl and PLC-γ2, which bind to phosphorylated tyrosines in the interdomain B of the kinase (Ota et al, 1996; Furlong et al, 1997; Zhang et al, 2002; Groesch et al, 2006). While the interaction with c-Cbl downregulates Syk activity, interaction with PLC-γ2 has a positive role in Syk-mediated lymphocyte activation (Hong et al, 2002; Simon et al, 2005). The Syk/SLP-65 complex we describe in this study has an activating role in connecting the BCR to downstream signalling.
A recent reconstitution study of the SLP adaptor and Syk in Jurkat T cells deficient for SLP-76 reveals a requirement for both SLP-65 and Syk expression to couple the TCR to Ca2+ mobilization (Abudula et al, 2007). Residual Syk phosphorylation we observe in experiments with SLP-65R373L (Figure 2F, lane 3) hints at the existence of an alternate mode of SLP-65 binding to Syk. Both Syk and SLP-65 can interact with PLC-γ2 (Chiu et al, 2002; Groesch et al, 2006) and could collaborate in the binding, membrane recruitment and activation of PLC-γ2, thus resulting in increased Ca2+ mobilization. The Syk/SLP-65 complex we characterize here has a greater effect on the late rather than the early phase of Ca2+ mobilization. Our finding that either Syk kinase inhibition or mutation of the tail inhibits Ca2+ influx (Figure 7A and B) is in line with a recent study showing that SLP-65 and tyrosine kinase activity of Lyn and Syk kinases are required for CRAC channel function, which is induced in a BCR-independent manner by IP3 (Chung et al, 2007). To mediate calcium entry upon store depletion, a Ca2+ sensor in the ER (STIM-1) must communicate with the CRAC channel protein Orai (Liou et al, 2005; Peinelt et al, 2006). The molecular details of this interaction are not known at present, but in B cells, it may require a tyrosine phosphorylation step mediated by the Syk/SLP-65 complex. The signalling and developmental defects in Syk3F-expressing cells are likely due to defective Ca2+ entry, as activation of NFAT, ERK and NF-κB depends on Ca2+ signalling; however, we cannot rule out an effect of this complex on other signalling pathways.
At present, we cannot exclude the possibility that other molecules bind to the tail of Syk or that the SLP-65-Syk interaction is indirect. However, the fact that the SH2 domains of the SLP family have a strong preference for the YXDV motif and together with our mutational analyses, and the peptide pull-down data make it likely that the SH2 domain of SLP-65 binds directly to Syk. The SH2 domain of SLP-65 can have several binding partners in B cells besides Syk and Ig-α (Engels et al, 2001; Sauer et al, 2001; Tsuji et al, 2001; Kabak et al, 2002). In this respect, it is interesting that combined mutation of the SLP-65-binding sites in Ig-α and Syk, results in a signalling defect that is similar to that of the SH2 domain mutant of SLP-65 (Figures 3 and 8B).
In summary, our data show that the molecules Syk and SLP-65 can have diverse roles in controlling signalling at distinct time points during BCR signalling (Figure 8C). Prior to BCR stimulation, Syk resides mostly in the cytosol in an autoinhibitory state, while SLP-65 is constitutively attached to the plasma membrane via its N-terminal leucine zipper (Figure 8C). BCR activation and phosphorylation results in ITAM binding and Syk activation allowing efficient phosphorylation of Y204 of Ig-α (Figure 8C). The SH2 domain of SLP-65 then binds to phosphorylated Y204 to couple the BCR to early Ca2+ signalling (Patterson et al, 2006). After its activation, the BCR is rapidly internalized, and this process could also abolish Syk activity (Pure and Tardelli, 1992; Stoddart et al, 2002). However, the binding of the SH2 domain of SLP-65 to the tail of Syk ensures that Syk remains active as a membrane-associated kinase even in the absence of a BCR (Figure 8C). The leucine zipper and other interaction sequences of SLP-65 may then localize the Syk/SLP-65 complex at a subcellular region, where this complex can regulate CRAC channel function and sustained signalling for maximal B-cell activation. The identification of yet unknown phosphorylation targets of the SLP-65/Syk complex may allow us to learn more about the pathways mediating differentiation of pre-B cells and the regulation of Ca2+ channels in B cells.
