Abstract
Interannual variations in CO2 exchange across Amazonia, as deduced from atmospheric inversions, correlate with El Niño occurrence. They are thought to result from changes in net ecosystem exchange and fire incidence that are both related to drought intensity. Alterations to net ecosystem production (NEP) are caused by changes in gross primary production (GPP) and ecosystem respiration (Reco). Here, we analyse observations of the components of Reco (leaves, live and dead woody tissue, and soil) to provide first estimates of changes in Reco during short-term (seasonal to interannual) moisture limitation. Although photosynthesis declines if moisture availability is limiting, leaf dark respiration is generally maintained, potentially acclimating upwards in the longer term. If leaf area is lost, then short-term canopy-scale respiratory effluxes from wood and leaves are likely to decline. Using a moderate short-term drying scenario where soil moisture limitation leads to a loss of 0.5 m2 m−2 yr−1 in leaf area index, we estimate a reduction in respiratory CO2 efflux from leaves and live woody tissue of 1.0 (±0.4) t C ha−1 yr−1. Necromass decomposition declines during drought, but mortality increases; the median mortality increase following a strong El Niño is 1.1% (n=46 tropical rainforest plots) and yields an estimated net short-term increase in necromass CO2 efflux of 0.13–0.18 t C ha−1 yr−1. Soil respiration is strongly sensitive to moisture limitation over the short term, but not to associated temperature increases. This effect is underestimated in many models but can lead to estimated reductions in CO2 efflux of 2.0 (±0.5) t C ha−1 yr−1. Thus, the majority of short-term respiratory responses to drought point to a decline in Reco, an outcome that contradicts recent regional-scale modelling of NEP. NEP varies with both GPP and Reco but robust moisture response functions are clearly needed to improve quantification of the role of Reco in influencing regional-scale CO2 emissions from Amazonia.
Keywords: net ecosystem exchange, drought, respiration, leaf, woody tissue, soil
1. Introduction
Respiration returns carbon dioxide (CO2) to the atmosphere that was originally removed from it by photosynthesis. CO2 is also released to the atmosphere episodically in single oxidation events, most notably fire. In combination, these processes govern the short-term carbon economy of a forested region. However, while terrestrial photosynthesis is performed by one main organ, the leaf, respiration occurs in all living cells and this simple distinction demands consideration of the metabolism of a mosaic of ecosystem components.
Atmospheric inversion studies have provided indirect evidence that Amazonia responds to climatic perturbation at large scale: the interannual flux anomaly is globally significant for tropical South America, showing CO2 emissions of up to 1.5 Pg C yr−1 during some years, and net CO2 acquisition during others (Rodenbeck et al. 2003; Zeng et al. 2005). Large net emissions have been associated with strong El Niño events impacting Amazonia (Zeng et al. 2005). Ecosystem modelling analyses are capable of approximately replicating this interannual pattern in regional-scale CO2 fluxes (Tian et al. 1998; Zeng et al. 2005) and have indicated that El Niño drought-related reductions in net ecosystem production (NEP) with respect to non-El Niño years can be as large as 1 Pg C yr−1 or more, owing to declines in photosynthesis coupled with increases in respiration. However, fire incidence also increases under drought stress and thus contributes to the observed El Niño-correlated emission anomalies. Fire-sourced CO2 emissions during drought have been difficult to quantify with high precision, but the available data indicate that they have the potential to be at least as large as the modelled declines in NEP (van der Werf et al. 2004). The size of the modelled change in regional NEP is also uncertain: although a reduction in gross primary production (GPP) is expected under moisture limitation, there is little evidence to support the short-term drought-correlated increases in ecosystem respiration reported in modelling studies and some evidence to the contrary (e.g. Saleska et al. 2003).
Good estimates of NEP require accurate representation of both photosynthesis and respiration, but an historical bias in research towards the former has left respiration less well parametrized, with consequent uncertainty in our understanding of the feedbacks resulting from land–atmosphere CO2 exchange across Amazonia. If we are to understand climatic influences on the Amazon carbon balance over the coming decades, then we must first be confident of how alterations in NEP take place at short time scales such as seasonally, or interannually, under episodic climate perturbations like El Niño. NEP represents the difference between carbon that is acquired and respired by an ecosystem (equation (1.1)). Our analysis focuses on using field data from Amazon rainforests as the basis for estimating respiration in each component, and how it changes under moisture limitation, particularly at the interannual time scale,
| (1.1) |
where Rl, Rw and Rs represent the aggregate component flux contributions to Reco from leaves, woody tissue (live and dead) and soil, respectively.
