Abstract
Recombination is essential for the recovery of stalled/collapsed replication forks and therefore for the maintenance of genomic stability. The situation becomes critical when the replication fork collides with an unrepaired single-strand break and converts it into a one-ended double-strand break. We show in fission yeast that a unique broken replication fork requires the homologous recombination (HR) enzymes for cell viability. Two structure-specific heterodimeric endonucleases participate in two different resolution pathways. Mus81/Eme1 is essential when the sister chromatid is used for repair; conversely, Swi9/Swi10 is essential when an ectopic sequence is used for repair. Consequently, the utilization of these two HR modes of resolution mainly relies on the ratio of unique and repeated sequences present in various eukaryotic genomes. We also provide molecular evidence for sister recombination intermediates. These findings demonstrate that Mus81/Eme1 is the dedicated endonuclease that resolves sister chromatid recombination intermediates during the repair of broken replication forks.
Keywords: recombination, replication collapse, sister chromatid recombination
Introduction
Increasing evidence from bacteria to mammalian cells indicates that the DNA replication period is the most active phase of the cell cycle for homologous recombination (HR) repair in non-pathological conditions. When the replication fork collides with an unrepaired single-strand break (SSB), a polar one-ended double-strand break (DSB) is formed. Such a polar one-ended DSB is a priori not a substrate for non-homologous end-joining (NHEJ), for single-strand annealing or for the classical double-strand break repair pathways, as in these processes two free double-stranded ends are required. It is generally accepted that a one-ended DSB uses the intact sister chromatid for repair, as it is the closer homologous sequence and may simultaneously repair the broken end and restore the fork structure, allowing replisome reassembly and replication to restart. The current understanding of how disintegrated replication forks are repaired by HR comes from genetic, biochemical and cytological studies in diverse organisms (reviewed by McGlynn and Lloyd, 2002; Vilenchik and Knudson, 2003; Lisby and Rothstein, 2004). The product of HR between sister chromatids is genetically invisible, because DNA replication by definition produces two identical chromosomes. Cytogenetic studies have strongly suggested that sister chromatid repair occurs through HR, although the evidence is indirect. Duplicated or repetitive intact homologous sequences, when they are available, can also be used for repair. However, utilization of an ectopic homologous sequence is a venturesome issue in regard to genetic stability and can occasionally result in genome rearrangements or loss of heterozygosity in diploid cells. The synthesis-dependent strand annealing (SDSA) and break-induced replication (BIR) pathways are thought to follow similar HR initiation events and involve DNA synthesis in the repair process. For BIR, DNA synthesis can proceed several hundred kilobases to the telomere end (Lydeard et al, 2007; Smith et al, 2007) and, for SDSA, the newly synthesized strands must be able to anneal to a complementary sequence in a way that precludes crossing over (see, for review, Pâques and Haber, 1999).
The HR proteins in the S. cerevisiae Rad52 epistasis group (Rad50, Mre11S.p.Rad32, Xrs2S.p.Nbs1, Rad52S.p.Rad22A, Rad51S.p.Rhp51, Rad54S.p.Rhp54, Rad57S.p.Rhp57, Rad55S.p.Rhp55, S.p. means Schizosaccharomyces pombe, which is used in this work) are recruited to sites of DSBs during the S and G2 phases of the cell cycle to form a nucleoprotein filament (see, for review, Krogh and Symington, 2004). Once assembled, the nucleoprotein filament is competent to search, pair and eventually exchange DNA with an intact homologous double-stranded DNA molecule. The joint molecule or D-loop, when stabilized, can promote DNA repair synthesis. In humans, the Rad51C- and XRCC3-containing complex, a probable counterpart of Rhp55/Rhp57 (Tsutsui et al, 2000), has been shown to be involved in Holliday junction (HJ) processing in mammalian cell-free extracts (Liu et al, 2004). Recently, in fission yeast, other Rhp51 mediator complexes have been identified. Swi5S.c.SAE3/Sfr1S.c.MEI5 (S.c. means S. cerevisiae) is involved in global HR repair and Swi5/Swi2 is dedicated to mating-type (MT) switching in fission yeast (Egel et al, 1984; Akamatsu et al, 2007).
The last activity for DNA repair is provided by the structure-specific endonucleases from the XPF family, the heterodimer Swi9S.c.RAD1/Swi10S.c.RAD10 (homologous to XPF/ERCC1 in mammals) (Rodel et al, 1997) and the heterodimer Mus81/Eme1S.c.MMS4 (Boddy et al, 2000; Interthal and Heyer, 2000). In vitro, Rad1/Rad10 has a major role in numerous DNA repair pathways and has been implicated in the SDSA pathway in S. cerevisiae and S. pombe by cleaving heterologous 3′ tails from branch intermediates (Pâques and Haber, 1999). In vitro, the Mus81/Eme1 complex (called Mus81) cleaves a variety of branched molecules, including D-loops, nicked HJs and also intact HJs, although less efficiently (Boddy et al, 2001; Gaillard et al, 2003; Osman et al, 2003; Gaskell et al, 2007).
We have used the fission yeast S. pombe to study the fate of a unique broken fork of replication at a specific locus. In S. pombe, a stable, site- and strand-specific DNA lesion has been found at the MT locus, mat1 (Arcangioli, 1998). The lesion was described as an SSB, with 3′OH and 5′OH termini, or with one or two ribonucleotides (Kaykov and Arcangioli, 2004; Vengrova and Dalgaard, 2004). In the following report, we will refer to the lesion as an SSB for simplicity.
The polarity of mat1 DNA replication is controlled by a strong replication block (RTS1) on the proximal side of the mat1 locus constraining mat1 to be replicated in a unique direction. In this configuration, the mat1 pause site (MPS1) localized distal to mat1 will be proficient for SSB formation (Dalgaard and Klar, 2001). By using an inducible MT switching system, it was shown that the SSB appears on the neo-synthesized lagging strand during mat1 DNA replication and remains stable. During the following round of DNA replication, the leading-strand DNA polymerase converts the SSB into a polar blunt-ended DSB (Kaykov et al, 2004; Holmes et al, 2005; Figure 1A and Supplementary Figure S3). The fate of the polar DSB will depend on the presence or absence of homologous sequences for repair. In the wild-type strain, the silent donor alleles (mat2P and mat3M), embedded in the heterochromatin, provide intact DNA templates for recombinational repair, allowing MT switching (Figure 1B; reviewed by Egel, 2005; Arcangioli et al, 2007). In the absence of donor loci, the steady-state SSB level is similar to the level observed in wild-type cells and the donorless strain is perfectly viable, indicating an alternative repair process (Klar and Miglio, 1986).
Figure 1.
Replication of the mat1 locus and MT loci of strains used. (A) A site- and strand-specific break, SSB (white arrow), at mat1 is shown. RTS1 at the proximal side of mat1 constrains replication in a unique direction. During replication, the leading strand converts the SSB into a polar DSB. (B) In the wild-type strain, the DSB allows MT switching: mat2P (white square) provides the intact DNA template in M cells and mat3M (grey square) in P cells. (C) We used five tester strains with (+SSB) or without (−SSB) the SSB, shown as a white arrow and black triangle, respectively, and with (+donors) or without (−donors) silent donor loci.
In this report, we provide genetic evidence that, in the absence or presence of homologous DNA sequences, the HR machinery is essential for viability when the replication fork collides with the unique mat1-SSB. Furthermore, we show that Mus81 is dispensable for MT switching, but essential when the sister chromatid is used for repair; conversely, Swi10 is dispensable in the absence of the silent donors loci, but essential for MT switching. The mechanism of choice/exclusion of one or the other nuclease is unknown. Consistent with these results, we found that Mus81 accumulates in vivo at mat1 in an SSB-dependent manner in the absence of donors. Furthermore, an inducible SSB formation system allowed us to observe the accumulation of sister recombination intermediates in the absence of Mus81.
Results
The Rad22A epistasis group is essential for MT switching
To determine the contribution of the HR gene products when a replication fork encounters the SSB at mat1, we analysed the phenotypes of several HR mutants in four related strains, with or without (+/−) the SSB and with or without (+/−) donors (mat2P and mat3M), which serve as template donor for repair. The strains used in this study are as follows: the wild-type or h90 strain (+SSB, +donors); the mat1-Msmt-0 strain (−SSB, +donors), in which mat1-M contains a deletion of the cis-acting elements essential for SSB formation (Styrkarsdottir et al, 1993); the mat1-M(2,3Δ) strain (+SSB, −donors), in which mat1-M contains a deletion of the mat2-P and mat3-M silent donors (Klar and Miglio, 1986); and the mat1-Msmt-0(2,3Δ) strain (−SSB, −donors), which combines the modifications of the two previous variants (Dalgaard and Klar, 1999) (Figure 1C). These four tester strains are isogenic, fully viable and have similar growth rates.
First the rad22A, rhp51, rhp54, rhp50, exo1, rhp57 and swi5 null mutations have been introduced into the stable mat1-PΔ17 strains, containing a similar deletion as the mat1-Msmt-0 strain inhibiting break formation, except that mat1 expresses the P allele (Arcangioli and Klar, 1991). Then, we crossed the mat1-PΔ17 strains, containing the null mutations, with the wild-type h90 strain. Following mating, several diploids were selected, incubated on sporulation plates and four spores from several diploids were dissected and analysed. MT switching efficiency was monitored by the iodine vapour staining method. Iodine stains black the starch produced before sporulation and therefore indirectly indicates the MT switching efficiency in individual colonies. The results obtained are listed in Table I and representative tetrads from the rhp51Δ cross are shown in Figure 2A, left panel).
Table 1.
Phenotypes of the mutants studied in this work
| S. pombe | Orthologues | Mutants | Comment | ||
|---|---|---|---|---|---|
| S. cerevisiae | Human | +SSB, +donors | +SSB, −donors | ||
| Rhp51 | RAD51 | RAD51 | Lethal | Lethal | Search for homology |
| Rad22A | RAD52 | RAD52 | Lethal | Lethal | |
| Rhp54 | RAD54 | RAD54 | Lethal | Lethal | |
| Rhp57 | RAD57 | XRCC3 | sd | ok | |
| Swi5 | SEA3 | AAH21748.1 | sd | ok | |
| Rhp57–Swi5 | Co-lethal | Co-lethal | |||
| Rad50 | RAD50 | RAD50 | sg sd | sg | DSB processing |
| Exo1 | EXO1 | EXO1 | ok | ok | |
| Rad50–Exo1 | Microcolony | Co-lethal | |||
| Swi8 | MSH2 | MSH2 | sd | ok | MMR |
| Swi10 | RAD10 | ERCC1 | sd | ok | Endonuclease |
| Mus81 | MUS81 | Mus81 | ok | Lethal | |
| Mus81–Swi10 | sg sd | Lethal | |||
| Rad2 | RAD27 | FEN1 | ok | ok | |
| Slx1(Slx4) | SLX1(4) | GIYD1(nf) | ok | ok | |
| Ku70 | YKU70 | KU70 | ok | ok | NHEJ |
| Rqh1 | SGS1 | BLM, WRN | ok | ok | Helicases |
| Srs2 | SRS2 | nf | ok | ok | |
| Fbh1 | nf | FBH1 | ok | ok | |
| Pfh1 | PIF1, RRM3 | PIF1 | sd | ok | |
| Rqh1–Top3 | SGS1-TOP3 | BLM/WRN-TOP3A | ok | ND | |
| Rad3 | MEC1 | ATR | ok | ok | Checkpoint |
| Tel1 | TEL1 | ATM | ok | ok | |
| Cds1 | RAD53 | CHK2 | ok | ok | |
| Chk1 | CHK1 | CHK1 | ok | ok | |
| Crb2 | RAD9 | 53BP1 | ok | ok | |
| Dcc1 | DCC1 | DCC1 | ok | ok | RFC-like |
| Elg1 | ELG1 | ELG1 | ok | ok | |
| sg: slow growth, sd: switching defect, ok: switching and growth similar ±SSB, ND: not determined, nf: not found. | |||||
Figure 2.
The Rad22A epistasis group is essential for MT switching and sister chromatid recombination. (A) Tetrad dissections of PΔ17 rhp51Δ crossed with h90 (left) or with M(2,3Δ) (right). (B) Tetrad dissections of PΔ17 rad50Δ crossed with h90 and M(2,3Δ) exo1Δ mutants. (C) Tetrad dissections of PΔ17 swi5Δ crossed with h90 and M(2,3Δ) rhp57Δ mutants. A circle, rhombus or hexagon surrounds mutants of interest with the SSB (h90 or M(2,3Δ)), whereas squares surround mutants without the SSB (PΔ17). (D) Alignment of mat1 distal and mat2P distal sequences from wild type and rhp57Δ variants with a stable MT locus.
rhp22AΔ, rhp51Δ and rhp54Δ mutants do not form colonies in the wild-type h90 strain, as already shown for rhp22AΔ (Ostermann et al, 1993), although they are viable in mat1-Msmt-0 and mat1-PΔ17 backgrounds. Further observation of the germinating spores showed that the mutated h90 cells elongated and eventually divided, but never formed visible colonies (data not shown). Altogether, these results indicate that the presence of the SSB at mat1 leads to cell death after few cell divisions in the absence of HR gene products.
In contrast to the three mutants described above, the h90rad50Δ or h90 mre11Δ (data not shown) mutant forms small colonies. However, these colonies contain many dead cells and few spores. The h90 exo1Δ mutant is viable and does not exhibit MT switching defects, whereas the double h90rad50Δ exo1Δ mutant is not viable, but can eventually form micro-colonies (Figure 2B, left panel). We next analysed the Rhp51 mediators, Rhp57- and Swi5-containing complex (Akamatsu et al, 2003, 2007; Hope et al, 2007). The h90 rhp57Δ strain produces colonies with a mild defect in MT switching and the h90 swi5Δ mutant produces healthy colonies but MT switching is drastically reduced (Egel et al, 1984; Jia et al, 2004). However, the h90rhp57Δ swi5Δ double mutant is not viable (Figure 2C). Interestingly, upon re-streaking, the h90 rhp57Δ cells progressively produced colonies defective in MT switching, indicating that Rhp57 participates in efficient MT switching. The mat1 sequences of several independent streaky and white rhp57Δ colonies were sequenced and found to contain the same mutation, in which 8 bp of the H1 distal sequence from mat2P was transferred to mat1 (Figure 2D). As expected, mat1-Msmt-0 and mat1-PΔ17 strains, which do not exhibit SSBs, are fully viable regardless of the mutant status. Altogether, these results clearly show that in the wild-type h90 strain, the major players of the HR pathway of DSB repair are also essential for MT switching.
The Rad22A epistasis group is essential for sister chromatid recombination
In the mat1-M(2,3Δ) donorless strain (+SSB, −donors), the SSB is observed at wild-type levels and cells are perfectly viable. This observation clearly indicates that MT switching is not the only possible outcome for repair (Klar and Miglio, 1986). We genetically investigated the role of the HR gene products in the absence of donor loci. All of the single mutants and combinations of mutants studied above exhibit similar viabilities as observed for the wild-type h90 strain (Table I; Figure 2A–C, right panels). Altogether, these results provide evidence that only one unrepaired SSB, subsequently transformed into a DSB during the replication period, requires the HR enzymes for repair. In the presence of the donors, HR uses the opposite donor allele for repair, and in the absence of donors, HR uses the sister chromatid, which is the only homologous template available for repair. Because the converging replication fork coming from the centromere-proximal side of mat1 is arrested by the RTS1 element, independently of the presence of the donors (Dalgaard and Klar, 2000), the HR repair process must re-establish a replication structure suitable to restart and complete mat1 DNA replication.
Mus81 is not required for MT switching but is essential for sister chromatid recombination
Genetic epistasis studies with the rad22 group have suggested that Mus81 also participates in HR pathway to properly replicate broken DNA (Boddy et al, 2000). Consequently, we introduced the mus81Δ mutation into the two strains containing the SSB with or without donors to analyse viability and/or MT switching of the segregants. The h90mus81Δ mutant strain produces homogeneous iodine-black colonies (Figure 3A, left panel), whereas the germinating donorless mat1-M(2,3Δ) mus81Δ mutant barely produces visible colonies (Figure 3A, right panel). Similar growth defects were observed with the nuclease-dead mus81-DD mutant (Boddy et al, 2001), demonstrating that Mus81 endonuclease activity is essential for viability in the mat1-M(2,3Δ) (+SSB, −donors) background (data not shown). We microscopically observed the germinating spores of mat1-M(2,3Δ) mus81Δ from the tetrad dissection plates and showed that the germinating cells elongate, indicating an active cell cycle checkpoint arrest and they eventually divide with a quasi-linear division mode (Figure 3B). An example of the lineage of mus81Δ strains, with or without donors, is presented (Figure 3C, right panel) and shows that only one of the two daughter cells inherits the potential to grow. Knowing that the SSB is formed on only one of the two mat1-containing chromatids during DNA replication, we conclude that the dividing daughter cell follows the segregation of the unbroken mat1 chromatid, whereas its daughter, inheriting the broken mat1 chromatid, rapidly dies in the absence of Mus81. These findings showed that the Mus81 endonuclease complex is necessary at the collapsed replication fork for sister chromatid recombination repair to complete replisome reassembly, as proposed previously (reviewed by Whitby, 2004). Notably, Rad2S.c.RAD27 (5′–3′ flap exo/endonuclease), required for Okazaki fragment processing, and the Slx1/Slx4 structure-specific endonuclease required for maintaining ribosomal DNA (Mullen et al, 2001; Coulon et al, 2004) are not necessary for viability and MT switching (Table I).
Figure 3.
Mus81 is essential for sister chromatid recombination. (A) Tetrad dissections of PΔ17 mus81Δ crossed with h90 (left) or M(2,3Δ) (right). The circles and the squares surround mus81Δ mutants with (h90 or M(2,3Δ)) or without (PΔ17) SSB, respectively. (B) Time course of germinating wild type and mus81Δ mutants with (M(2,3Δ)) or without (PΔ17) the SSB. Numbers of cells are indicated for mus81Δ M(2,3Δ) mutants. (C) Pedigree of the mus81Δ mutant with or without donors. The empty circles indicate dividing cells, the grey circles indicate elongated cells and the black circles indicate undividing cells. (D) Tetrad dissections of h90 mus81Δ crossed with PΔ17 swi10Δ. A circle, rhombus or hexagon surrounds mutants of interest with the SSB (h90 or M(2,3Δ)), whereas squares surround mutants without the SSB (PΔ17). (E) Iodine staining of wild-type, swi10Δ, mus81Δ and swi10Δ mus81Δ h90 colonies, after 4 days at 33°C and 4 days at 25°C.
The h90 wild-type strain lacking either Swi9S.c.Rad10/ERCC1 (also named Rad16) or Swi10S.c.Rad1/XPF endonuclease subunits rapidly produces MT region rearrangements containing duplications and extrachromosomal circles of the MT region, probably resulting from HR resolution errors (Egel et al, 1984). Similar results have been observed in strains lacking Swi4S.c.Msh3 or Swi8S.c.Msh2, two proteins related to the mismatch repair proteins (Egel et al, 1984; Fleck et al, 1992, 1994). When the swi10Δ or swi8Δ mutations were genetically introduced into the other three tester strains, no loss of viability or significant slow growth phenotypes were observed (Table I). These results indicated that in the donorless mat1-M(2,3Δ) (+SSB, −donors) strain, the Swi9/10 nuclease and Swi4/8 complexes are not required, or can be replaced by other activities, when the sister chromatid is used for HR repair.
Next, we analysed MT switching and viability of the wild-type h90 strain in the absence of both Swi10 and Mus81 by crossing the mat1-PΔ17 swi10Δ strain with the h90 mus81Δ mutant strains (Figure 3D). Among the segregants, the h90 mus81Δ swi10Δ double mutant gives rise to small irregular colonies, containing many dead cells, and exhibits a white/pale iodine staining, indicative of an MT switching inhibition associated with deadly recombination events (Figure 3D). Therefore, we analysed by Southern blot the MT region in the single and double mutants and showed that indeed the double mutant exhibits a higher level of DNA rearrangements (Supplementary Figure S1, h+N*). These phenotypes (Figure 3E) are different for both single mutants and indicate that the Mus81 endonuclease might act to prevent further aberrant chromosomal rearrangements, when Swi10 is absent.
RusA allows sister chromatid recombination
Previous work has shown that RusA, an Escherichia coli HJ resolvase (Chan et al, 1997), suppresses mus81Δ phenotypes (Boddy et al, 2001; Doe et al, 2002). In contrast, RusA does not rescue the genotoxic sensitivities of swi10Δ mutant (Doe et al, 2002). The experiments described in Supplementary data indicate that RusA does not participate in MT switching in the absence of Swi10. However, RusA suppresses, although partially, the lethality of the mus81Δ mat1-M(2-3Δ) mutant strain, indicating that in the absence of Mus81, HJs accumulate, which can be resolved by RusA.
The Rqh1, Srs2 and Fbh1 helicases, NHEJ and the DNA damage checkpoints
DNA helicases and Mus81 endonuclease are thought to function in different pathways for restarting stalled or collapsed replication forks. Therefore, we analysed the phenotypes of the rqh1Δ, srs2Δ, fbh1Δ, pfh1Δ and the top3Δ mutations (Morishita et al, 2005; Osman et al, 2005; Boulé and Zakian, 2006; Osman and Whitby, 2007) in our tester strains. None of the single helicase and essential topoisomerase 3 (in rqh1Δ background) mutant strains exhibit MT switching and/or viability defect, except for the pfh1 mutant where a mild MT switching defect was observed (Table I, data not shown). The absence of any role for the helicases in replication fork repair at mat1 is consistent with their anti-recombinational function. The rqh1Δ srs2Δ and srs2Δ fbh1Δ double mutants exhibit poor growth and rqh1Δ fbh1Δ double mutant is dead. The growth defect is independent of the presence of the SSB at mat1 and thus prevents us from testing if these DNA helicases could substitute for each other. To definitively exclude the NHEJ process, the pku70Δ mutation was also introduced into the tester strains and no defects in MT switching or viability were observed. Finally, we analysed the DNA damage checkpoints and showed that the four tester strains are equally viable in the absence of Rad3S.c.MEC1, Cds1S.c.RAD53, Chk1S.c.CHK1 and Crb2S.c.RAD9 null mutants (human ATR, CHK2, CHK1 homologues and 53BP1-related protein, respectively) and exhibit a wild-type level of MT switching in the h90 strain (Noguchi et al, 2003; Table I). Taken together, these results indicate that a process not requiring the DNA damage checkpoint efficiently repairs the collapsed fork at mat1.
Mus81 interacts with mat1 during sister chromatid recombination
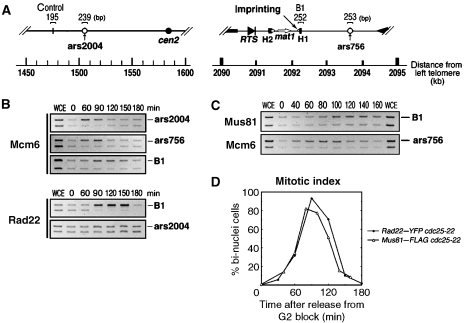
The lethality of the mus81Δ mat1-M(2,3Δ) strain implies that Mus81 is required for resolving sister chromatid recombination intermediates that form during fork recapture. To test this, we used chromatin immunoprecipitation to assay whether Mus81 interacts in vivo with mat1 during the replication period. We used the temperature-sensitive cdc25-22 mutant in the donorless background (+SSB, −donors) and proceeded through a block and release experiment. We first arrested the cells in the late G2 phase at the nonpermissive temperature of 36°C and allowed cells to re-enter synchronously into the cell cycle at the permissive temperature of 25°C. Then, we followed by chromatin immunoprecipitation (ChIP) the binding of Mcm6, Rad22–YFP and Mus81–FLAG to mat1 and the flanking replication origins, ars2004 and ars756 (Figure 4A). Upon release from G2 arrest, Mcm6 begins to accumulate at the origins at the 60 min time point to form pre-RC. Subsequently, Mcm6 is detected at the break site at the 90 min time point, indicating replication fork movement. Its persistence at mat1 for 30–60 min is consistent with an active MPS1 element. Rad22A is not present at the two origins and appears at the 90 min time point, accompanying the Mcm6 kinetics (Figure 4B). Mus81 also accumulates at mat1 starting at the 80–100 min time point and stays until the 160 min point (Figure 4C). Importantly, neither Rad22A nor Mus81 associates at mat1 in the absence of the SSB (mat1-Msmt-0(2,3Δ) strain), as shown in Supplementary Figure S2. Altogether, these results indicate that Rad22A and Mus81 accumulate rapidly at mat1 concomitantly with the replication fork collapse and not with the MPS1 pause, which is still active in the mat1-Msmt-0(2,3Δ) strain.
Figure 4.
Mus81 accumulates at the collapsed replication fork. Cells with a cdc25 background are first incubated at 36°C for 3 h to arrest at G2/M and then shifted to 25°C to release from G2/M block. (A) Positions of regions studied in this assay are shown along with the distance from the left telomere. PCR is carried out using a combination of two primer sets that amplify either replication origins or H1 (shown as B1) and control region. (B) Mcm6 and Rad22A ChIP. Chromatin was immunoprecipitated with anti-Mcm6 antisera and anti-GFP antibody from YYY023 (-donor Rad22A–YFP cdc25) as a function of time after G2/M release. DNA was analysed by PCR with the indicated primer sets. DNA amplified from total cellular DNA is shown in lane WCE. (C) Mus81 and Mcm6 ChIP. Chromatin was immunoprecipitated with anti-Mcm6 antisera and anti-FLAG antibody from YYY172 (-donor Mus81-5xFLAG cdc25). (D) Mitotic index was determined by analysing cells with bi-nuclei. YYY023 and YYY172 are represented by filled diamond and open triangle, respectively.
Sister replication/recombination intermediates
Having established genetically the essential role of the HR enzymes at a unique collapsed replication fork, we wanted to confirm by Southern blot that mat1 is still cleaved in several mutant backgrounds (rhp51Δ, mus81Δ and rad50Δ). By introducing the thiamine repressible promoter (nmt1) in a neutral region upstream of the mat1 locus, we can force transcription through the broken strand, repairing the SSB. This inducible system allows us to maintain good viability in the three mutant backgrounds, as long as the nmt1 promoter is transcribing. Subsequently, by simply adding thiamine to the medium, we can turn off the transcription and follow SSB formation on one of the sister strands during the first mat1 DNA replication and MT switching during the following DNA replication (Holmes et al, 2005). In this experiment, we extracted the DNA following the traditional DNA purification procedure, which breaks the DNA containing SSBs (Arcangioli, 1998). The break is observed in the three mutants, although with kinetics slightly slower than that for the wild type, consistent with their slower generation time (Figure 5A and B).
Figure 5.
Break formation in mat1P:nmt1:KAN (2,3Δ) strains. (A) Schematic representation of the MT locus in the mat1P:nmt1:KAN (2,3Δ) strain. The strong nmt1 thiamine repressible promoter (dark grey box) was inserted just distal to mat1 (Holmes et al, 2005). The transcript is turned on (−thiamine) and the break is repaired at mat1. When the promoter is turned off (+thiamine), one can follow the timing of break formation. The size (in kbp) of the XhoI–PvuII DNA fragments is indicated and the probe is shown. (B) Genomic DNA of wild-type (PB157), rhp51Δ (LR95), mus81Δ (LR27) and rad50Δ (LR164) mutants was digested with XhoI and PvuII and analysed by Southern blot.
Next, to identify the replication/recombination intermediates that might accumulate in the absence of Mus81, we used thiamine repressible promoter in the donorless strain system coupled with the 2D gel electrophoresis method (Brewer and Fangman, 1987), which allows the detection of replicating and recombining molecules. In this experiment, low-melting agarose plugs have been used to preserve the replication forks and branched molecules from shearing. The replicating DNA, containing single-stranded regions, was enriched on BND cellulose, making cell cycle synchronization unnecessary. Previous work showed that mat1 is replicated by a replication fork coming from its distal side and pauses at MPS1 identified as a spot on the ascending side of the Y-arc (Dalgaard and Klar, 1999, 2000) a few minutes after thiamine addition in this inducible system (Holmes et al, 2005; Figure 6A). The position of MPS1 is consistent with a pause inside H1, located at 1.2 and 0.9 kb from the proximal and distal NdeI restriction sites, respectively. Interestingly, we also observed a secondary weaker pause structure just below MPS1. The position of this structure is consistent with the Sap1 binding site (SAS1) localized 120 bp distal to MPS1 (Arcangioli and Klar, 1991), also known to participate in replication fork pausing at the rDNA loci (Krings and Bastia, 2005; Mejia-Ramirez et al, 2005). This weak pause signal is not observed in the Msmt0 mutant, containing a deletion of SAS1 (Dalgaard and Klar, 1999). We also observed DNA accumulating in the descending side of the Y-arc in both the wild-type and mus81Δ strains, which might correspond to replication forks slowly restarting from MPS1 (Vengrova and Dalgaard, 2004), as they are observed after thiamine addition during the first DNA replication, one generation before one-ended DSB formation and HR repair. Strikingly, a new structure forming a dot at the tip of the spike accumulates within 5–6 h in mus81Δ strain but not in the wild type (Figure 6C), giving an estimation of the kinetics of this repair process. This new structure is absent in the rhp51Δ single and rhp51Δ mus81Δ double mutant strains (Figure 6A), and this supports the genetic conclusion and indicates that Mus81 acts later in the recombination process. This structure, appearing during the second replication period, is indicative of unresolved sister recombination intermediates that are accumulating in the absence of Mus81. The accumulation of recombination intermediates strongly argues that DNA synthesis/replication restart can occur in the absence of cleavage by Mus81/Eme1.
Figure 6.
mus81Δ strain accumulates sister recombination intermediates. (A) DNA from mitotic time courses of PB157 (wt), LR27 (mus81Δ), LR95 (rhp51Δ) and LR294 (rhp51Δ mus81Δ) strains, in the absence of donors, was digested with NdeI, separated by 2D gel electrophoresis, Southern blotted and probed for mat1-P. Double-stranded DNA that migrates as a major spot has been used as reference to standardize the 2D gels. (B) Replication–recombination-coupled models at mat1, in the presence (left/ectopic) or absence of donors (right/sister). (a) Following formation of the polar DSB, two homologous templates can be used for repair. (b) With donors, the Swi2/Swi5 mediator complex recruits the broken end for strand invasion using the H1 sequence homology box and D-loop formation. (c) Following DNA synthesis copying the opposite silent mat2 (or mat3) sequences, the Swi9/Swi10 endonuclease resolves the recombination intermediate at the non-homologous DNA junctions, allowing DNA synthesis of the second strand and resetting of the replication fork structure. (d) Lagging-strand re-initiation. (e) Generation of unbroken mat1 switched MT allele and broken mat1* unswitched allele. (f) Without donors, the sister chromatid is the only available template for HR repair, and DNA synthesis followed by ligation of the imprinted strand is required. (g) Invasion and D-loop formation. (h) Mus81/Eme1 resolves the D-loop and resets the replication fork structure, without crossovers. (i) Lagging-strand re-initiation. (j) Generation of unbroken mat1 and broken mat1*. The right panel shows the two possible representations of the mat1 nicked HJ in mus81Δ strain. (C) Identity of the molecular intermediates observed by 2D gel analysis.
Discussion
Checkpoint responses to a single polar one-ended DSB
Previous works have shown that the polar DSB is formed when the fork collides at mat1 with the SSB (Arcangioli, 1998; Kaykov et al, 2004; Supplementary Figure S3). The poor viability inflicted by this DSB (in mre11Δ or rad50Δ mutants), together with the synthetic lethality observed for the rad50Δ exo1Δ double mutant, indicates that the function of the MRN complex can be partially substituted by Exo1 (reviewed by Tran et al, 2004). Given the fact that recombination factors such as Rhp51 and Rad22 are required for sister chromatid recombination, RPA is also likely to be involved; however, the lack of extensive 5′ to 3′ resection may not generate a strong enough signal for Rad3/ATR checkpoint activation (Zou and Elledge, 2003).
Finally, under physiological conditions, Mus81 associates with chromatin throughout S phase and dissociates from chromatin in a Cds1-dependent manner in the presence of HU, which stalls replication forks but not in the presence of camptothecin (CPT), which breaks replication forks (Boddy et al, 2000; Kai et al, 2005). Collectively, these data are consistent with the absence of a requirement for the intra-S checkpoint and Cds1 activation during mat1 replication runoff. This may, in turn, ensure that Mus81 is available for repair if MT switching has failed or if it is impossible, as in the swi2Δ, swi5Δ or donorless mutant strains.
MT switching process
The Swi6-dependent (heterochromatin protein 1 homologue) positioning/spreading of the Swi5/Swi2 heterodimer at one of the two donor loci, depending on the allele expressed at mat1, together with the physical interaction between Swi2 and Rhp51 might allow the capture of the Rhp51 nucleoprotein filament (Thon and Klar, 1993; Akamatsu et al, 2003; Jia et al, 2004). These interactions have been proposed to direct the choice of the donor to guarantee MT switches, before DNA sequence recognition. A secondary consequence of this developmental bias is also to avoid sister chromatid usage, which would reduce MT switching efficiency. This interpretation is supported by the slow growth phenotype of the swi5Δ mus81Δ double mutant (data not shown), where the absence of Swi5 mimics to a certain extent the absence of donors, leaving the sister chromatid as the main template for repair, hence requiring Mus81. During MT switching, a second Rhp51 mediator Rhp55/Rhp57 complex is also involved and progressively accumulates mutations at mat1. Indeed, h90 swi8Δ (Fleck et al, 1994), h90 rhp55Δ (Vagin et al, 2006) and h90 rhp57Δ (this work) mutant strains generate identical small 8 bp substitution mutations (in bold in Figure 2D) next to mat1, due to another small homology of 12 bp common to mat1 and mat2 (Figure 2D). This substitution mutation removes the cis-acting element SAS2 next to mat1 required for efficient SSB formation and switching, stabilizing the mutation (Arcangioli and Klar, 1991; Kaykov et al, 2004). These results suggest that the mismatch repair Swi8/Swi4 and Rhp57/Rhp55 mediator complexes work together to stabilize the invading single end allowing for efficient gene conversion. The proposed D-loop structure, joining the H1 sequence of mat1 with the H1 sequence of the appropriate donor, uses the invading 3′-end as a primer to allow DNA synthesis to proceed through the donor template. As the donor contains the opposite allele of mat1, DNA synthesis has to extend to the other end of the silent cassette (about 1 kb) and reach the H2 homologous sequence. The annealing between the two H2 sequences forms a structure with two non-homologous 3′ tails. The new strand can be recognized/stabilized by the Swi4/8 complex and clipped off by Swi9/10 and the old mat1 strand can also be cleaved by Swi9/10 or degraded by the MRN complex (Figure 6B, left panel).
Recent work indicates that cohesin complexes that hold sister chromatids together are important for equal sister chromatid recombination on plasmids and rDNA loci (Sjogren and Nasmyth, 2001; Unal et al, 2004; Cortés-Ledesma and Aguilera, 2006). Here, we do not know if the cohesins are absent or inactive at mat1 in order to preferentially use the donors instead of the sister chromatid to favour MT switching.
Mus81 resolves sister chromatid recombination
This work does not address the proposed early role of Mus81 at stalled replication forks but instead examines the role of Mus81 after the fork is broken and the HR machinery has engaged. At mat1, in the absence of donors when the sister chromatid is used for repair, the Rhp55/57 and Swi5/Sfr1 mediators can replace each other (reviewed by Haruta et al, 2008) allowing initial strand invasion and D-loop formation. In S. cerevisiae, Mus81 was initially found by two-hybrid analysis to interact with Rad54 (Interthal and Heyer, 2000) and might be positioned very early by Rad54 following D-loop formation. Biochemical studies have shown that Mus81 exhibits a similar DNA structure specificity among different organisms and that its preferred substrate in vitro is nicked HJs and D-loops, although intact HJs are also cleaved, but less efficiently (reviewed by Osman and Whitby, 2007). Formally, resolution of the recombination intermediate does not require DNA synthesis from the 3′ invading end and the simplest model proposes a resolution whereby Mus81 cleaves the D-loop (Figure 6B, right panel). As shown in vitro, the Mus81 endonuclease subunit from S. cerevisiae or S. pombe preferentially cleaves 5′ to the junction point (Gaskell et al, 2007). Subsequently, the 3′ end of the cleaved molecule, annealed to the initial invading strand, can prime DNA synthesis to accommodate ligation, restoring the replication fork structure and potentially allowing replication restart (Figure 6B, step i). Another alternative, which does not require Mus81 activity at an early step, might be that the D-loop initiates only leading-strand DNA synthesis, whereby full DNA replication of mat1 will be completed by the release of the leading-strand polymerase blocked at RTS1 or carrying along re-initiation of the lagging strand as in the BIR process (Lydeard et al, 2007). These three models are not exclusive and require a single cleavage by Mus81 of a D-loop or nicked HJ to prevent sister chromatid exchanges. This view is supported by the observation that mammalian Eme1 is not required for sister chromatid exchanges (Abraham et al, 2003). The X-shaped structures accumulating in the mus81Δ mutant (Figure 6C) indicated that DNA synthesis initiates without Mus81 cleavage, supporting the last two models. A dual specificity (D-loop and nicked HJ) for Mus81 is consistent with the unstable strand invasion intermediates, followed by a hypothetical endonuclease cleavage to establish a stable replication fork during the BIR process (Smith et al, 2007). As only 2 kb of DNA synthesis is required before reaching RTS1, reconstruction of a mature replication fork might not be necessary. Finally, the nicked HJ shown in Figure 6B (right panel) could branch-migrate in either direction in a Rad54-dependent fashion (Bugreev et al, 2006) to form an intact HJ, consistent with the ability of RusA to replace Mus81.
Importantly, S. cerevisiae and S. pombe mus81 mutants are hypersensitive to CPT but not to ionizing radiation. The drug CPT works by trapping the catalytic intermediate of the topoisomerase I–DNA complexes and thus introduces SSBs (reviewed by Pommier, 2006). Prolonged incubation with CPT allows collision of the replication forks with the Top1–SSB complexes, leading to the formation of polar one-ended DSBs, revealing the role of Mus81 in DNA replication. In contrast, mouse mus81−/− cells are not hypersensitive to CPT, indicating that another endonuclease is able to maintain viability (Liu et al, 2004; Dendouga et al, 2005). Our work suggests that the ratio of unique and repeated sequences, found in various eukaryotic genomes, determines the utilization of two HR modes of resolution (Mus81 versus Swi10). This may explain, at least in part, the weak sensitivity of mus81 mutant to CPT in mammals as compared to yeast. During meiosis in fission yeast, Mus81 is required for crossovers (Boddy et al, 2001; Osman et al, 2003; Smith et al, 2003; Cromie et al, 2006) induced by DSBs created by Rec12, the S. pombe orthologue of Spo11. It was recently proposed that two sequential and asymmetric single-end invasions are cleaved by Mus81 (Cromie et al, 2006 and references therein). The resolution by Mus81 of a single nicked HJ, as shown in Figure 6B, does not generate sister chromatid exchanges, whereas the resolution of two independent nicked HJs would lead to one crossover event as observed during meiosis in S. pombe.
Materials and methods
Fission yeast strains, media, techniques and plasmids
The strains used are listed in Supplementary Table S1. Media and genetic methods for studying S. pombe were as described by Moreno et al (1991).
The pREP-rus plasmids are described by Doe et al (2002). They express RusA (pMW413) and RusAD70N (pMW415) under the control of the inductive nmt1 promoter. The h90/mat1-M(2,3Δ) mus81Δ/+ diploid strain was transformed with these plasmids.
ChIP assay
ChIP assay was performed as described previously (Ogawa et al, 1999). Anti-FLAG antibody (Sigma), anti-GFP antibody (Abcam) and anti-Mcm6 antisera (gift from H Masukata) were used for immunoprecipitation. The nucleotide sequences of the primers used in this study are available on request.
Preparation of S. pombe genomic DNA
DNA was isolated by a classical method (Moreno et al, 1991), digested with XhoI and PvuII (or with HindIII in Supplementary Figure S1) enzymes and analysed by Southern blots, using a 32P-labelled mat1-distal (or HindIII—HindIII mat1 fragment in Supplementary Figure S1) specific probe.
When required, the genomic DNA was prepared into low-melting agarose plugs. The agarose-embedded DNA was digested overnight with NdeI, as recommended by New England Biolabs.
2D gel
The mat1P:nmt1:KAN (2,3Δ) strains (PB157, LR27, LR95 and LR294) were grown in Edinburgh minimal medium without thiamine. Following the addition of thiamine, samples were taken at different time points, as described by Holmes et al (2005). DNA was prepared and digested with NdeI in agarose plugs, and agarose was removed by agarase treatment.
The replicating DNA was enriched on BND cellulose columns and separated by 2D gel electrophoresis (Brewer and Fangman, 1987). Gels were hybridized with a 1 kb mat1-P-specific fragment.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Table
Supplementary data
Acknowledgments
We thank the scientific community for yeast strains, in particular H Masukata (Osaka University, Japan) for anti-Mcm6 antibody, E Hartsuiker for Rad50s and M Whitby for RusA expression plasmids; we thank A Holmes and B Llorente for critical reading of the manuscript. This work was supported by Grants from the region Martinique, the ARC to LR and ANR-06-BLAN-0271 to BA. YY is supported by a fellowship from The Uehara Memorial Foundation. Work in PR's lab was supported by NIH grant GM59447.
References
- Abraham J, Lemmers B, Hande MP, Moynahan ME, Chahwan C, Ciccia A, Essers J, Hanada K, Chahwan R, Khaw AK, McPherson P, Shehabeldin A, Laister R, Arrowsmith C, Kanaar R, West SC, Jasin M, Hakem R (2003) Eme1 is involved in DNA damage processing and maintenance of genomic stability in mammalian cells. EMBO J 22: 6137–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu Y, Dziadkowiec D, Ikeguchi M, Shinagawa H, Iwasaki H (2003) Two different Swi5-containing protein complexes are involved in mating-type switching and recombination repair in fission yeast. Proc Natl Acad Sci USA 100: 15770–15775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu Y, Tsutsui Y, Morishita T, Siddique MS, Kurokawa Y, Ikeguchi M, Yamao F, Arcangioli B, Iwasaki H (2007) Fission yeast Swi5/Sfr1 and Rhp55/Rhp57 differentially regulate Rhp51-dependent recombination outcomes. EMBO J 26: 1352–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B (1998) A site- and strand-specific DNA break confers asymmetric switching potential in fission yeast. EMBO J 17: 4503–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B, Klar AJ (1991) A novel switch-activating site (SAS1) and its cognate binding factor (SAP1) required for efficient mat1 switching in Schizosaccharomyces pombe. EMBO J 10: 3025–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B, Roseaulin L, Holmes A (2007) Mating-type switching in S. pombe. In Topics in Current Genetics, Aguilera A, Rothstein R (eds), Vol. 17, pp 251–284. Springer: Berlin/Heidelberg [Google Scholar]
- Boddy MN, Gaillard PH, McDonald WH, Shanahan P, Yates JR III, Russell P (2001) Mus81–Eme1 are essential components of a Holliday junction resolvase. Cell 107: 537–548 [DOI] [PubMed] [Google Scholar]
- Boddy MN, Lopez-Girona A, Shanahan P, Interthal H, Heyer WD, Russell P (2000) Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol Cell Biol 20: 8758–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule JB, Zakian VA (2006) Roles of Pif1-like helicases in the maintenance of genomic stability. Nucleic Acids Res 34: 4147–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer BJ, Fangman WL (1987) The localization of replication origins on ARS plasmids in S. cerevisiae. Cell 51: 463–471 [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Mazina OM, Mazin AV (2006) Rad54 protein promotes branch migration of Holliday junctions. Nature 442: 590–593 [DOI] [PubMed] [Google Scholar]
- Chan SN, Harris L, Bolt EL, Whitby MC, Lloyd RG (1997) Sequence specificity and biochemical characterization of the RusA Holliday junction resolvase of Escherichia coli. J Biol Chem 272: 14873–14882 [DOI] [PubMed] [Google Scholar]
- Cortes-Ledesma F, Aguilera A (2006) Double-strand breaks arising by replication through a nick are repaired by cohesin-dependent sister-chromatid exchange. EMBO Rep 7: 919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon S, Gaillard PH, Chahwan C, McDonald WH, Yates JR III, Russell P (2004) Slx1–Slx4 are subunits of a structure-specific endonuclease that maintains ribosomal DNA in fission yeast. Mol Biol Cell 15: 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie GA, Hyppa RW, Taylor AF, Zakharyevich K, Hunter N, Smith GR (2006) Single Holliday junctions are intermediates of meiotic recombination. Cell 127: 1167–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard JZ, Klar AJ (1999) Orientation of DNA replication establishes mating-type switching pattern in S. pombe. Nature 400: 181–184 [DOI] [PubMed] [Google Scholar]
- Dalgaard JZ, Klar AJ (2000) swi1 and swi3 perform imprinting, pausing, and termination of DNA replication in S. pombe. Cell 102: 745–751 [DOI] [PubMed] [Google Scholar]
- Dalgaard JZ, Klar AJ (2001) A DNA replication-arrest site RTS1 regulates imprinting by determining the direction of replication at mat1 in S. pombe. Genes Dev 15: 2060–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendouga N, Gao H, Moechars D, Janicot M, Vialard J, McGowan CH (2005) Disruption of murine Mus81 increases genomic instability and DNA damage sensitivity but does not promote tumorigenesis. Mol Cell Biol 25: 7569–7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CL, Ahn JS, Dixon J, Whitby MC (2002) Mus81–Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J Biol Chem 277: 32753–32759 [DOI] [PubMed] [Google Scholar]
- Egel R (2005) Fission yeast mating-type switching: programmed damage and repair. DNA Repair (Amst) 4: 525–536 [DOI] [PubMed] [Google Scholar]
- Egel R, Beach DH, Klar AJ (1984) Genes required for initiation and resolution steps of mating-type switching in fission yeast. Proc Natl Acad Sci USA 81: 3481–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck O, Michael H, Heim L (1992) The swi4+ gene of Schizosaccharomyces pombe encodes a homologue of mismatch repair enzymes. Nucleic Acids Res 20: 2271–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck O, Rudolph C, Albrecht A, Lorentz A, Schar P, Schmidt H (1994) The mutator gene swi8 effects specific mutations in the mating-type region of Schizosaccharomyces pombe. Genetics 138: 621–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard PH, Noguchi E, Shanahan P, Russell P (2003) The endogenous Mus81–Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol Cell 12: 747–759 [DOI] [PubMed] [Google Scholar]
- Gaskell LJ, Osman F, Gilbert RJ, Whitby MC (2007) Mus81 cleavage of Holliday junctions: a failsafe for processing meiotic recombination intermediates? EMBO J 26: 1891–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta N, Akamatsu Y, Tsutsui Y, Kurokawa Y, Murayama Y, Arcangioli B, Iwasaki H (2008) Fission yeast Swi5 protein, a novel DNA recombination mediator. DNA Repair (Amst) 7: 1–9 [DOI] [PubMed] [Google Scholar]
- Holmes AM, Kaykov A, Arcangioli B (2005) Molecular and cellular dissection of mating-type switching steps in Schizosaccharomyces pombe. Mol Cell Biol 25: 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope JC, Cruzata LD, Duvshani A, Mitsumoto J, Maftahi M, Freyer GA (2007) Mus81–Eme1-dependent and -independent crossovers form in mitotic cells during double-strand break repair in Schizosaccharomyces pombe. Mol Cell Biol 27: 3828–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interthal H, Heyer WD (2000) MUS81 encodes a novel helix–hairpin–helix protein involved in the response to UV- and methylation-induced DNA damage in Saccharomyces cerevisiae. Mol Gen Genet 263: 812–827 [DOI] [PubMed] [Google Scholar]
- Jia S, Yamada T, Grewal SI (2004) Heterochromatin regulates cell type-specific long-range chromatin interactions essential for directed recombination. Cell 119: 469–480 [DOI] [PubMed] [Google Scholar]
- Kai M, Boddy MN, Russell P, Wang TS (2005) Replication checkpoint kinase Cds1 regulates Mus81 to preserve genome integrity during replication stress. Genes Dev 19: 919–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaykov A, Arcangioli B (2004) A programmed strand-specific and modified nick in S. pombe constitutes a novel type of chromosomal imprint. Curr Biol 14: 1924–1928 [DOI] [PubMed] [Google Scholar]
- Kaykov A, Holmes AM, Arcangioli B (2004) Formation, maintenance and consequences of the imprint at the mating-type locus in fission yeast. EMBO J 23: 930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJ, Miglio LM (1986) Initiation of meiotic recombination by double-strand DNA breaks in S. pombe. Cell 46: 725–731 [DOI] [PubMed] [Google Scholar]
- Krings G, Bastia D (2005) Sap1p binds to Ter1 at the ribosomal DNA of Schizosaccharomyces pombe and causes polar replication fork arrest. J Biol Chem 280: 39135–39142 [DOI] [PubMed] [Google Scholar]
- Krogh BO, Symington LS (2004) Recombination proteins in yeast. Annu Rev Genet 38: 233–271 [DOI] [PubMed] [Google Scholar]
- Lisby M, Rothstein R (2004) DNA damage checkpoint and repair centers. Curr Opin Cell Biol 16: 328–334 [DOI] [PubMed] [Google Scholar]
- Liu Y, Masson JY, Shah R, O'Regan P, West SC (2004) RAD51C is required for Holliday junction processing in mammalian cells. Science 303: 243–246 [DOI] [PubMed] [Google Scholar]
- Lydeard JR, Jain S, Yamaguchi M, Haber JE (2007) Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature 448: 820–823 [DOI] [PubMed] [Google Scholar]
- McGlynn P, Lloyd RG (2002) Recombinational repair and restart of damaged replication forks. Nat Rev Mol Cell Biol 3: 859–870 [DOI] [PubMed] [Google Scholar]
- Mejia-Ramirez E, Sanchez-Gorostiaga A, Krimer DB, Schvartzman JB, Hernandez P (2005) The mating type switch-activating protein Sap1 is required for replication fork arrest at the rRNA genes of fission yeast. Mol Cell Biol 25: 8755–8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Morishita T, Furukawa F, Sakaguchi C, Toda T, Carr AM, Iwasaki H, Shinagawa H (2005) Role of the Schizosaccharomyces pombe F-Box DNA helicase in processing recombination intermediates. Mol Cell Biol 25: 8074–8083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ (2001) Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 157: 103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi E, Noguchi C, Du LL, Russell P (2003) Swi1 prevents replication fork collapse and controls checkpoint kinase Cds1. Mol Cell Biol 23: 7861–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Takahashi T, Masukata H (1999) Association of fission yeast Orp1 and Mcm6 proteins with chromosomal replication origins. Mol Cell Biol 19: 7228–7236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman F, Dixon J, Barr AR, Whitby MC (2005) The F-Box DNA helicase Fbh1 prevents Rhp51-dependent recombination without mediator proteins. Mol Cell Biol 25: 8084–8096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman F, Dixon J, Doe CL, Whitby MC (2003) Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81–Eme1 in meiosis. Mol Cell 12: 761–774 [DOI] [PubMed] [Google Scholar]
- Osman F, Whitby MC (2007) Exploring the roles of Mus81–Eme1/Mms4 at perturbed replication forks. DNA Repair (Amst) 6: 1004–1017 [DOI] [PubMed] [Google Scholar]
- Ostermann K, Lorentz A, Schmidt H (1993) The fission yeast rad22 gene, having a function in mating-type switching and repair of DNA damages, encodes a protein homolog to Rad52 of Saccharomyces cerevisiae. Nucleic Acids Res 21: 5940–5944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pâques F, Haber JE (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 63: 349–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y (2006) Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 6: 789–802 [DOI] [PubMed] [Google Scholar]
- Rodel C, Jupitz T, Schmidt H (1997) Complementation of the DNA repair-deficient swi10 mutant of fission yeast by the human ERCC1 gene. Nucleic Acids Res 25: 2823–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren C, Nasmyth K (2001) Sister chromatid cohesion is required for postreplicative double-strand break repair in Saccharomyces cerevisiae. Curr Biol 11: 991–995 [DOI] [PubMed] [Google Scholar]
- Smith CE, Llorente B, Symington LS (2007) Template switching during break-induced replication. Nature 447: 102–105 [DOI] [PubMed] [Google Scholar]
- Smith GR, Boddy MN, Shanahan P, Russell P (2003) Fission yeast Mus81.Eme1 Holliday junction resolvase is required for meiotic crossing over but not for gene conversion. Genetics 165: 2289–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrkarsdottir U, Egel R, Nielsen O (1993) The smt-0 mutation which abolishes mating-type switching in fission yeast is a deletion. Curr Genet 23: 184–186 [DOI] [PubMed] [Google Scholar]
- Thon G, Klar AJ (1993) Directionality of fission yeast mating-type interconversion is controlled by the location of the donor loci. Genetics 134: 1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PT, Erdeniz N, Symington LS, Liskay RM (2004) EXO1—a multi-tasking eukaryotic nuclease. DNA Repair (Amst) 3: 1549–1559 [DOI] [PubMed] [Google Scholar]
- Tsutsui Y, Morishita T, Iwasaki H, Toh H, Shinagawa H (2000) A recombination repair gene of Schizosaccharomyces pombe, rhp57, is a functional homolog of the Saccharomyces cerevisiae RAD57 gene and is phylogenetically related to the human XRCC3 gene. Genetics 154: 1451–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal E, Arbel-Eden A, Sattler U, Shroff R, Lichten M, Haber JE, Koshland D (2004) DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Mol Cell 16: 991–1002 [DOI] [PubMed] [Google Scholar]
- Vagin DA, Khasanov FK, Bashkirov VI (2006) [The role of recombinational repair proteins in mating type switching in fission yeast cells]. Genetika 42: 487–493 [PubMed] [Google Scholar]
- Vengrova S, Dalgaard JZ (2004) RNase-sensitive DNA modification(s) initiates S. pombe mating-type switching. Genes Dev 18: 794–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilenchik MM, Knudson AG (2003) Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA 100: 12871–12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby MC (2004) Junctions on the road to cancer. Nat Struct Mol Biol 11: 693–695 [DOI] [PubMed] [Google Scholar]
- Zou L, Elledge SJ (2003) Sensing DNA damage through ATRIP recognition of RPA–ssDNA complexes. Science 300: 1542–1548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Table
Supplementary data