Abstract
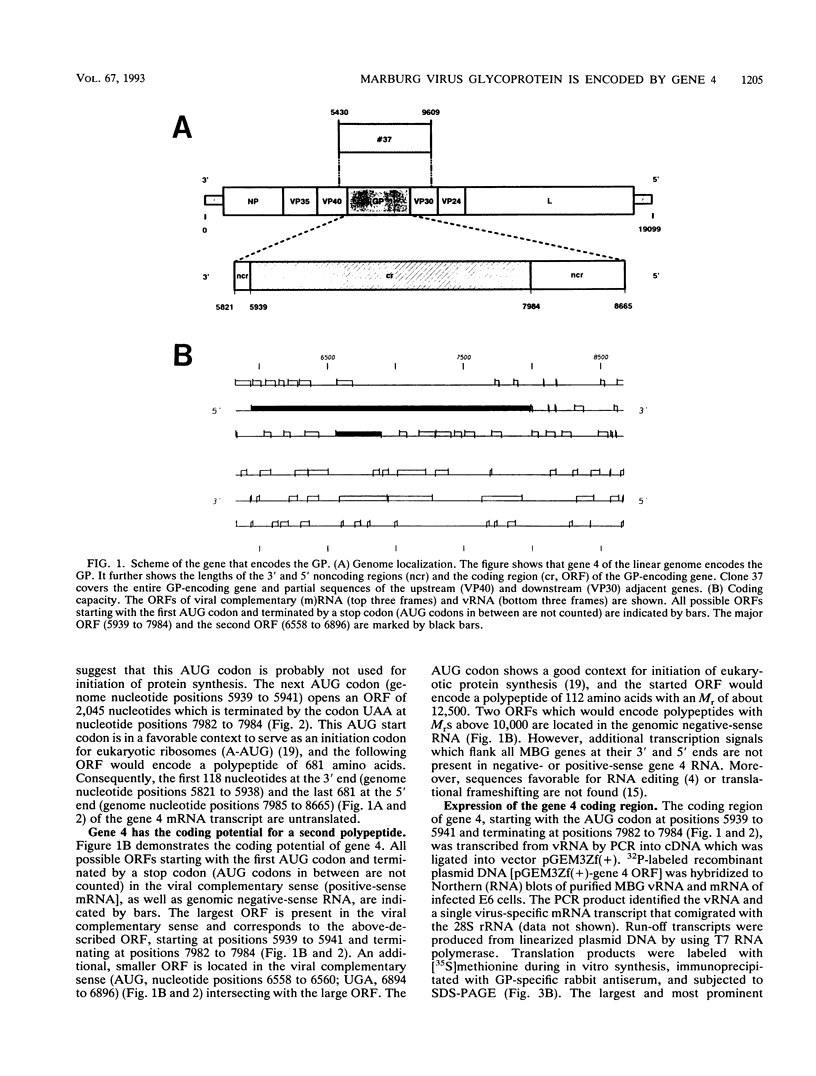
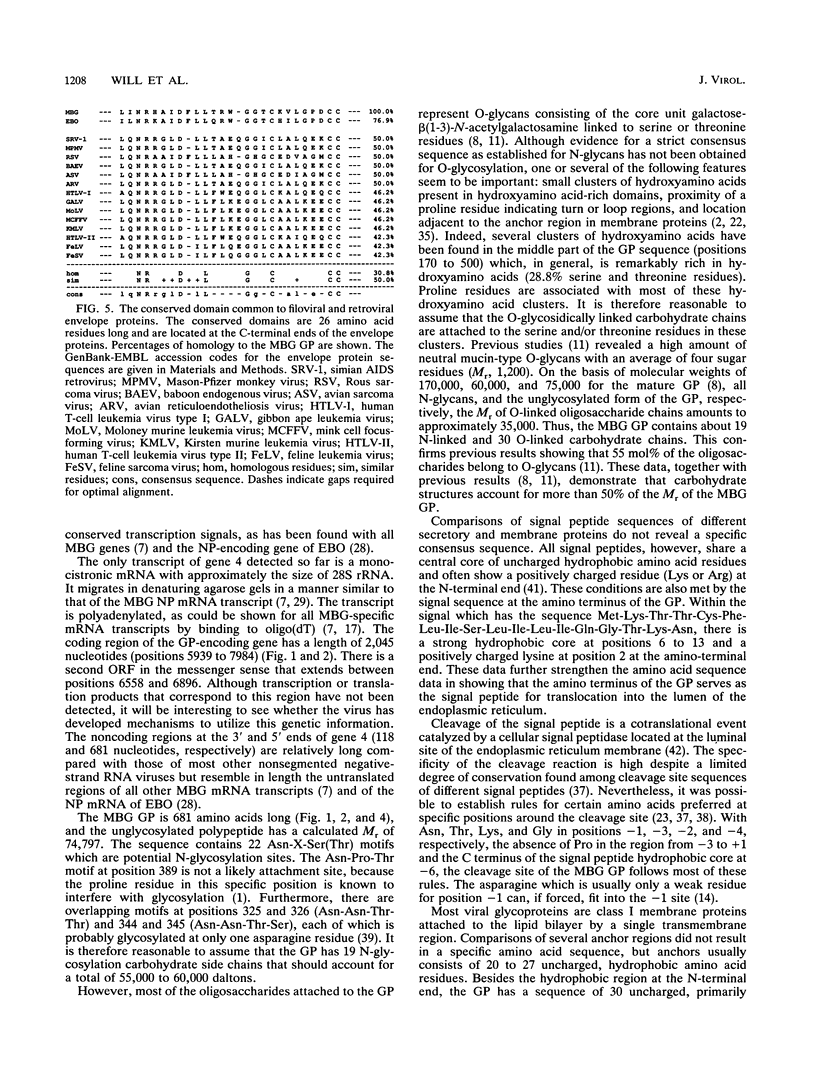
Gene 4 of Marburg virus, strain Musoke, was subjected to nucleotide sequence analysis. It is 2,844 nucleotides long and extends from genome position 5821 to position 8665 (EMBL Data Library, emnew: MVREPCYC [accession no. Z12132]). The gene is flanked by transcriptional signal sequences (start signal, 3'-UACUUCUUGUAAUU-5'; termination signal, 3'-UAAUUCUUUUU-5') which are conserved in all Marburg virus genes. The major open reading frame encodes a polypeptide of 681 amino acids (M(r), 74,797). After in vitro transcription and translation, as well as expression in Escherichia coli, this protein was identified by its immunoreactivity with specific antisera as the unglycosylated form of the viral membrane glycoprotein (GP). The GP is characterized by the following four different domains: (i) a hydrophobic signal peptide at the amino terminus (1 to 18), (ii) a predominantly hydrophilic external domain (19 to 643), (iii) a hydrophobic transmembrane anchor (644 to 673), and (iv) a small hydrophilic cytoplasmic tail at the carboxy terminus (674 to 681). Amino acid analysis indicated that the signal peptide is removed from the mature GP. The GP therefore has the structural features of a type I transmembrane glycoprotein. The external domain of the protein has 19 N-glycosylation sites and several clusters of hydroxyamino acids and proline residues that are likely to be the attachment sites for about 30 O-glycosidic carbohydrate chains. The region extending from positions 585 to 610 shows significant homology to a domain observed in the envelope proteins of several retroviruses and Ebola virus that has been suspected to be responsible for immunosuppressive properties of these viruses. A second open reading frame of gene 4 has the coding capacity for an unidentified polypeptide 112 amino acids long.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983 Feb 1;209(2):331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochberger T. C., Sabatine J. M., Lee Y. C., Hughey R. P. O-linked glycosylation of rat renal gamma-glutamyltranspeptidase adjacent to its membrane anchor domain. J Biol Chem. 1989 Dec 5;264(34):20718–20722. [PubMed] [Google Scholar]