2. Climatic influences on respiration
What are the magnitudes of the relevant climatic drivers? Although there is variability among predictions of twenty-first century climatic change for Amazonia, most climate models predict some kind of drying and warming, with the strongest drying scenario indicating a 65% reduction in precipitation (Cubasch et al. 2001). Long-term climate data from the twentieth century are much less extreme: they show non-significant changes in precipitation allied with slight warming, although these overall patterns mask important regional and interannual differences (Malhi & Wright 2004). Focusing on the shorter-term climatic impacts of the principal interannual climate perturbation of El Niño, during the 1997/1998 event, precipitation reductions were large, especially for the eastern Amazon, reaching 50% of the long-term mean (Uppala et al. 2005). Maximum temperature anomalies were larger during the daytime (average anomaly of maximum=+1.7°C) than the night-time (average anomaly of minimum=+0.5°C), with an average increase in solar radiation of 30.7 W m−2 and concomitant decreases in atmospheric humidity yielding a mean midday water vapour pressure deficit anomaly of +0.4 kPa (Uppala et al. 2005). The effects of these climatic anomalies on NEP are surprisingly poorly understood. For example, observed changes to NEP in European forests during widespread drought in 2003 were, on average, non-significant (Reichstein et al. 2007) because declines in photosynthesis were partially balanced by declines in Reco. These measured temperate zone declines in respiration during drought contradict recent modelling of the same processes in Amazonia, and thus further prompt the need to consider the response to drought by Reco in tropical rainforest ecosystems using direct observations.
3. Leaves
Leaf respiration (Rl) comprises 10–40% of the respiratory emissions of CO2 to the atmosphere by terrestrial ecosystems (Wright et al. 2006). In ecosystems where leaf area is large and temperatures high, the contribution to the carbon budget from foliar respiration can be significant. Although respiration occurs during both day and night, measurement is usually made on leaves in the absence of light so that the respiratory flux of CO2 during the dark can be distinguished from that released during photosynthesis, which itself varies with the photosynthetic process, often declining strongly with irradiance (Brooks & Farquhar 1985). Rl during the night ranges between 2.0 and 7.0 t C ha−1 yr−1 on a ground area basis in tropical rainforests (Meir 1996; Malhi et al. 1999; Chambers et al. 2004; R. Vale 2002, unpublished data).
Respiration in leaves yields energy used for the maintenance of leaf metabolism and structure in mature leaves, and for the construction of new leaves. Maintenance respiration is the larger component, is temperature sensitive (Atkin et al. 2005), and correlates positively with irradiance, with the tissue concentration of nutrients, such as nitrogen or phosphorus, and thus also with photosynthetic capacity (Meir et al. 2001; Wright et al. 2006). The impact of moisture limitation on leaf respiration has received less attention. Stomatal closure in response to drought can lead to higher leaf temperatures, and lower photosynthetic rates, but the reported effects on maintenance respiration in leaves differ. Laboratory experiments have mostly suggested that the net effect is small (Flexas et al. 2006). For example, at a level of moisture limitation sufficient to cause a general failure of photochemistry and biochemistry, instead of declining, leaf respiration rates were unaltered while electron flow to the alternative oxidative pathway increased from 10 to 40%, with a concomitant decrease by 32% in electron flow to the mitochondrial cytochrome c pathway (Ribas-Carbo et al. 2005). By contrast, in field measurements on 208 species from sites varying widely in rainfall, Wright et al. (2006) showed woody plants in dry environments to have higher leaf respiration rates, and indicated an average 50% increase in leaf respiration for a rainfall reduction from 2000 to 500 mm yr−1. These higher leaf respiration rates possibly reflect adaptation, or longer-term acclimation, to drier conditions where, although stomatal closure may constrain photosynthesis, more respiration occurs owing to an increased need to maintain high vacuolar concentrations of osmotically active solutes (Wright et al. 2006), or a need to avoid the accumulation of cellular redox equivalents and free oxygen radicals resulting from decreased photosynthesis and increased photoinhibition (Macherel & Atkin in review). The general physiological response to drought by Rl is thus unclear, although initial experimental data from an eastern Amazon site suggest increases in respiration rate on a leaf area basis after extended artificial soil moisture limitation of 4–5 years (table 1), and this is consistent with dry season measurements at a transition forest in Sinop (Miranda et al. 2005).