Materials and methods
Cell lines, cell culture and transfections
Schneider S2 cells (gift from K Karjalainen) were grown in Schneider's Drosophila medium (Invitrogen Life Technologies) and transfected using CellFectin (Invitrogen Life Technologies) as described earlier (Rolli et al, 2002). The SLP-65−/− pre-B cell line has been described previously (Flemming et al, 2003). The Syk−/− pre-B cell line was established from cell suspensions derived from bone marrow of Sykfl/f × mb-1/Cre-knockin mouse. Ig-α−/−Syk−/− double-deficient pro-B cell line was generated from bone marrow cultures of Sykfl/fl mice homozygous for the mb-1/Cre allele (Hobeika et al, 2006). The pro-B cell lines were cultured in Iscove's medium supplemented with 10% fetal calf serum (FCS) (Vitromex), 100 U/ml penicillin, 100 U/ml streptomycin (Invitrogen Life Technologies), 5 × 10−5 M 2-ME and IL-7. Syk-deficient DT40 cells were grown as described earlier (Takata et al, 1994; Brummer et al, 2003). Transfection of the Phoenix retroviral producer line using GeneJuice (Novagen) was performed according to the manufacturer's instructions.
Plasmids and mutagenesis
The vectors pRmHa3, pMOWS and pMIG have been described earlier (Rolli et al, 2002; Kohler et al, 2005). The vectors used in S2 transfections, encoding for SLP-65, Syk and the BCR components—Ig-α, Ig-β and scδm—have been described earlier (Rolli et al, 2002). pRmHa3 vector encoding nLyn-Syk was created by PCR amplifying the first 24 amino acids of Lyn using the primers 5′-CCGGAATTCCGCCACCATGGGATGTATTAAATC-3′ as the forward primer and 5′-CGCGGATCCCTACTGGTTGAGTCTTCG-3′ as the reverse primer. The PCR fragment was cut with EcoRI and BamHI and inserted into pRmHa3 Syk cut with the same enzymes. Site-directed mutagenesis was performed by sequential PCR steps using nLyn-Syk as template (primer sequences are available upon request). cDNA encoding mouse ecotropic receptor (mEcoR), a gift from Dr Michael Gold, was inserted into pMOWS-IRES-CD8 vector (Herzog and Jumaa, 2007). cDNA encoding Ig-αwt and Ig-αY204F (Storch et al, 2007) were PCR amplified and cloned into pM-IRES-CD8.
Retroviral transduction of DT40 cell lines
DT40Syk−/− cells (2 × 107) were electroporated with 20 μg of retroviral vector pMOWS-mEcoR-IRES CD8 plasmid (Dr Thomas Wossning) DNA using a Bio-Rad Gene Pulser II set at 300 V/975 μF. At 24 h after electroporation, cells were mixed with retroviral supernatants from Phoenix cells transfected with pMOWS/CD8-mEcoR and centrifuged at 1800 r.p.m. at 37°C for 3 h. Cells were expanded and sorted for CD8 expression. DT40Syk−/− cells expressing mouse ecotropic receptor were then used for retroviral transduction with pMIG vectors and GFP-positive cells were sorted.