- Bressan G. M., Stanley K. K. pUEX, a bacterial expression vector related to pEX with universal host specificity. Nucleic Acids Res. 1987 Dec 10;15(23):10056–10056. doi: 10.1093/nar/15.23.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Different types of messenger RNA editing. Annu Rev Genet. 1991;25:71–88. doi: 10.1146/annurev.ge.25.120191.000443. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Elliott L. H., Kiley M. P., McCormick J. B. Descriptive analysis of Ebola virus proteins. Virology. 1985 Nov;147(1):169–176. doi: 10.1016/0042-6822(85)90236-3. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Mühlberger E., Randolf A., Will C., Kiley M. P., Sanchez A., Klenk H. D. Marburg virus, a filovirus: messenger RNAs, gene order, and regulatory elements of the replication cycle. Virus Res. 1992 Jun;24(1):1–19. doi: 10.1016/0168-1702(92)90027-7. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Will C., Schikore M., Slenczka W., Klenk H. D. Glycosylation and oligomerization of the spike protein of Marburg virus. Virology. 1991 May;182(1):353–356. doi: 10.1016/0042-6822(91)90680-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear J. S., Cassel G. A., Gear A. J., Trappler B., Clausen L., Meyers A. M., Kew M. C., Bothwell T. H., Sher R., Miller G. B. Outbreake of Marburg virus disease in Johannesburg. Br Med J. 1975 Nov 29;4(5995):489–493. doi: 10.1136/bmj.4.5995.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer H., Holschbach C., Hunsmann G., Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988 Aug 25;263(24):11760–11767. [PubMed] [Google Scholar]
- Geyer H., Will C., Feldmann H., Klenk H. D., Geyer R. Carbohydrate structure of Marburg virus glycoprotein. Glycobiology. 1992 Aug;2(4):299–312. doi: 10.1093/glycob/2.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hortin G., Boime I. Miscleavage at the presequence of rat preprolactin synthesized in pituitary cells incubated with a threonine analog. Cell. 1981 May;24(2):453–461. doi: 10.1016/0092-8674(81)90336-6. [DOI] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley M. P., Bowen E. T., Eddy G. A., Isaäcson M., Johnson K. M., McCormick J. B., Murphy F. A., Pattyn S. R., Peters D., Prozesky O. W. Filoviridae: a taxonomic home for Marburg and Ebola viruses? Intervirology. 1982;18(1-2):24–32. doi: 10.1159/000149300. [DOI] [PubMed] [Google Scholar]
- Kiley M. P., Cox N. J., Elliott L. H., Sanchez A., DeFries R., Buchmeier M. J., Richman D. D., McCormick J. B. Physicochemical properties of Marburg virus: evidence for three distinct virus strains and their relationship to Ebola virus. J Gen Virol. 1988 Aug;69(Pt 8):1957–1967. doi: 10.1099/0022-1317-69-8-1957. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Mühlberger E., Sanchez A., Randolf A., Will C., Kiley M. P., Klenk H. D., Feldmann H. The nucleotide sequence of the L gene of Marburg virus, a filovirus: homologies with paramyxoviruses and rhabdoviruses. Virology. 1992 Apr;187(2):534–547. doi: 10.1016/0042-6822(92)90456-y. [DOI] [PubMed] [Google Scholar]