Table 1.
Leaf dark respiration throughout the vertical profile of a rainforest at Caxiuana, in eastern Amazonia, following 4 years of artificial soil drought. Values represent the mean leaf dark respiration in μmol m−2 s−1±s.d. (n), at 25°C, and compare vegetation subjected to artificial soil drought and normal rainfall (Metcalfe et al. in preparation).
| leaf height | control | drought |
|---|---|---|
| greater than 20 m | 0.52±0.13 (14) | 0.70 (1) |
| 10–20 m | 0.46±0.10 (14) | 0.67±0.25 (12) |
| 0–10 m | 0.30±0.03 (5) | 0.49±0.08 (15) |
| mean | 0.46±0.15 (33) | 0.57±0.19 (28) |
To fully account for changes in leaf respiration on a ground area basis, the effect of alterations in canopy structure must also be considered. Moisture limitation can cause a decline in leaf area index (LAI) in dry or dry–wet transition zones between savannah and rainforest (Pennington et al. 2000). Although seasonal LAI measurements are scarce in tropical rainforests (Carswell et al. 2002), two recent artificial droughting experiments in the eastern Amazon (at Tapajos and Caxiuana) have shown LAI reductions of 20% or more (approx. 1 m2 m−2 over 2 or more years) after reducing rainfall input by approximately 50% (Nepstad et al. 2004; Fisher et al. 2007). The effect of a loss in LAI is linear in terms of the change in respiration caused by a reduction in leaf biosynthesis, although alterations to leaf longevity and the decline in maintenance respiration with height in the canopy (Domingues et al. 2005) could modify this linearity. The metabolic cost of biosynthesis is approximately 0.25(±0.02) g C respired per gram of biomass constructed (Penning de Vries 1975). Therefore, assuming mean leaf maintenance respiration at 25°C to be 0.6(±0.2) μmol C m−2 s−1 (Chambers et al. 2004; Domingues et al. 2005), leaf longevity to be 12 months, and an average leaf mass per unit area of 100 g m−2, a loss in LAI of 0.5 m2 m−2 in 1 year would accrue a reduction in construction respiration of 0.07(±0.01) t C ha−1 yr−1 and a reduction in maintenance respiration of 0.6(±0.2) t C ha−1 yr−1. Combined with the effect of a 0.5°C night-time warming anomaly (1997/1998 El Niño; Uppala et al. 2005), this yields an overall reduction in Rl of 0.5(±0.2) t C ha−1 yr−1. Such short-term alterations in canopy-scale leaf respiration are similar in magnitude to the (annual) NEP reported for some Amazonian rainforests (Ometto et al. 2005), although this only accounts for changes during darkness; changes in daytime leaf respiration have not been estimated (i.e. assumed=0) given the lack of available data on drought responses in this variable. Over the longer term, sustained moisture limitation (greater than 2 years) could lead to different effects of Rl on NEP: leaves formed entirely under conditions of significant moisture limitation may be altered in structure (e.g. increased mass per unit area), with consequent increases in leaf respiration on a leaf area basis and, potentially, a canopy basis (cf. table 1)
4. Woody tissue
Emissions of CO2 from woody tissue in forest ecosystems (Rw) are divided into two main components: the flux from living woody tissue (Rwc) and the flux from decomposing coarse necromass (Rwn). Fluxes from Rwc are thought to comprise up to 18% of GPP, or 5.1 t C ha−1 yr−1 (Cavaleri et al. 2006). Those from Rwn vary strongly with precipitation and contain uncertainty of up to 50% (Rice et al. 2004). Where detailed measurements have been made, annualized mass loss rates in tropical rainforests suggest Rwn emissions of 0.5–1.9 t C ha−1 yr−1 (Chambers et al. 2004; Rice et al. 2004; Hutyra et al. 2007).