Luciferase assays
For the measurement of NFAT or NF-κB activity, 2 × 107 cells were transiently transfected with 2 μg of Renilla luciferase plasmid and 10 μg of the NFAT reporter containing the NFAT-AP1 elements of the minimal human IL-2 promotor (Durand et al, 1988), pNFATluc Firefly (Dr Gerald Crabtree) or the NF-κB reporter plasmid containing 2κB elements from the long terminal repeat of the human immunodeficiency virus type 1, p2xHIVκB-Luc (from Dr Yinon Ben-Neriah). After electroporation, the cells were cultured for 18 h in culture medium and then starved (1% FCS, 0.1% chicken serum) for 30 min, following which the cells were treated with M4 antibody for 6 h, lysed and luciferase activities (Renilla and firefly) assayed with the Dual Luciferase assay kit from Promega. Luciferase activity was standardized against Renilla luciferase activity as an internal control for transfection efficiency.
HSC reconstitution experiments
Sykfl/f × mb-1/Cre were injected with 5-fluorouracil (5-FU) and mice were killed 3 days later, and bone marrows put in culture in Iscove's medium supplemented with 10% fetal bovine serum, insulin, primatone, penicillin, streptomycin, IL-3, IL-6 and Stem cell factor. Cells were retrovirally transduced, enriched for GFP-expressing cells and expanded in culture. Rag−/−/γC−/− mice were sublethally irradiated at 450 rads and were injected intraveneously 24 h later with 1 × 106 HSCs. Mice were bled 4 weeks later and B cells were analysed by flow cytometry. Animal experiments were carried out in accordance with guidelines of the German law and the Max-Planck Institute for Immunobiology.
Antibodies
The following antibodies were used for western blotting: Syk (4D10 and N19) from Santa Cruz Biotech.; the anti-phosphotyrosine Ab 4G10 from Upstate Biotech.; phosphorylated ERK detected with anti-activated MAPK 12D4 (Nanotools) and phosphorylated IκBα (S32), total IκBα and Akt (S473) from Cell Signaling. The antibody used to detect SLP-65 has been described previously (Wienands et al, 1998). The anti-pY630 Syk antibody was generated by immunizing rabbits with phosphopeptides, and the antiserum was affinity purified to generate a phospho-specific antibody (Eurogentec).
Cell harvesting, phosphotyrosine analysis and peptide pull-down assay
S2 transfectants were collected and lysed as described previously (Rolli et al, 2002). For peptide pull-down assays, transfected cells were lysed in lysis buffer (50 mM Tris–HCl pH 7.4, 137.5 mM NaCl, 10% glycerol, 1 mM sodium orthovanadate, 1 mM sodium fluoride, protease inhibitor cocktail (Sigma)) containing 0.1% NP-40. An aliquot of total cell lysate was used for western blot analysis, and the remainder was incubated with biotinylated peptides (IRIS Biotech) bound to streptavidin agarose beads (Pierce) for 1 h at 4°C. Beads were then washed thrice in lysis buffer, following which the proteins were denatured, separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE), transferred onto nitrocellulose membranes and analysed using enhanced chemiluminescence detection system (ECL, Amersham Biosciences) for SLP-65.
Stimulation of B cells
DT40 cells were stimulated with 5 μg/ml of anti-IgM Ab M4 (Southern Biotechnology) as described previously. Cells were lysed either in lysis buffer with 0.1% NP-40 or RIPA buffer (Brummer et al, 2003), and cleared lysates were boiled in Laemmli buffer for 5 min. Aliquots equivalent to 1 × 106 cell were separated by SDS–PAGE and western blot analysis was performed using the enhanced chemiluminescence (ECL, Amersham Biosciences).
Flow cytometry
Cells were stained for FACS analysis (FACSCalibur and LSRII; BD Biosciences) using Cy5-anti-IgM (μ chain specific; Jackson Labs), PE-anti-Ig-D (Southern Biotech), CD19 (BD Biosciences) or biotin-anti-κ (Southern Biotech) and streptavidin-Cy5 (Dianova).
Measurement of Ca2+ release
Transiently transfected cells (1 × 106) were loaded with 5 mg/ml indo-1 acetoxymethyl ester and 0.5 mg/ml pluoronic F-127 (Molecular Probes) in medium supplemented with 1% FCS at 37°C for 45 min. Following loading, cells were washed and resuspended in medium containing 1% FCS and fluorometric analysis was performed at 37°C. The Ca2+ response was induced by the addition of 20 μg/ml goat anti-mouse μ or mouse anti-chicken μ M4 (Southern Biotech). The Syk inhibitor R406 was provided by Rigel Pharmaceuticals Inc. to Dr Hassan Jumaa.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr A Tarakhovsky for generously providing the Syk floxed mice (all requests concerning the mice may be addressed to tarakho@mail.rockefeller.edu), Dr T Kurosaki for Syk−/− DT40 cells and Dr H Jumaa for SLP-65−/− deficient cells and protocols and reagents. Andreas Wurch for help with FACS, Uta Stauffer and Christa Kalmbach Zuern for technical assistance. We also thank Mahima Swamy and Dr Peter Nielsen, Dr Lise Leclercq and Dr Susana Minguet for helpful discussions. This work was supported by Grants SFB746 and SFB620 from the Deutsche Forschungsgemeinschaft and FORSYS from the Bundesministerium fuer Bildung and Forschung.
References
- Abudula A, Grabbe A, Brechmann M, Polaschegg C, Herrmann N, Goldbeck I, Dittmann K, Wienands J (2007) SLP-65 signal transduction requires SH2-mediated membrane anchoring and a kinase-independent adaptor function of Syk. J Biol Chem 282: 29059–29066 [DOI] [PubMed] [Google Scholar]
- Bogin Y, Ainey C, Beach D, Yablonski D (2007) SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci USA 104: 6638–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer T, Naegele H, Reth M, Misawa Y (2003) Identification of novel ERK-mediated feedback phosphorylation sites at the C-terminus of B-Raf. Oncogene 22: 8823–8834 [DOI] [PubMed] [Google Scholar]
- Cha HS, Boyle DL, Inoue T, Schoot R, Tak PP, Pine P, Firestein GS (2006) A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J Pharmacol Exp Ther 317: 571–578 [DOI] [PubMed] [Google Scholar]
- Cheetham GM (2004) Novel protein kinases and molecular mechanisms of autoinhibition. Curr Opin Struct Biol 14: 700–705 [DOI] [PubMed] [Google Scholar]
- Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T (1995) Syk tyrosine kinase required for mouse viability and B-cell development. Nature 378: 303–306 [DOI] [PubMed] [Google Scholar]
- Chiu CW, Dalton M, Ishiai M, Kurosaki T, Chan AC (2002) BLNK: molecular scaffolding through ‘cis'-mediated organization of signaling proteins. EMBO J 21: 6461–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SC, Limnander A, Kurosaki T, Weiss A, Korenbrot JI (2007) Coupling Ca2+ store release to Icrac channel activation in B lymphocytes requires the activity of Lyn and Syk kinases. J Cell Biol 177: 317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J (2007) Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell 129: 735–746 [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI (1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858 [DOI] [PubMed] [Google Scholar]
- Durand DB, Shaw JP, Bush MR, Replogle RE, Belagaje R, Crabtree GR (1988) Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol 8: 1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels N, Wollscheid B, Wienands J (2001) Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-α. Eur J Immunol 31: 2126–2134 [DOI] [PubMed] [Google Scholar]
- Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A (2001) Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol 2: 316–324 [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185 [DOI] [PubMed] [Google Scholar]
- Flemming A, Brummer T, Reth M, Jumaa H (2003) The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nat Immunol 4: 38–43 [DOI] [PubMed] [Google Scholar]
- Fu C, Turck CW, Kurosaki T, Chan AC (1998) BLNK: a central linker protein in B cell activation. Immunity 9: 93–103 [DOI] [PubMed] [Google Scholar]
- Furlong MT, Mahrenholz AM, Kim KH, Ashendel CL, Harrison ML, Geahlen RL (1997) Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim Biophys Acta 1355: 177–190 [DOI] [PubMed] [Google Scholar]
- Gallo EM, Cante-Barrett K, Crabtree GR (2006) Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol 7: 25–32 [DOI] [PubMed] [Google Scholar]
- Goitsuka R, Fujimura Y, Mamada H, Umeda A, Morimura T, Uetsuka K, Doi K, Tsuji S, Kitamura D (1998) BASH, a novel signaling molecule preferentially expressed in B cells of the bursa of Fabricius. J Immunol 161: 5804–5808 [PubMed] [Google Scholar]
- Gold MR, Scheid MP, Santos L, Dang-Lawson M, Roth RA, Matsuuchi L, Duronio V, Krebs DL (1999) The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J Immunol 163: 1894–1905 [PubMed] [Google Scholar]
- Groesch TD, Zhou F, Mattila S, Geahlen RL, Post CB (2006) Structural basis for the requirement of two phosphotyrosine residues in signaling mediated by Syk tyrosine kinase. J Mol Biol 356: 1222–1236 [DOI] [PubMed] [Google Scholar]
- Herzog S, Jumaa H (2007) The N terminus of the non-T cell activation linker (NTAL) confers inhibitory effects on pre-B cell differentiation. J Immunol 178: 2336–2343 [DOI] [PubMed] [Google Scholar]
- Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M (2006) Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA 103: 13789–13794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JJ, Yankee TM, Harrison ML, Geahlen RL (2002) Regulation of signaling in B cells through the phosphorylation of Syk on linker region tyrosines. A mechanism for negative signaling by the Lyn tyrosine kinase. J Biol Chem 277: 31703–31714 [DOI] [PubMed] [Google Scholar]
- Hsueh RC, Hammill AM, Lee JA, Uhr JW, Scheuermann RH (2002) Activation of the Syk tyrosine kinase is insufficient for downstream signal transduction in B lymphocytes. BMC Immunol 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Craxton A, Kurosaki T, Clark EA (1998) Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1, and p38 mitogen-activated protein kinase. J Exp Med 188: 1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Pleiman CM, Pao L, Schneringer J, Hippen K, Cambier JC (1995) Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol 155: 4596–4603 [PubMed] [Google Scholar]
- Jumaa H, Hendriks RW, Reth M (2005) B cell signaling and tumorigenesis. Annu Rev Immunol 23: 415–445 [DOI] [PubMed] [Google Scholar]
- Kabak S, Skaggs BJ, Gold MR, Affolter M, West KL, Foster MS, Siemasko K, Chan AC, Aebersold R, Clark MR (2002) The direct recruitment of BLNK to Ig-α couples the B-cell antigen receptor to distal signaling pathways. Mol Cell Biol 22: 2524–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Sakamoto H, Appella E, Siraganian RP (1996) Conformational changes induced in the protein tyrosine kinase p72syk by tyrosine phosphorylation or by binding of phosphorylated immunoreceptor tyrosine-based activation motif peptides. Mol Cell Biol 16: 1471–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler F, Storch B, Kulathu Y, Herzog S, Kuppig S, Reth M, Jumaa H (2005) A leucine zipper in the N terminus confers membrane association to SLP-65. Nat Immunol 6: 204–210 [DOI] [PubMed] [Google Scholar]
- Kolanus W, Romeo C, Seed B (1993) T cell activation by clustered tyrosine kinases. Cell 74: 171–183 [DOI] [PubMed] [Google Scholar]
- Koretzky GA, Abtahian F, Silverman MA (2006) SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat Rev Immunol 6: 67–78 [DOI] [PubMed] [Google Scholar]
- Kurosaki T (1999) Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol 17: 555–592 [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Y, Beitz LO, Scharenberg AM, Donovan JA, Kinet JP, Samelson LE (1996) Characterization of Cbl tyrosine phosphorylation and a Cbl–Syk complex in RBL-2H3 cells. J Exp Med 184: 1713–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson HC, Kraus M, Kim YM, Ploegh H, Rajewsky K (2006) The B cell receptor promotes B cell activation and proliferation through a non-ITAM tyrosine in the Ig-α cytoplasmic domain. Immunity 25: 55–65 [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP (2006) Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat Cell Biol 8: 771–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E, Tardelli L (1992) Tyrosine phosphorylation is required for ligand-induced internalization of the antigen receptor on B lymphocytes. Proc Natl Acad Sci USA 89: 114–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WW, Zurn C, Reth M (2002) Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell 10: 1057–1069 [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada K, Takano T, Yanagi S, Yamamura H (2001) Structure and function of Syk protein-tyrosine kinase. J Biochem (Tokyo) 130: 177–186 [DOI] [PubMed] [Google Scholar]
- Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A (2003) Essential role of Src-family protein tyrosine kinases in NF-κB activation during B cell development. Nat Immunol 4: 274–279 [DOI] [PubMed] [Google Scholar]
- Sauer K, Liou J, Singh SB, Yablonski D, Weiss A, Perlmutter RM (2001) Hematopoietic progenitor kinase 1 associates physically and functionally with the adaptor proteins B cell linker protein and SLP-76 in lymphocytes. J Biol Chem 276: 45207–45216 [DOI] [PubMed] [Google Scholar]
- Schamel WW, Reth M (2000) Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity 13: 5–14 [DOI] [PubMed] [Google Scholar]
- Schweighoffer E, Vanes L, Mathiot A, Nakamura T, Tybulewicz VL (2003) Unexpected requirement for ZAP-70 in pre-B cell development and allelic exclusion. Immunity 18: 523–533 [DOI] [PubMed] [Google Scholar]
- Simon M, Vanes L, Geahlen RL, Tybulewicz VL (2005) Distinct roles for the linker region tyrosines of Syk in FcepsilonRI signaling in primary mast cells. J Biol Chem 280: 4510–4517 [DOI] [PubMed] [Google Scholar]
- Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM (2002) Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17: 451–462 [DOI] [PubMed] [Google Scholar]
- Storch B, Meixlsperger S, Jumaa H (2007) The Ig-α ITAM is required for efficient differentiation but not proliferation of pre-B cells. Eur J Immunol 37: 252–260 [DOI] [PubMed] [Google Scholar]
- Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T (1994) Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J 13: 1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR (1996) Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 383: 837–840 [DOI] [PubMed] [Google Scholar]
- Tsuji S, Okamoto M, Yamada K, Okamoto N, Goitsuka R, Arnold R, Kiefer F, Kitamura D (2001) B cell adaptor containing src homology 2 domain (BASH) links B cell receptor signaling to the activation of hematopoietic progenitor kinase 1. J Exp Med 194: 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL (1995) Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature 378: 298–302 [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen PJ, Reth M (1998) SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med 188: 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wossning T, Reth M (2004) B cell antigen receptor assembly and Syk activation in the S2 cell reconstitution system. Immunol Lett 92: 67–73 [DOI] [PubMed] [Google Scholar]
- Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM (2005) Lyn tyrosine kinase: accentuating the positive and the negative. Immunity 22: 9–18 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Hayashi K, Nojima T, Matsuzaki Y, Kawano Y, Karasuyama H, Goitsuka R, Kitamura D (2006) BASH-novel PKC-Raf-1 pathway of pre-BCR signaling induces kappa gene rearrangement. Blood 108: 2703–2711 [DOI] [PubMed] [Google Scholar]
- Zeitlmann L, Knorr T, Knoll M, Romeo C, Sirim P, Kolanus W (1998) T cell activation induced by novel gain-of-function mutants of Syk and ZAP-70. J Biol Chem 273: 15445–15452 [DOI] [PubMed] [Google Scholar]
- Zhang J, Berenstein E, Siraganian RP (2002) Phosphorylation of Tyr342 in the linker region of Syk is critical for Fc epsilon RI signaling in mast cells. Mol Cell Biol 22: 8144–8154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information