- Niemann H., Geyer R., Klenk H. D., Linder D., Stirm S., Wirth M. The carbohydrates of mouse hepatitis virus (MHV) A59: structures of the O-glycosidically linked oligosaccharides of glycoprotein E1. EMBO J. 1984 Mar;3(3):665–670. doi: 10.1002/j.1460-2075.1984.tb01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Sanchez A., Kiley M. P., Holloway B. P., McCormick J. B., Auperin D. D. The nucleoprotein gene of Ebola virus: cloning, sequencing, and in vitro expression. Virology. 1989 May;170(1):81–91. doi: 10.1016/0042-6822(89)90354-1. [DOI] [PubMed] [Google Scholar]
- Sanchez A., Kiley M. P. Identification and analysis of Ebola virus messenger RNA. Virology. 1987 Apr;157(2):414–420. doi: 10.1016/0042-6822(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Sanchez A., Kiley M. P., Klenk H. D., Feldmann H. Sequence analysis of the Marburg virus nucleoprotein gene: comparison to Ebola virus and other non-segmented negative-strand RNA viruses. J Gen Virol. 1992 Feb;73(Pt 2):347–357. doi: 10.1099/0022-1317-73-2-347. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuy W., Will C., Kuroda K., Scholtissek C., Garten W., Klenk H. D. Mutations blocking the transport of the influenza virus hemagglutinin between the rough endoplasmic reticulum and the Golgi apparatus. EMBO J. 1986 Nov;5(11):2831–2836. doi: 10.1002/j.1460-2075.1986.tb04576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Malagolini N., Nanni M., Dall'Olio F., Campadelli-Fiume G., Tanner J., Kieff E. Characterization of N- and O-linked oligosaccharides of glycoprotein 350 from Epstein-Barr virus. Virology. 1989 May;170(1):1–10. doi: 10.1016/0042-6822(89)90345-0. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Johnson B. K., Isaacson M., Swanapoel R., Johnson K. M., Killey M., Bagshawe A., Siongok T., Keruga W. K. Marburg-virus disease in Kenya. Lancet. 1982 Apr 10;1(8276):816–820. doi: 10.1016/s0140-6736(82)91871-2. [DOI] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Volchkov V. E., Blinov V. M., Netesov S. V. The envelope glycoprotein of Ebola virus contains an immunosuppressive-like domain similar to oncogenic retroviruses. FEBS Lett. 1992 Jul 6;305(3):181–184. doi: 10.1016/0014-5793(92)80662-z. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Collins P. L., Huang Y., Gruber C., Levine S., Ball L. A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]