The CO2 comprising the flux from living woody tissue has two origins, the woody tissue itself and the soil. Mitochondria in living parenchymatic, cambium and phloem cells in wood respire and release CO2 into the surrounding fluid, where it dissolves and dissociates according to pH and temperature, following Henry's law. Part of this CO2 ultimately diffuses radially and evolves into the atmosphere, but part of it is transported away from the locus of production dissolved in water columns in the xylem, to evolve elsewhere in the canopy or to be refixed by photosynthesis. However, the xylem stream originates in the soil where CO2 respired by both roots and microbes dissolves to saturation point, creating an additional and strong CO2 source within woody tissue. Hence, the CO2 that is released from bark is a product of a mixture of autotrophic and heterotrophic respiration, is dependent on the diffusion properties of live and dead cells in wood, and is sensitive to rates of sap flux (Levy et al. 1999; Bowman et al. 2005). Different organ-scale metrics by which canopy-scale live woody tissue CO2 effluxes (Rwc) may be calculated have been suggested (Ryan 1990; Levy & Jarvis 1998). More recently, Cavaleri et al. (2006) made measurements throughout the vertical profile of a Costa Rican rainforest canopy and demonstrated that small diameter branches and lianas high in the canopy were significant sources of CO2, especially during the day, the period when CO2 evolves from the transpiration stream, diffusing rapidly to the atmosphere through the thinnest sections of tissue and bark.
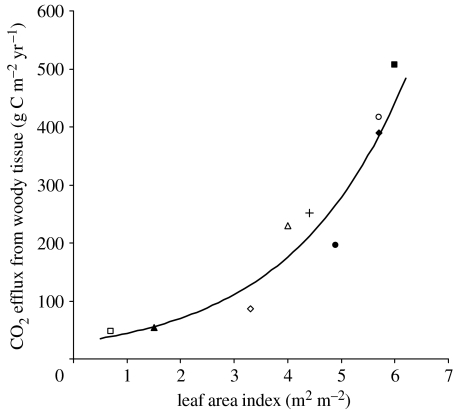
One solution to quantify Rwc is to measure changes in emissions caused individually through alterations in maintenance and growth respiration in all above-ground woody tissue elements, and in the transport of heterotrophic and autotrophic CO2 by the xylem stream. An alternative and functionally appropriate method is to use the strong observed relationship between Rwc and LAI for forest ecosystems (figure 1). Rwc probably scales with LAI for reasons of economy in respiration and transpiration (Shinozaki 1964; Meir & Grace 2002), and this relationship should thus hold functionally as well as biogeographically. Since LAI varies with strong moisture limitation, the Rwc–LAI relationship can be used to quantify the change in CO2 efflux from live woody tissue associated with short-term drought. Applying the same reduction in LAI (0.5 m2 m−2 yr−1) used to estimate changes in Rl, figure 1 predicts a reduction in Rwc of 0.5(±0.1) t C ha−1 yr−1. These estimates suggest that the impact of drought on Rwc is similar in direction and magnitude to changes in Rl.
Figure 1.
Variation with leaf area index (LAI) in CO2 efflux from above-ground woody tissue (Rwc). Graph redrawn from Meir & Grace (2002), using new data for site 7 (Rwc, Cavaleri et al. 2006; LAI, D. Clark 2007, personal communication) and data for two additional tropical rainforests, sites 8 and 9, near Manaus (C. Amazonia) and at Reserva Jaru (SW Amazonia), respectively (McWilliam et al. 1993; Kruijt et al. 1996; Meir 1996; Chambers et al. 2004). LAI was obtained directly for sites 1 and 5–8 and estimated indirectly for sites 2–4 and 9; the LAI for site 8 is taken from a nearby destructive harvest (McWilliam et al. 1993) because this estimate includes all tree sizes. Rwc was obtained from measurements of woody tissue CO2 efflux at all sites. Original , p=0.003, n=7 (Meir & Grace 2002); revised fit , p<0.0001, n=9 (Rwc=29.1e(0.45.LAI)). TRF is tropical rainforest. Solid line, model; open square, site 1: Sahelian shrub, Niger; filled triangle, site 2: Ponderosa pine, USA; open diamond, site 3: Black spruce, Canada; plus sign, site 4: TRF, Cameroon; filled circle, site 5: deciduous broadleaf, USA; filled diamond, site 6: TRF, Brazil (Manaus); filled square, site 7: TRF, Costa Rica; open circle, site 8: TRF, Brazil (Manaus); open triangle, site 9: TRF, Brazil (Jaru).
The breakdown of necromass leading to Rwn is estimated as a function of the rate of loss of dry mass over time, described by a decay constant, k (yr−1), where k for coarse necromass varies among studies between 0.1 and 0.3 yr−1 (mean=0.197 (±0.04) yr−1, n=6; Kira 1976; Yoneda 1990; Chambers et al. 2000; Eaton & Lawrence 2006; Palace et al. 2007). Irrigation experiments have demonstrated that k declines strongly under moisture limitation by a factor of 2.4 or more (Vasconcelos et al. 2007), and despite variation in tissue density, moisture also dominates the rate of respiration in decomposing wood, with up to fivefold differences in CO2 efflux rates for woody tissue moisture contents varying between 0.5 and 1.5 g H2O g−1 dry woody biomass (Chambers et al. 2000; Keller et al. 2004). Accounting for natural spatial variation in necromass moisture content and consequent decomposition rates, Rice et al. (2004) proposed a stand-scale value for k of 0.113 yr−1 for an eastern Amazon forest at Tapajos.
Although drought reduces the rate of decomposition, where sufficient moisture limitation increases tree mortality, then the net effect on Rwn is positive. Mortality measurements are dependent on the death of only a few trees per 1 ha plot, so the use of data from multiple plots is essential for estimating the overall effects of drought on Rwn. Mortality impacts following short-term drought caused by strong El Niño events vary for tropical rainforests globally, with a median increase of 1.2% for observational studies both in Amazonia and globally (Meir & Grace 2005; n=46 plots). Mean standing biomass varies substantially across the Amazon basin (Baker et al. 2004) and assuming an increase in mortality of 1.1%, a stand scale k of 0.113 (Rice et al. 2004) and the lower estimate of 2.4 for the factor by which k declines under drought (Chambers et al. 2001; Vasconcelos et al. 2007), the estimated maximum effect on Rwn is an increase of 0.13–0.18 t C ha−1 yr−1, although up to 25% of this flux may instead join the soil organic matter pool (Chambers et al. 2001). Thus, the short-term impact of drought on respiration in necromass is likely to marginally increase Reco during a drought, although this small increase in Rwn may be followed by larger fluxes under subsequent increased rainfall, reflecting the increase in the necromass carbon pool under the original period of drought.
5. Soil
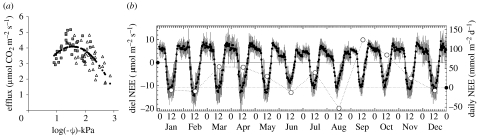
The efflux of CO2 from the soil (Rs) is the largest component of the respiration budget, ranging in size from approximately 11 to 22 t C ha−1 yr−1, so small fractional changes in this component can influence NEP significantly. Rs comprises respiration by plant roots (autotrophic respiration, Rsa) and microbes (heterotrophic respiration, Rsh). Recent focus (Trumbore 2006; Metcalfe et al. 2007) has emphasized the need to separate Rsa and Rsh to improve the mechanistic understanding of Rs. Evidence at seasonal and interannual time scales suggests that the effects of variation in moisture availability on Rs in tropical rainforests are much larger than temperature. Using chamber measurements, Davidson et al. (2000) demonstrated declines of more than 50% in Rs with changes in soil matric potential of −0.001 to −10 MPa. Subsequent studies have made similar findings (e.g. Schwendenmann et al. 2003; Sotta et al. 2004, 2007), although conflicting evidence of moisture limitation on Rs has been reported in one case (Saleska et al. 2003; Davidson et al. 2004). Using eddy covariance measurements of net ecosystem exchange from a seasonally dry forest at Sinop in Mato Grosso, Vourlitis et al. (2005) showed that ecosystem respiration, probably dominated by Rs, declined more strongly in response to moisture constraints than did maximum photosynthesis (figure 2). This moisture sensitivity in net ecosystem exchange is not restricted to climatically marginal sites: eddy covariance data from an eastern Amazon site at Tapajos on deeply weathered oxisols have also been used to show how dry season declines in Rs contribute to increased net CO2 assimilation rates when compared with wet season values (Saleska et al. 2003; Hutyra et al. 2007).
Figure 2.
Moisture constraints on Rs and Reco. (a) The relationship between soil water potential and soil respiration (Rs, μmol m−2 s−1; R2=0.43, p<0.001); data are from a soil drought experiment at an eastern Amazon rainforest, Caxiuana (Sotta et al. 2007). (b) Net ecosystem exchange (NEE) in a transitional forest, Sinop, Mato Grosso (Vourlitis et al. 2005). During the four-month dry season (May–August), Reco is more sensitive to moisture stress than is maximum photosynthesis, Amax (a decline of 28% in Reco versus 5% in Amax; Vourlitis et al. 2005). Data show changes in diel and daily NEE of CO2. Positive values indicate CO2 release (mean NEE (±s.d.) of 30-min data (filled circle) and average daily NEE by month (open circles); horizontal solid line is diel NEE=0 and horizontal dashed line is daily NEE=0).
A fully mechanistic analysis of the impact of moisture limitation on respiration in soil is complicated by the need to quantify both Rsh and Rsa in the context of vertical differences in soil moisture along the soil profile, of differences in the supply of respiratory substrate and owing to the as yet largely unquantified role of mycorrhizae (Davidson et al. 2006; Meir et al. 2006). As a consequence, Rs is often still represented as an empirical function of environmental constraints. Large-scale modelling studies of Amazonian NEP during El Niño-caused drought have tended to emphasize the enhancement of Rsh under marginal associated warming (Zeng et al. 2005) even though any theoretical temperature response by Rs is dominated in field data by the availability of moisture (Sotta et al. 2007). The response to moisture has been found to be a linear (Davidson et al. 2000) or parabolic (Schwendenmann et al. 2003; Sotta et al. 2007) function of soil water potential, although mixtures of weak and strong responses in Rs to experimental changes in soil moisture have also been noted (Vasconcelos et al. 2004). Recent multi-scale field data are thus establishing the importance of low soil moisture as the dominant short-term climatic constraint on Rs, and indicate that moisture limitations can cause reductions in Rs of 10–30%, potentially equating to 2.0(±0.5) t C ha−1 y−1 or more (e.g. Saleska et al. 2003; Vourlitis et al. 2005; Sotta et al. 2007), notwithstanding the potential for efflux pulses following dry period rainfall events (Hutyra et al. 2007). Overall, field evidence shows that if strong short-term moisture limitation is experienced, for example, during El Niño-caused drought, it is very likely to cause significant reductions in Rs. The key now will be to specify more accurately the Rs-soil moisture response across the Amazon basin as part of a mechanistically and spatially consistent model of Rs.
6. Conclusions
Process modelling of NEP has historically been informed by more data on photosynthesis than on respiration. Although new flux measurements at the ecosystem scale are emerging, improved insight into the component respiratory processes is necessary to properly test and specify model outcomes because these separate processes respond to environmental changes in different and nonlinear ways, sometimes also interacting. Appropriate field data remain relatively sparse, but this analysis shows that the short-term drought responses of the component processes of Reco are larger than, or show notable deviations from, previous estimates.
The clearest of the impacts of drought on Reco takes place in the soil and this has been observed at seasonal and 1–2 year time scales. Part of the drought-related decline in Rs results from the decline in GPP. Additionally, some carbon that is not respired owing to moisture limitations on Rsh is probably retained temporarily in the soil as labile organic material. Improved modelling of the impact of these processes on Rs will require better understanding of the interactions between Rs and GPP, and of the effects of moisture limitation on water and substrate supply to respiring cells in soils that differ hydraulically across Amazonia.
The separate impacts of short-term drought on respiration in woody and leafy tissue are directly altered by structural change in leaf area and mortality. Leaf respiration and CO2 effluxes from live woody tissue will both decline over the short term if leaf area is lost during drought, although physiological acclimation to drought may modulate the impact on Reco over the longer term. The total emissions of CO2 from necromass increase when mortality increases, but the main impact on emissions is held in check until the moisture content of the decomposing tissue rises.
To summarize drought-related alterations to Reco, in the 1- to 2-year drought scenario used for our calculations above, we estimate a loss in LAI of 0.5 m2 m−2 y−1; declines in Rl, Rwc and Rs of 0.5(±0.2), 0.5(±0.1) and 2.0(±0.5) t C ha−1 y−1, respectively; and an increase in Rwn of 0.13–0.18 t C ha−1 yr−1. Drought is thus very likely to cause an overall decline in Reco of tropical rainforests over seasonal or interannual time scales, with differing effects on each component of Reco. The largest component, Rs, is especially sensitive to the water-holding characteristics of soil, and understanding this variation over biogeographic space is a research priority: GPP and Rs are both affected if water is unavailable to plants.
In the specific case of El Niño impacts, the intensity of the associated climatic perturbations differs among years, but the probable sequence of principal effects on Reco is: first, a reduction in heterotrophic respiration in soil and woody tissue in combination with variable limitations on GPP; and, secondly, structural and physiological impacts developing following extended strong moisture limitation. The impact of any drought on NEP will vary where climatic and edaphic constraints on Reco and GPP differ, but in general, the effects are very likely to oppose each other, dampening change in NEP rather than amplifying it, as previously modelled (e.g. Zeng et al. 2005). Where drought also leads to fire, then this large abiotic addition to Reco could dominate the short-term carbon budget (van der Werf et al. 2004), rather than changes in NEP. Over decadal or longer time scales, continued drought can be expected lead to large emissions of CO2 from the land owing to substantial shifts in vegetation and soil properties. But in order to have confidence in longer-term predictions of rainforest–atmosphere interactions, we must first correctly understand and model short-term variations in the biophysical functioning of these ecosystems.
Acknowledgments
The authors would like to thank D. Clark for generously making available new LAI measurement results for La Selva, and the anonymous referees for useful critical appraisal. This work was supported by NERC, EU-FP5 and LBA.
Footnotes
One contribution of 27 to a Theme Issue ‘Climate change and the fate of the Amazon’.
References
- Atkin O.K, Bruhn D, Hurry V.M, Tjoelker M.G. The hot and the cold: unraveling the variable response of plant respiration to temperature. Funct. Plant Biol. 2005;32:87–105. doi: 10.1071/FP03176. doi:10.1071/FP03176 [DOI] [PubMed] [Google Scholar]
- Baker T.R, et al. Increasing biomass in Amazonian forest plots. Phil. Trans. R. Soc. B. 2004;359:353–365. doi: 10.1098/rstb.2003.1422. doi:10.1098/rstb.2003.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W.P, Barbour M.M, Turnbull M.H, Tissue D.T, Whitehead D, Griffin K.L. Sap flow rates and sapwood density are critical factors in within- and between-tree variation in CO2 efflux from stems of mature Dacrydium cupressinum trees. New Phytol. 2005;167:815–828. doi: 10.1111/j.1469-8137.2005.01478.x. doi:10.1111/j.1469-8137.2005.01478.x [DOI] [PubMed] [Google Scholar]
- Brooks A, Farquhar G.D. Effect of temperature on the CO2–O2 specificity of ribulose 1,5-bisphosphate carboxylase oxygenase and the rate of respiration in the light. Estimates from gas exchange measurements on spinach. Planta. 1985;165:397–406. doi: 10.1007/BF00392238. doi:10.1007/BF00392238 [DOI] [PubMed] [Google Scholar]
- Carswell F.E, et al. Seasonality in CO2 and H2O flux at an eastern Amazonian rain forest. J. Geophys. Res. Atmos. 2002;107:8066. doi:10.1029/2001JD000524 [Google Scholar]
- Cavaleri M.A, Oberbauer S.F, Ryan M.G. Wood CO2 efflux in a primary tropical rain forest. Glob. Change Biol. 2006;12:2442–2458. doi:10.1111/j.1365-2486.2006.01269.x [Google Scholar]
- Chambers J.Q, Higuchi N, Schimel J.P, Ferreira L.V, Melack J.M. Decomposition and carbon cycling of dead trees in tropical forests of the central Amazon. Oecologia. 2000;122:380–388. doi: 10.1007/s004420050044. doi:10.1007/s004420050044 [DOI] [PubMed] [Google Scholar]
- Chambers J.Q, Schimel J.P, Nobre A.D. Respiration from coarse wood litter in central Amazon forests. Biogeochemistry. 2001;52:115–131. doi:10.1023/A:1006473530673 [Google Scholar]
- Chambers J.Q, et al. Respiration from a tropical forest ecosystem: partitioning of sources and low carbon use efficiency. Ecol. Appl. 2004;14:S72–S88. doi:10.1890/01-6012 [Google Scholar]
- Cubasch U, et al. Projections of future climate change. In: Houghton J.T, editor. The scientific basis. Contribution of Working Group I to the Third Assessment report of the IPCC. Cambridge University Press; Cambridge, UK: 2001. pp. 525–582. [Google Scholar]
- Davidson E.A, Verchot L.V, Cattânio J.H, Ackerman I.L, Carvalho J.E.M. Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry. 2000;48:53–69. doi:10.1023/A:1006204113917 [Google Scholar]
- Davidson E.A, Ishida F.Y, Nepstad D.C. Effects of an experimental drought on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Glob. Change Biol. 2004;10:718–730. doi:10.1111/j.1365-2486.2004.00762.x [Google Scholar]
- Davidson E.A, Janssens I.A, Luo Y. On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob. Change Biol. 2006;12:154–164. doi:10.1111/j.1365-2486.2005.01065.x [Google Scholar]
- Domingues T.F, Berry J.A, Martinelli L.A, Ometto J.P.H.B, Ehleringer J.R. Parameterization of canopy structure and leaf-level gas exchange for an eastern Amazonian tropical rain forest. Earth Interact. 2005;9:1–23. doi:10.1175/EI149.1 [Google Scholar]
- Eaton J.M, Lawrence D. Woody debris stocks and fluxes during succession. Forest Ecol. Manage. 2006;232:46–55. doi:10.1016/j.foreco.2006.05.038 [Google Scholar]
- Fisher R.A, Williams M, da Costa A.L, Malhi Y, da Costa R.F, Almeida S, Meir P. The response of an eastern Amazonian rain forest to drought stress: results and modelling analyses from a through-fall exclusion experiment. Glob. Change Biol. 2007;13:2361–2378. doi:10.1111/j.1365-2486.2007.01417.x [Google Scholar]
- Flexas J, Bota J, Galmés J, Medrano H, Ribas-Carbó M. Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006;127:343–352. doi:10.1111/j.1399-3054.2006.00621.x [Google Scholar]
- Hutyra L.R, Munger J.W, Saleska S.R, Gottlieb E, Daube B.C, Dunn A.L, Amaral D.F, de Camargo P.B, Wofsy S.C. Seasonal controls on the exchange of carbon and water in an Amazonian rain forest. J. Geophys. Res. Atmos. 2007;112:G04099. doi:101029/2006JG000365 [Google Scholar]
- Keller M, Palace M, Asner G.P, Pereira R, Jr, Silva J.N.M. Coarse woody debris in undisturbed and logged forests in the eastern Brazilian Amazon. Glob. Change Biol. 2004;10:784–795. doi:10.1111/j.1529-8817.2003.00770.x [Google Scholar]
- Kira T. Cambridge University Press; Cambridge, UK: 1976. Community arhitecture and organic matter dynamics in tropical lowland rain forests of Southeast Asia; pp. 561–590. [Google Scholar]
- Kruijt B, Lloyd J, Grace J, McIntyre J, Raupach M.R, Farquhar G.D, Miranda A.C, McCraken P. Sources and sinks of CO2 in Rondônian tropical forest. In: Gash J.H.C, et al., editors. Amazonian deforestation and climate. Wiley; Chichester, UK: 1996. pp. 331–351. [Google Scholar]
- Levy P.E, Jarvis P.G. Stem CO2 fluxes in two Sahelian shrub species (Guiera senegalensis and Combretum micranthum) Funct. Ecol. 1998;12:107–116. doi:10.1046/j.1365-2435.1998.00156.x [Google Scholar]
- Levy P.E, Meir P, Allen S.J, Jarvis P.G. The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiol. 1999;19:53–58. doi: 10.1093/treephys/19.1.53. [DOI] [PubMed] [Google Scholar]
- Macherel, D. & Atkin, O. K. In review. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot [DOI] [PMC free article] [PubMed]
- Malhi Y, Wright J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Phil. Trans. R. