Abstract
The G-protein-coupled receptor (GPCR) activated by the neurotransmitter GABA is made up of two subunits, GABAB1 and GABAB2. GABAB1 binds agonists, whereas GABAB2 is required for trafficking GABAB1 to the cell surface, increasing agonist affinity to GABAB1, and activating associated G proteins. These subunits each comprise two domains, a Venus flytrap domain (VFT) and a heptahelical transmembrane domain (7TM). How agonist binding to the GABAB1 VFT leads to GABAB2 7TM activation remains unknown. Here, we used a glycan wedge scanning approach to investigate how the GABAB VFT dimer controls receptor activity. We first identified the dimerization interface using a bioinformatics approach and then showed that introducing an N-glycan at this interface prevents the association of the two subunits and abolishes all activities of GABAB2, including agonist activation of the G protein. We also identified a second region in the VFT where insertion of an N-glycan does not prevent dimerization, but blocks agonist activation of the receptor. These data provide new insight into the function of this prototypical GPCR and demonstrate that a change in the dimerization interface is required for receptor activation.
Keywords: allosteric modulators, anxiety, baclofen, class C GPCRs, drug addiction
Introduction
GABA is the main inhibitory neurotransmitter in the central nervous system, regulating many physiological and psychological processes. It mediates fast synaptic inhibition through ionotropic GABAA receptors, as well as slow and prolonged synaptic inhibition through both pre- and post-synaptic metabotropic GABAB receptors (Couve et al, 2004; Bettler and Tiao, 2006). GABAB receptors represent promising drug targets for the treatment of epilepsy, pain, drug addiction, anxiety and depression (Cryan and Kaupmann, 2005; Bowery, 2006). GABAB agonists have demonstrated beneficial effects in humans, as illustrated by the anti-spastic activity of baclofen (Lioresal®). Recently, GABAB-positive allosteric modulators (PAM) have been identified as potentially better alternatives to agonists, as they can limit the development of tolerance and avoid the adverse effects observed with agonists (Pin and Prézeau, 2007).
At the structural level, the GABAB receptor is composed of two homologous subunits, GABAB1 and GABAB2 (Jones et al, 1998; Kaupmann et al, 1998; White et al, 1998; Kuner et al, 1999), also called GB1 and GB2 (Figure 1A). Heterodimerization of GB1 and GB2 is required for the formation of functional receptors, both in recombinant systems and in native tissues. Cell-surface trafficking of the GABAB receptor is controlled by an endoplasmic reticulum retention signal (RSR motif) located in the intracellular C-terminal region of GB1. This signal can be masked through a coiled-coil (CC) interaction with the intracellular tail of GB2, such that GB1 reaches the cell surface only when associated with GB2 (Margeta-Mitrovic et al, 2000; Calver et al, 2001). The GABAB receptor is an allosteric complex similar to other class C G-protein-coupled receptors (GPCRs). Each subunit is composed of an extracellular domain, called Venus flytrap domain (VFT), which is linked to the N-terminus of a prototypical heptahelical transmembrane domain (7TM) (Pin et al, 2004). GABAB agonists and competitive antagonists (the orthosteric ligands) bind to the GB1 VFT, whereas the GB2 VFT is not bound by GABA nor, most likely, by any other ligand (Kniazeff et al, 2002). In contrast, the 7TM of GB2 is responsible for G-protein activation (Galvez et al, 2001) and contains the site of action of PAMs (Binet et al, 2004).
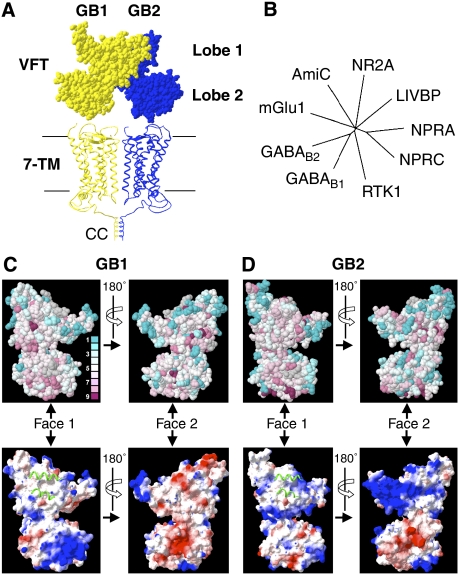
Figure 1.
Models and bioinformatic analysis of the GABAB VFTs. (A) Structural model of the dimeric GABAB receptor in the resting state. Corey–Pauling–Koltun representation of the GABAB VFT dimer model generated according to the resting state of the dimeric mGlu receptors (Protein Data Bank (PDB) accession number 1EWT), and apposition of two heptahelical domains (7TM) according to the rhodopsin dimer structure (PDB accession number 1n3m). GB1 (yellow) and GB2 (blue) are in the front and the back, respectively. The C-terminal regions of the two subunits are associated through a CC interaction that masks the RSR intracellular retention signal of GB1. (B) The phylogenetic tree was constructed using the sequences of the VFTs of the mGlu1 receptor, the amide-binding protein (AmiC) from the amidase operon, the NR2A subunit of the rat N-methyl-D-aspartate (NMDA) receptor, the leucine–isoleucine–valine-binding protein (LIVBP), the natriuretic peptide receptor types A and C (NPRA and NPRC, respectively), RTK1 from Schistosoma mansoni and the rat GB1 and GB2 subunits. Only branches with bootstrap values >600 are shown. (C, D) Evolutionary conservation of residues (upper panels) and electrostatic surfaces (lower panels) of the GB1 and GB2 VFTs visualized on both faces of the VFTs (Face 1 and Face 2). Conservation scores are indicated according to a colour scale, from variable (blue) to conserved (purple) residues. No conservation scores were calculated for the residues in grey. Electrostatic surface representations are provided (negative, red; neutral, white; positive, blue) for the VFT faces, in which the green ribbons correspond to the helices of the associated subunit in the inactive state, illustrating the possible dimerization interface.
To better understand both the molecular functioning of the GABAB receptor and the mechanism of action of orthosteric and allosteric ligands, it is important to know how the GABAB VFTs dimerize and control 7TM activity. We recently demonstrated that the VFTs of the two subunits interact with each other, and also that the GB2 VFT controls agonist affinity for GB1 (Liu et al, 2004); this was recently confirmed using purified VFTs (Nomura et al, 2008). However, it is unknown whether the GABAB VFT dimer functions similarly to the VFT of mGlu receptors (Kunishima et al, 2000; Muto et al, 2007), of ANP receptors (He et al, 2001; van den Akker, 2001) or of tyrosine kinase receptors in Schistosoma (Vicogne et al, 2003), with which it shares equal evolutionary distance (Figure 1B).
Here, we have identified the VFT dimerization interface and used a glycan wedge scanning approach to analyse its functional relevance. Our data demonstrate that a direct interaction between the VFTs of the GABAB subunits is required for cell-surface targeting and agonist activation of the receptor. We also provide direct evidence that a change in the dimerization interface takes place during agonist activation of the receptor.
Results
Bioinformatic prediction of the dimerization interface of GABAB VFTs
Previous results have shown that GABAB VFTs form heterodimers at the surface of cells, even in the absence of the receptors' 7TM and C-terminal domains (Liu et al, 2004). To localize the dimerization interfaces of the GABAB VFTs, we first analysed the conservation of residues at their surfaces using a set of 20 GB1 VFT sequences and 22 GB2 VFT sequences, from Dictyostelium to mammals (see Supplementary Figure 1); the sequences were selected based on our previously established 3D models of the GABAB VFTs (Kniazeff et al, 2002). This analysis revealed that one face of the subunits is more conserved than the others (Face 1 in Figure 1C and D), suggesting that it might correspond to the dimerization interface, as observed with mGlu receptor dimers (Rondard et al, 2006). This possibility was also supported by an analysis of the charge distribution at the surface of the VFTs. Indeed, the conserved Face 1 is composed of a large patch of hydrophobic residues in both subunits (Figure 1C and D), and corresponds to the hydrophobic dimerization interface of the mGlu VFT dimer.
In view of this correspondence, the 3D structure of the mGlu VFT dimer was used to build a model of the GB1–GB2 VFT heterodimer (Figure 2A). Interestingly, none of the putative N-glycosylation sites (consensus sequence Asn-X-Ser/Thr or NXS/T, where X can be any natural amino acid except proline) found in the GB1 and GB2 sequences from different species, from nematodes to mammals, were located within the proposed dimerization interface (Figure 2A). In contrast, most other faces contained at least one putative glycosylation site in at least one of the species examined. This further supported our model of GB1 and GB2 VFT interaction. Taken together, these observations were consistent with the VFT dimer interface in the GABAB receptor being similar to that in mGlu receptors, involving the same two helices of lobe 1.
Figure 2.
Native and engineered N-glycan sites in the heterodimeric GABAB VFTs. (A) Ribbon views of the heterodimeric VFTs are shown, with the putative N-glycosylation sites (Cα of Asn residue) in mammalian VFTs in cyan and orange for GB1 and GB2, respectively. Additional putative N-glycosylation sites in other species are in dark blue and magenta for GB1 and GB2, respectively. (B) The GABAB VFT interface is mainly composed of two helices (green) in lobe 1 of GB1 and GB2 that interact together. The positions of Cα of Asn residues modified by an N-glycan and resulting in a nonfunctional or functional receptor are depicted in red and blue, respectively.
Introduction of N-glycans at the VFT interface abolishes receptor activity
To examine the functional importance of the interaction between the VFTs, we tried to block the interaction by introducing N-glycans at the possible dimer interface in GB1 or GB2 (Figure 2B), creating a steric wedge. Experimentally, we introduced the consensus sequence NXS/T (where X can be any natural amino acid except proline), which typically results in the attachment of a bulky N-glycan moiety to the side chain of the Asn residue. These N-glycosylation sites were introduced at different positions within GB1 (225, 229, 232, 251, 255 and 258) and GB2 (110, 114, 118, 137, 141 and 145) (Figure 2B and see Supplementary Table 1). To ensure the correct trafficking of GB1 to the cell surface when expressed alone, these mutations were first introduced into a GB1 subunit that had a mutated ER retention signal (ASA instead of RSR) (Pagano et al, 2001; Brock et al, 2005).
Western blot experiments revealed that all mutated subunits were expressed at their expected molecular weights (Figures 3A and 4A). Owing to the large size of the full-length subunits (130 kDa), it was impossible to detect any shifts in their apparent molecular weights due to the presence of additional N-glycan moieties (Figures 3A and 4A). However, such shifts were clearly seen with most mutants of GB1 and GB2 VFTs attached to the plasma membrane with a single transmembrane domain (Liu et al, 2004) (Figures 3B and 4B; see Supplementary Table 1). Moreover, treatment with glycosidase PNGase F restored gel mobility similar to that of wild-type VFT (Figures 3B and 4B), demonstrating that the mutants were indeed glycosylated.
Figure 3.
Analysis of the N-glycosylated GB1 mutants. (A) Western blot analysis of the full-length HA–GB1 mutants from membrane fractions of cells coexpressing HA–GB1 mutants and Flag–GB2-WT. Cartoons depict the GABAB subunits wild-type (white) and mutant (grey), and engineered N-glycan is indicated by a white star. (B) Western blot analysis of the truncated GB1 subunits deleted of both HD and C-terminal regions, with or without treatment with PNGase F, and comparison to the wild-type construct. (C) The amount of HA-tagged GB1 mutants coexpressed with Flag-tagged GB2-WT at the cell surface as measured by ELISA. (D) Inositol phosphate (IP) production for the HA-tagged GB1ASA mutants coexpressed with GB2-WT. Data are means±s.e. of at least three independent determinations.
Figure 4.
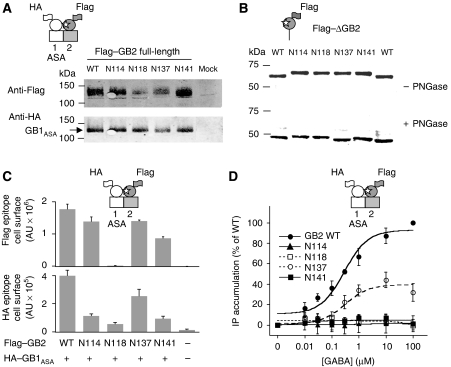
Analysis of the N-glycosylated GB2 mutants. (A) Western blot analysis of the full-length Flag–GB2 mutants from membrane fractions of cells coexpressing Flag–GB2 mutants and HA–GB1ASA. As indicated in Figure 3, cartoons depict the GABAB subunits wild-type (white) and mutant (grey), and engineered N-glycan is indicated by a white star. (B) Western blot analysis of the truncated GB2 subunits deleted of both HD and C-terminal regions, with or without treatment with PNGase F, and comparison to the wild-type construct. (C) The amount of Flag–GB2-WT mutants coexpressed with HA–GB1ASA at the cell surface as measured by ELISA. (D) IP production for the Flag-tagged GB2 mutants coexpressed with GB1ASA. Data are means±s.e. of at least three independent determinations.
The surface expression of all the full-length glycosylation mutants was also examined using haemagglutinin (HA) or Flag epitopes inserted into the extracellular N-terminal end (after the signal peptide) of GB1ASA and GB2, respectively. ELISA assays performed on intact cells revealed that all mutants reached the cell surface. Although a lower level was observed with some of them at the cell surface (Figures 3C and 4C), their total expression level as measured on western blots was similar to that of the wild-type proteins, suggesting that some mutants had difficulties passing the quality control system. This was confirmed by quantifying both the surface and total expression levels of these subunits using ELISA performed on intact and permeabilized cells, respectively (Supplementary Figure 3A and B).
The functional consequences of these additional glycosylation sites were then analysed by coexpressing either the mutated GB1 subunits together with wild-type GB2 (Figure 3D), or the mutated GB2 forms with GB1ASA (Figure 4D). In most cases, GABA was unable to generate a response in cells coexpressing the subunits, despite a sufficient expression level of both proteins at the cell surface. Among the different mutants tested, only two GB1 mutants (HA–GB1ASA-N225 and -N232) and one GB2 mutant (Flag–GB2-N137) were able to form a functional receptor when coexpressed with the wild-type partner. Notably, for those positions whose mutation generated nonfunctional receptors, it was sterically impossible to add an N-acetyl glucosamine group to the Asn residue in the 3D model of the GB1–GB2 VFT dimer, in contrast to those residues whose mutation did not interfere with the formation of a functional heterodimer (Supplementary Figure 2A and B).
To address whether the negative effect of the NXS/T mutations was indeed due to the introduction of an additional glycan on the VFT, we first tested the effect of the N-glycosylation inhibitor tunicamycin (Luo et al, 2003). However, this molecule was toxic to our electroporated cells, preventing us from examining its effect on receptor function. Indeed, even the response mediated by the wild-type receptor could no longer be measured. Therefore, we compared the properties of certain N-glycosylation site mutants (HA–GB1ASA-N229 or -N251 and Flag–GB2-N114 or -N141) with those of analogous mutants in which the Asn residue was replaced by a Gln residue, which cannot be glycosylated. The Gln-containing mutants (HA–GB1ASA-Q229 and -Q251; and Flag–GB2-Q114 and -Q141) generated similar responses upon activation with GABA to those obtained with the wild-type receptor (Figure 5A and B). These data demonstrated that the lack of activity of the HA–GB1ASA-N229 or -N251 and Flag–GB2-N114 or -N141 mutants was likely due to the presence of the N-glycan on the VFT and not mutation per se.
Figure 5.
Loss of function is due to the N-glycan at the interface between the VFTs. (A) Comparison of the IP production stimulated by the GB1ASA NXS/T and QXS/T mutants. (B) Similar comparison of IP stimulation for the GB2 mutants. Data are means±s.e. of at least three independent measurements. (C) Effect of CGP7930 on Ca2+ signals in cells expressing the indicated GB2 mutants. Data are means±s.e. of triplicates from a typical experiment.
Binding and G-protein coupling properties of GB1 and GB2 mutants
Among the mutations introduced into GB1, those that led to nonfunctional receptors were unable to bind the radiolabelled antagonists 3H-CGP54626 and 125I-CGP64213, suggesting that the mutant proteins were not folded correctly (Supplementary Figure 4). It has recently been reported that the correct association of soluble GB1 and GB2 VFTs is required for the GB1 VFT to be able to bind ligands (Nomura et al, 2008). Then, the absence of antagonist binding on GB1-N229 and -N251 could well be the consequence of the lack of possible association with GB2 rather than a misfolding due to the N-glycan. We therefore introduced nine additional glycosylation sites into the GB1 VFT in a region devoid of any natural sites in the various GB1 subunits identified in different species (Figure 2). All these mutations generated GB1 VFTs with an additional N-glycan, as shown by a decrease in mobility in acrylamide gels (Supplementary Figure 5A). Moreover, all were expressed correctly to the cell surface, and eight led to a functional GABAB receptor upon coexpression with GB2, with GABA EC50 values in the same range as that of the wild-type receptor (Supplementary Figure 5B and C). Further information on the nonfunctional N315 mutant is provided at the end of the Results section (see Figure 11).
Regarding the GB2 mutants, the absence of activation by GABA of the GB2-N114 and -N141 mutants was not due to the uncoupling of these subunits from G proteins. We previously reported that the PAM of the GABAB receptor, CGP7930, has agonist activity and can activate both GB2 expressed alone and a truncated version of GB2 that is deleted for the VFT (GB2 7TM) (Binet et al, 2004). Interestingly, the GB2 subunits carrying an additional N-glycan could still be activated by CGP7930 (Figure 5C), demonstrating that they all retained their ability to activate G proteins.
These data revealed that perturbation of the putative dimer interface in both the GB1 and GB2 VFTs has important consequences for the activity of the receptor, but does not prevent the 7TM of GB2 from reaching an active state.
N-glycan in GB2 lobe 1 VFT interface prevents receptor heterodimerization
To further analyse the molecular mechanism by which the N-glycan modification of GB2 abolishes receptor activity, we focused our study on three mutants that were well expressed (N114, N137 and N141), but of which only one, GB2-N137, forms a functional GABAB receptor when coexpressed with GB1ASA. Using FRET measurements, we found that GB2-N114 and -N141 do not interact with GB1 at the cell surface in contrast to GB2-N137. These experiments were conducted on intact cells, using anti-HA antibodies conjugated with the energy donor fluorophore (europium cryptate PBP) to label the HA-tagged GB1 and anti-Flag antibodies linked to the fluorophore acceptor (d2) to label the Flag-tagged GB2 proteins (Figure 6A). Such an approach enables the detection of receptor dimers at the cell surface only, as previously shown with GB1 and GB2 subunits coexpressed in the same cells (Maurel et al, 2004). As shown in Figure 6B, a very low FRET signal was measured between Flag–GB2-N114 or -N141 and HA–GB1ASA and was not significantly different from a negative control (e.g. FRET between Flag–GB2 and HA–CD4; data not shown). In contrast, large FRET signals were obtained between HA–GB1ASA and either Flag–GB2-N137, -Q114, -Q141 or wild-type Flag–GB2 (Figure 6B). These experiments were conducted with similar amounts of GB1ASA and GB2 subunits at the cell surface for all the constructs tested, to ensure that differences in FRET signals did not reflect differences in the level of expression of one of the subunits (Figure 6C and D). These data strongly suggest that the glycosylation site prevents direct interaction of GB1 with GB2. Co-immunoprecipitation experiments confirmed that, among the GB2 mutants, only GB2-N137 interacts with GB1 (Supplementary Figure 6).
Figure 6.
N-glycan at the GB2 VFT interface prevents dimerization with GB1. (A) The schemes depict the experimental approach used to monitor receptor dimers at the cell surface using time-resolved FRET. The FRET signal is measured between an anti-HA antibody linked to a donor molecule (D) and an anti-Flag antibody linked to an acceptor molecule (A). (B) FRET signal between HA-tagged GB1ASA and Flag-tagged GB2 subunits in cells coexpressing the indicated constructs. (C, D) Amount of HA- and Flag-tagged subunits expressed at the cell surface, respectively, as measured by ELISA. Data are means±s.e. of triplicates from a typical experiment.
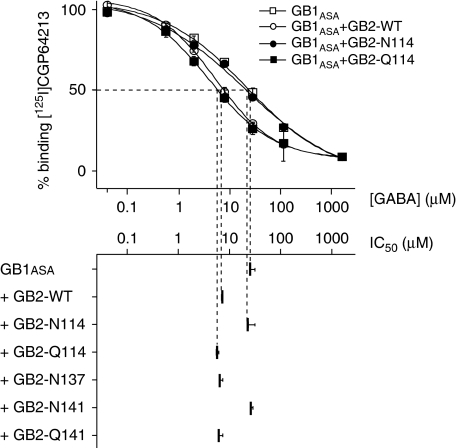
One well-established consequence of GB1–GB2 interaction is an increase in agonist affinity for GB1. Indeed, GABA affinity, as measured by the displacement of 125I-CGP64213 on GB1ASA expressed alone, was 5- to 10-fold lower than the affinity measured in the presence of GB2 (Liu et al, 2004) (Figure 7). Coexpression of GB1ASA with GB2-N114 or -N141 did not increase agonist affinity for GB1ASA (Figure 7), whereas GB2-N137, -Q114 and -Q141 had the same effect as wild-type GB2. These data indicate that the presence of a glycosylation site at the GB2 VFT dimer interface suppresses the allosteric control of agonist affinity in GB1, consistent with a lack of interaction between GB2-N114 and -N141 and GB1 (Figure 6B).
Figure 7.
N-glycan at the GB2 VFT interface prevents allosteric interaction with GB1. Displacement of non-permeant antagonist [125I]CGP64213 by GABA on HA-tagged GB1ASA, expressed alone or in combination with the indicated Flag-tagged GB2 subunits.
Presence of N-glycan at the GB2 lobe 1 VFT interface abolishes cell-surface targeting of the heterodimer
Wild-type GB1 is retained in the endoplasmic reticulum in the absence of GB2 (Margeta-Mitrovic et al, 2000; Calver et al, 2001; Pagano et al, 2001). Till this point, all of the experiments had been conducted with a mutated version of GB1 in which the ER retention signal was changed to ASA. Therefore, we next examined if the glycosylated GB2 mutants could still target wild-type GB1 to the cell surface. Indeed, the inactive N-glycan-modified GB2-N114, -N118 and -N141 receptors were unable to target wild-type GB1 to the cell surface, as indicated by both ELISA and 125I-CGP64213 binding experiments on intact cells (Figure 8). In contrast, GB2-Q141 was able to target GB1 to the cell surface. These results indicate that the masking of the ER retention signal cannot occur if VFT interaction is prevented by the presence of an N-glycan, even at an early stage of protein processing. However, a GB2 deletion mutant lacking the VFT was still able to target GB1 to the cell surface (Figure 8), as can a GB2 CC domain expressed alone (Brock et al, 2005). Taken together, these data demonstrate that (i) the CC interaction between the GABAB subunits has a major role in controlling the trafficking of GB1 to the cell surface and (ii) this interaction can only occur if subunits are able to interact normally outside of the CC domain. In other words, the interaction of the CC domains within the full-length subunits can only take place if the VFTs are able to interact.
Figure 8.
N-glycan at the GB2 VFT interface abolishes cell-surface targeting of the heterodimer. Cell-surface targeting of HA-tagged GB1-WT, expressed alone or in combination with the indicated Flag-tagged GB2 mutants and measured by either antagonist binding to GB1 (left panel) or anti-HA ELISA (right panel). Data are means±s.e. of triplicates from a typical experiment.
N-glycan at the GB1 lobe 1 interface abolishes the interaction with GB2
One possible explanation for the observed absence of antagonist binding in the GB1 mutants carrying an N-glycan at the dimerization interface was that they were unable to associate with partner VFTs, as reported with the purified soluble VFTs (Nomura et al, 2008). As observed with the GB2 mutants, low FRET signals were obtained at the cell surface between Flag–GB2 and HA–GB1ASA-N229 or -N251, whereas significant FRET signals were measured with the functional GB1ASA mutants HA–GB1-N225, -Q229 and -Q251 (Supplementary Figure 7). Moreover, among these GB1 mutants carrying the intracellular retention signal, only the -N225 and -Q251 could reach the cell surface when coexpressed with GB2 (Supplementary Figure 8). In contrast, all GB1 mutants reached the cell surface when coexpressed with a GB2 deleted for its VFT (GB2-7TM; data not shown).
Introduction of an N-glycan into the VFT lobe 2 interface locks the receptor in an inactive state
According to the structure of the isolated dimeric mGlu VFTs determined in the presence and absence of agonist, the lobe 2 domains within the VFT dimer are far apart in the inactive state (Kunishima et al, 2000) but are in contact in the active receptor, constituting a Gd3+ binding site (Tsuchiya et al, 2002). Such a major movement of the mGlu VFTs relative to one another is assumed to have a critical role in the activation of these dimeric receptors, although this has never been firmly demonstrated with the native receptor dimer. We investigated whether the GABAB VFTs show a similar movement during receptor activation. To do this, we introduced an N-glycan wedge into lobe 2 of GB2, at position 209 (Flag–GB2-N209), in the region corresponding to the Gd3+ binding site in mGluR1 (Figure 9A). Interestingly, this GB2 mutant produced an inactive GABAB receptor when coexpressed with GB1, whereas the non-glycosylated version of this mutant (Flag–GB2-Q209) gave rise to a perfectly functional receptor (Figure 9B and C). This loss of activity was not due to impaired expression of Flag–GB2-N209 at the cell surface (Figure 9D) or defect in assembly with GB1, as shown by (i) the correct cell-surface targeting of wild-type GB1 (Figure 10A), (ii) a positive allosteric effect of GB2-N209 on the affinity of GABA for GB1 (Figure 10B) and (iii) a FRET signal similar to that measured with the wild-type receptor (Figure 10C). The lack of activity of the GB1:Flag–GB2-N209 dimer was also not due to misfolding of the GB2 subunit, as this receptor combination could still be activated by the PAM CGP7930 (Figure 10D).
Figure 9.
N-glycan at the GB2 VFT lobe 2 interface locks the receptor in the inactive state. (A) Structural model of the dimeric GABAB receptor based on the resting state of the dimeric mGlu1 receptors (Roo, PDB accession number 1EWT), with both VFTs open, and on the active state of mGlu1 (Aco, PDB accession number 1EWK), with the VFT of GB1 and GB2 closed and open, respectively. Note the position of residue 209 (red) in lobe 2 of GB2, where the engineered N-glycan prevents the receptor from reaching the active state, and the C-terminal ends of the VFTs (green). (B) Intracellular Ca2+ response mediated by the indicated wild-type and mutant Flag-tagged GB2, coexpressed with HA-tagged GB1-WT. (C) Western blot analysis of Flag–GB2 mutants in truncated subunits, as in Figure 4B. (D) The amount of Flag-tagged GB2 expressed at the cell surface as measured by ELISA.
Figure 10.
Loss of activity of the GB2-N209 subunit is not due to a defect in assembly with GB1. (A) Cell-surface targeting of HA-tagged GB1-WT by Flag-tagged GB2 mutants as indicated and measured by anti-HA ELISA. (B) Displacement of non-permeant antagonist [125I]CGP64213 by GABA on HA-tagged GB1ASA, expressed alone or in combination with the indicated GB2 subunits. (C) FRET signal between HA-tagged GB1ASA and Flag-tagged GB2 subunits in cells coexpressing the indicated constructs. (D) Effect of CGP7930 on Ca2+ signals in cells expressing the indicated GB2 subunit mutants. Data are means±s.e. of triplicates from a typical experiment.
Similar data were obtained with an equivalent mutant at the GB1 lobe 2 interface, GB1-N315 (Figure 11). Indeed, this GB1 mutant coexpressed with GB2 is not functional (Figure 11B), although it is well expressed and targeted to the surface in the presence of GB2 (Figure 11C and E), glycosylated (Figure 11D) and still able to bind CGP64213 with affinity similar to that of wild-type GB1 (Figure 11F).
Figure 11.
N-glycan in GB1 VFT lobe 2 interface locks the receptor in the inactive state. (A) Structural model of the dimeric GABAB receptor as shown in Figure 9. Note the position of residue 315 (red) in lobe 2 of GB1, where engineered N-glycan prevents the receptor activation to reach the active state, and the C-terminal ends of the VFTs (green). (B) Intracellular Ca2+ response mediated by the indicated wild-type and mutant HA-tagged GB1 coexpressed with Flag-tagged GB2-WT. (C) Amount of HA-tagged GB1 detected at the cell surface (non-perm) or when the cells are permeabilized (perm), as measured by ELISA. (D) Western blot analysis of the HA–GB1 mutants in truncated subunit as in Figure 3B. (E) Effect of CGP7930 on Ca2+ signals in cells expressing the indicated GB1 subunits. (F) Displacement of non-permeant antagonist [125I]CGP64213 binding by CGP64213 on HA-tagged GB1 subunits coexpressed with GB2-WT.
These data revealed that the presence of an N-glycan wedge in the lobe 2 interface prevents the heterodimer from reaching the active state upon agonist binding in GB1, but does not prevent the correct assembly of the receptor heterodimer.
Discussion
How the signal is transduced in the GABAB heterodimer, from the agonist-occupied GB1 VFT to the GB2 7TM responsible for G-protein activation, remains unknown. In this study, we have used a glycan wedge scanning approach to identify the dimerization interface within the VFT dimer. We also show that physical interaction between the subunits at the interface is necessary for receptor function, for allosteric interaction between the subunits and for the correct assembly and trafficking of the GABAB heterodimer to the cell surface. We also provide evidence that a change in the relative position between the VFTs is required for signal transduction to occur.
The glycan wedge approach
Random insertion of glycosylation sites has often been used to study the topology of transmembrane proteins, for instance to identify their extracellular portions. However, many mutants are not functional and are likely misfolded owing to the introduction of N-glycans in buried positions (see for example Hollmann et al, 1994). However, if the 3D protein structure is taken into account and the Asn side chain is well exposed at the surface of the protein, then the N-glycan is not expected to dramatically affect the folding of the protein. Indeed, natural glycosylation sites are typically found in loops, helices and beta strands. Significantly, among the 13 glycosylation sites introduced into the GB1 VFT, 11 resulted in the correct folding of the domain, as illustrated by their normal activity or binding.
The insertion of N-glycans at critical places can be used to prevent protein–protein interactions involving extracellular domains or to block specific conformational changes. Although this has never been used systematically, the insertion of an N-glycan was used to block the activation of the β-integrin receptor (Luo et al, 2003). In addition, a natural mutation creating a glycosylation site has been identified at the level of the dimerization interface of the sweet and umami taste receptor subunit T1R3, leading to non-tasting mice (Max et al, 2001). It was therefore speculated that the additional N-glycan prevented the association of the taste receptor subunits, thus preventing their normal functioning. This is consistent with our finding that glycosylation at the same place in either GB1 or GB2 prevents the association of these subunits and, consequently, the functioning of the GABAB receptor.
Conservation of the VFT dimerization interface
Despite the evolutionary distance of the GABAB receptor VFTs from those of other class C GPCRs, we provide evidence that their dimerization interface is conserved. This is nicely illustrated by the high degree of conservation of this interface between the mGlu and GABAB VFTs, by the hydrophobicity of the GABAB interface and, most importantly, by the demonstration that the presence of an N-glycan at this interface in either GB1 or GB2 prevents the interaction between the subunits. It may appear surprising that the insertion of N-glycan at this putative dimer interface in GB1 results in incorrectly folded mutant subunits, as shown by their inability to bind GABAB antagonists. Although one cannot exclude the possibility that the misfolding results from the N-glycan insertion per se, thus preventing dimer formation, the misfolding may simply result from a lack of interaction with the partner VFT at this level. Indeed, such a hydrophobic dimerization area may not be stable when exposed to aqueous solvent. In agreement with this hypothesis, it has recently been shown that the soluble GB1 VFT does not fold correctly in the absence of its partner GB2 VFT (Nomura et al, 2008); this provides further evidence that the region identified corresponds to the dimerization interface of the GABAB VFTs.
Interestingly, the same area also serves as a dimerization interface in other VFT-containing proteins, such as the atrial natriuretic peptide receptors (He et al, 2001; van den Akker, 2001), as well as in prokaryotic VFTs (Schumacher et al, 1994). This suggests that this mode of association between the VFTs arose early in evolution, likely before the appearance of class C GPCRs.
Conformational change of the GB1–GB2 VFT interface during activation
The current hypothesis for the ligand-induced activation of class C GPCRs is that a change in the relative position of the VFTs leads to a relative movement of the 7TMs and, as a result, the activation of one of them (Pin et al, 2005). The most compelling evidence supporting this idea comes from the crystal structure of the soluble dimeric mGlu1 VFTs (Kunishima et al, 2000; Tsuchiya et al, 2002). However, it remains to be proven that what was observed with the soluble mGlu1 VFTs is related to the activation process of the full-length receptor, and also that the findings would apply to the structurally distant GABAB receptor. Indeed, the specific lobe 2 interaction observed in the glutamate-bound form of the mGlu1 VFTs (Tsuchiya et al, 2002) is not observed in the soluble dimeric mGlu3 VFTs occupied with five different agonists (Muto et al, 2007) in which the lobes 2 are still apart.
Our data show that the insertion of an N-glycan at the lobe 2 interface does not prevent the two GABAB VFTs from interacting nor does it affect the cell-surface targeting of the heterodimer. In addition, and most importantly, the N-glycan does not impair the ability of GB2 to increase agonist affinity on GB1, indicating that the two subunits are correctly assembled. This finding indicates that an N-glycan at that position can well be accommodated in the inactive VFT dimer structure, in agreement with the idea that the lobes 2 do not interact with each other in the inactive receptor. However, the presence of this N-glycan does prevent agonist activation of the receptor, suggesting that N-glycan at this site interferes with the adoption of the active form of the VFT dimer. This is entirely consistent with the notion that there is a relative movement of the VFTs upon agonist binding, similar to what is observed in the crystal structure of the dimeric mGlu1 VFT. These data not only support the view that a relative movement of the GABAB VFTs is required for agonist activation, but also that this may be a general mechanism among class C GPCRs in spite of their structural differences.
Surprisingly, even though the GB2-N209 mutant does not allow agonist activation, it still enhances agonist affinity on GB1, indicating that this increased affinity is unrelated to the active state conformation. This is consistent with our previous results showing that this effect of GB2 mostly occurs indirectly, alleviating an inhibition of the GB1 VFT by the GB1 7TM as a result of the interaction between the VFTs, rather than from a direct allosteric action between the VFTs (Liu et al, 2004).
We also found that although agonists were no longer able to activate the GB2-N209-containing receptor, the PAM CGP7930 could. This shows that the effect of this molecule, which acts upon the GB2 7TM, does not require the relative movement of the VFTs.
Importance of VFTs for GABAB receptor assembly
Our results provide new insight into how GABAB subunits assemble together to form a functional heterodimer at the cell surface. One limiting step requires the masking of the ER retention signal in GB1 through a CC interaction involving the intracellular C-terminal portions of the two subunits (Margeta-Mitrovic et al, 2000; Calver et al, 2001; Pagano et al, 2001; Brock et al, 2005). Indeed, we previously reported that the GB2 CC domain is sufficient for masking the GB1 ER retention signal (Brock et al, 2005), as is a GB2 subunit deleted for its VFT (this study), indicating that the interaction between the VFTs is not required per se for this process. However, preventing the interaction between the VFTs is sufficient to prevent the masking of the GB1 ER retention signal, indicating that the CC interaction cannot occur if the two VFTs of the full-length subunits cannot assemble correctly. This indicates that the VFT interaction has a crucial role in the correct assembly of the functional GABAB receptor. Such a role for VFT dimerization in the assembly of oligomeric proteins has also been reported with ionotropic glutamate receptors (Leuschner and Hoch, 1999; Meddows et al, 2001; Ayalon et al, 2005).
Taken together, our data, obtained using an N-glycan wedge scanning approach, have revealed the critical importance of the correct association of the GABAB VFTs for receptor function and for passing the quality control checkpoints in the biosynthetic pathways. They have also shed new light on the activation process of this prototypical GPCR dimer, revealing the requirement for a relative movement between the VFTs for the action of agonists, but not for that of PAMs.
Materials and methods
Materials
GABA was obtained from Sigma (St Louis, MO, USA). CGP7930 and [125I]CGP64213 were purchased from Tocris (Fisher Scientific BioBlock, Illkrich, France) and Anawa (Zurich, Switzerland), respectively.
Plasmids and transfection
pRK5 plasmids encoding the wild-type GB1a, GB1ASA and GB2 subunits tagged with an HA or Flag epitope at their N-terminal end under the control of a cytomegalovirus promoter have been described previously (Galvez et al, 2001; Pagano et al, 2001). GB1 and GB2 mutants were generated using the QuikChange mutagenesis protocol (Stratagene, La Jolla, CA). For western blot analysis of N-glycan introduced by the mutations, EcoRI–BamHI fragments of pRK-HA-GB1 and pRK-Flag-GB2 containing the mutations were subcloned into pRK vectors encoding the GB1 and GB2 subunits deleted for the transmembrane and C-terminal tail, respectively, called ΔGB1 and ΔGB2 (Liu et al, 2004).
HEK-293 and COS-7 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS and transfected by electroporation as described elsewhere (Liu et al, 2004). Ten million cells were transfected with plasmid DNA containing GB1WT (2 μg), GB1ASA (2 μg), GB2WT (2 μg) or one of the mutants (6 μg) and made up to a total amount of 10 μg plasmid DNA with empty pRK5 vector. To allow efficient coupling of the receptor to the phospholipase C pathway, the cells were also transfected with the chimeric Gαqi9 (2 μg) (Galvez et al, 2001).
Deglycosylation assays
Twenty hours after transfection, COS-7 cells were washed with PBS (Ca2+- and Mg2+-free) and harvested. The membranes were prepared as previously described (Liu et al, 2004). For each sample, 50 μg of total protein was denatured using 0.5% (w/v) SDS, 1% Nonidet P-40, 1% (v/v) β-mercaptoethanol, Tris–HCl pH 7.4, 50 mM NaCl and protease inhibitor cocktail for 5 min at 50°C and incubated with 1 U of N-glycosidase F (Roche, Penzberg, Germany) for 2 h at 37°C. Reactions were stopped by adding 6 × sample buffer and heating the samples at 95°C, before western blotting.
Co-immunoprecipitation assays
As previously described (Terrillon et al, 2003; Michineau et al, 2006), 20 h after transfection, COS-7 cells were washed with PBS (Ca2+- and Mg2+-free) and incubated for 1 h in blocking buffer (PBS containing 0.2% BSA) and for 2 h with a monoclonal rat anti-HA antibody 3F10 (Roche) at 4°C. After two washes in blocking buffer, cells were lysed for 45 min at 4°C in RIPA buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail) and centrifuged at 12 000 g for 30 min at 4°C. Lysates were incubated with immobilized Protein A/G (Pierce, Rockford, IL) beads for 3 h at 4°C. After three washes with PBS, the beads was resuspended in 2 × sample buffer and heated at 95°C, before western blotting.
Western blotting
For each sample, 50 μg of total protein was subjected to SDS–PAGE using 10% polyacrylamide gels. Proteins were transferred to a nitrocellulose membrane (Hybond-C; Amersham Biosciences, Uppsala, Sweden). HA-tagged proteins were probed with a polyclonal anti-HA rabbit antibody (dilution 1/400; Zymed, San Francisco, CA) and then with an Alexa-Fluor® 700 goat anti-rabbit antibody (dilution 1/3000; Molecular Probes Invitrogen Corporation, Carlsbad, CA). Flag-tagged constructs were probed with the mouse monoclonal anti-Flag antibody M2 (Sigma) at 0.6 μg/ml and then with a DyLight™ 800 anti-mouse antibody (Rockman Immunochemicals Inc., Gilbertsville, PA) at 0.1 μg/ml. Proteins were visualized by the Odyssey® Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
Inositol phosphate and intracellular calcium measurements
Measurements of IP accumulation and the calcium signal in transfected HEK-293 cells were performed in 96-well microplates as previously described (Goudet et al, 2004).
Cell-surface quantification by ELISA and ligand binding assays
Experiments were conducted as described (Liu et al, 2004). HA-tagged constructs were detected with a monoclonal rat anti-HA antibody 3F10 (Roche) at 0.5 μg/ml and goat anti-rat antibodies coupled to horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA) at 1.0 μg/ml. Flag-tagged constructs were detected with the mouse monoclonal anti-Flag antibody M2 (Sigma) at 0.8 μg/ml and goat anti-mouse antibodies coupled to horseradish peroxidase (Amersham Biosciences) at 0.25 μg/ml.
The ligand binding assay on intact HEK-293 cells was performed as previously described using 0.1 nM [125I]CGP64213 (Anawa) (Liu et al, 2004). The radioligand was displaced by increasing concentrations of GABA (Sigma). The curves were fitted according to the equation y=[(ymax−ymin)/(1+(x/IC50)nH))+ymin using GraphPad Prism software (San Diego), where IC50 is the concentration of the compound that inhibits 50% of bound radioligand and nH is the Hill coefficient.
Time-resolved FRET measurements
Time-resolved FRET experiments were conducted as described (Maurel et al, 2004). This methodology is based on the non-radiative energy transfer between rare earth cryptates such as europium (Eu3+) cryptates and the acceptor fluorophore d2 (CisBio International, Bagnols-sur-Cèze, France). Briefly, COS-7 cells coexpressing the HA-tagged GB1 and Flag-tagged GB2 were incubated with 1 nM of monoclonal anti-HA (12CA5) antibodies carrying Eu3+-cryptate PBP and 3 nM of monoclonal anti-Flag (M2) d2 (provided by CisBio International). As a negative control, cells were incubated with the fluorescence donor antibodies only.
Molecular modelling
A homology model of the GABAB VFTs was generated using the crystal structure of mGlu1 VFT (PDB accession number 1EWK) as a template. Models were manually refined with ViTO (Catherinot and Labesse, 2004) using the sequence alignment of the GABAB VFTs. Final models were built using Modeller 7.0 (Sali and Blundell, 1993) and evaluated using the dynamic evolutionary trace as implemented in ViTO.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr P Paoletti for constructive discussions and N Liu, J He and X Li for initial work. We also thank F Maurin (CisBio International) for the preparation of the labelled antibodies and C Vol for the support with the Ca2+ assay. This work was made possible thanks to the screening facilities of the Institut Fédératif de Recherche (IFR) 3. This work was supported by a CNRS/NSFC collaboration project (PICS3604) (to PR and JL), the National Basic Research Program of China (Grant 2007CB914202 to JL), the National Natural Science Foundation of China (Grants 30530820 and 30470368 to JL) and the Hi-Tech Research and Development Program of China (863 project) (Grants 2006AA02Z326 and 2007AA02Z322 to JL). J-PP was supported by the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM) and by grants from the French Ministry of Research, Action Concertée Incitative ‘Biologie Cellulaire Moléculaire et Structurale' (ACI-BCMS 328), the Agence Nationale de la Recherche (ANR-BLAN06-3_135092) and by an unrestricted grant from Senomyx (La Jolla, CA, USA).
References
- Ayalon G, Segev E, Elgavish S, Stern-Bach Y (2005) Two regions in the N-terminal domain of ionotropic glutamate receptor 3 form the subunit oligomerization interfaces that control subtype-specific receptor assembly. J Biol Chem 280: 15053–15060 [DOI] [PubMed] [Google Scholar]
- Bettler B, Tiao JY (2006) Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol Ther 110: 533–543 [DOI] [PubMed] [Google Scholar]
- Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prézeau L (2004) The heptahelical domain of GABAB is activated directly by CGP7930, a positive allosteric modulator of the GABAB receptor. J Biol Chem 279: 29085–29091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG (2006) GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol 6: 37–43 [DOI] [PubMed] [Google Scholar]
- Brock C, Boudier L, Maurel D, Blahos J, Pin JP (2005) Assembly-dependent surface targeting of the heterodimeric GABAB receptor is controlled by COPI but not 14-3-3. Mol Biol Cell 16: 5572–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver AR, Robbins MJ, Cosio C, Rice SQ, Babbs AJ, Hirst WD, Boyfield I, Wood MD, Russell RB, Price GW, Couve A, Moss SJ, Pangalos MN (2001) The C-terminal domains of the GABAB receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J Neurosci 21: 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catherinot V, Labesse G (2004) ViTO: tool for refinement of protein sequence-structure alignments. Bioinformatics 20: 3694–3696 [DOI] [PubMed] [Google Scholar]
- Couve A, Calver AR, Fairfax B, Moss SJ, Pangalos MN (2004) Unravelling the unusual signalling properties of the GABAB receptor. Biochem Pharmacol 68: 1527–1536 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K (2005) Don't worry ‘B' happy!: a role for GABAB receptors in anxiety and depression. Trends Pharmacol Sci 26: 36–43 [DOI] [PubMed] [Google Scholar]
- Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prézeau L, Pin J-P (2001) Allosteric interactions between GB1 and GB2 subunits are required for optimal GABAB receptor function. EMBO J 20: 2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C, Gaven F, Kniazeff J, Vol C, Liu J, Cohen-Gonsaud M, Acher F, Prézeau L, Pin JP (2004) Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors. Proc Natl Acad Sci USA 101: 378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Chow DC, Martick MM, Garcia KC (2001) Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science 293: 1657–1662 [DOI] [PubMed] [Google Scholar]
- Hollmann M, Maron C, Heinemann S (1994) N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron 13: 1331–1343 [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C (1998) GABAB receptors function as a heteromeric assembly of the subunits GABAB R1 and GABAB R2. Nature 396: 674–679 [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B (1998) GABAB receptor subtypes assemble into functional heteromeric complexes. Nature 396: 683–687 [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Galvez T, Labesse G, Pin JP (2002) No ligand binding in the GB2 subunit of the GABAB receptor is required for activation and allosteric interaction between the subunits. J Neurosci 22: 7352–7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R, Köhr G, Grünewald S, Eisenhardt G, Bach A, Kornau HC (1999) Role of heteromer formation in GABAB receptor function. Science 283: 74–77 [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407: 971–977 [DOI] [PubMed] [Google Scholar]
- Leuschner WD, Hoch W (1999) Subtype-specific assembly of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits is mediated by their N-terminal domains. J Biol Chem 274: 16907–16916 [DOI] [PubMed] [Google Scholar]
- Liu J, Maurel D, Etzol S, Brabet I, Ansanay H, Pin JP, Rondard P (2004) Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J Biol Chem 279: 15824–15830 [DOI] [PubMed] [Google Scholar]
- Luo BH, Springer TA, Takagi J (2003) Stabilizing the open conformation of the integrin headpiece with a glycan wedge increases affinity for ligand. Proc Natl Acad Sci USA 100: 2403–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY (2000) A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27: 97–106 [DOI] [PubMed] [Google Scholar]
- Maurel D, Kniazeff J, Mathis G, Trinquet E, Pin JP, Ansanay H (2004) Cell surface detection of membrane protein interaction with HTRF® technology. Anal Chem 329: 253–262 [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF (2001) Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58–63 [DOI] [PubMed] [Google Scholar]
- Meddows E, Le Bourdelles B, Grimwood S, Wafford K, Sandhu S, Whiting P, McIlhinney RA (2001) Identification of molecular determinants that are important in the assembly of N-methyl-D-aspartate receptors. J Biol Chem 276: 18795–18803 [DOI] [PubMed] [Google Scholar]
- Michineau S, Alhenc-Gelas F, Rajerison RM (2006) Human bradykinin B2 receptor sialylation and N-glycosylation participate with disulfide bonding in surface receptor dimerization. Biochemistry 45: 2699–2707 [DOI] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H (2007) Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci USA 104: 3759–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura R, Suzuki Y, Kakizuka A, Jingami H (2008) Direct detection of the interaction between recombinant soluble extracellular regions in the heterodimeric metabotropic gamma-aminobutyric acid receptor. J Biol Chem 283: 4665–4673 [DOI] [PubMed] [Google Scholar]
- Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, Stein T, Prézeau L, Blahos J, Pin J, Froestl W, Kuhn R, Heid J, Kaupmann K, Bettler B (2001) C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABAB receptors. J Neurosci 21: 1189–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Prézeau L (2007) Allosteric modulators of GABAB receptors: mechanism of action and therapeutic perspective. Curr Neuropharmacol 5: 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Kniazeff J, Binet V, Liu J, Maurel D, Galvez T, Duthey B, Havlickova M, Blahos J, Prézeau L, Rondard P (2004) Activation mechanism of the heterodimeric GABAB receptor. Biochem Pharmacol 68: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Pin JP, Kniazeff J, Liu J, Binet V, Goudet C, Rondard P, Prézeau L (2005) Allosteric functioning of dimeric class C G-protein-coupled receptors. FEBS J 272: 2947–2955 [DOI] [PubMed] [Google Scholar]
- Rondard P, Liu J, Huang S, Malhaire F, Vol C, Pinault A, Labesse G, Pin JP (2006) Coupling of agonist binding to effector domain activation in metabotropic glutamate-like receptors. J Biol Chem 281: 24653–24661 [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234: 779–815 [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Choi KY, Zalkin H, Brennan RG (1994) Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by alpha helices. Science 266: 763–770 [DOI] [PubMed] [Google Scholar]
- Terrillon S, Durroux T, Mouillac B, Breit A, Ayoub MA, Taulan M, Jockers R, Barberis C, Bouvier M (2003) Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol 17: 677–691 [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K (2002) Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+. Proc Natl Acad Sci USA 99: 2660–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker F (2001) Structural insights into the ligand binding domains of membrane bound guanylyl cyclases and natriuretic peptide receptors. J Mol Biol 311: 923–937 [DOI] [PubMed] [Google Scholar]
- Vicogne J, Pin JP, Lardans V, Capron M, Noel C, Dissous C (2003) An unusual receptor tyrosine kinase of Schistosoma mansoni contains a venus flytrap module. Mol Biochem Parasitol 126: 51–62 [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH (1998) Heterodimerization is required for the formation of a functional GABAB receptor. Nature 396: 679–682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information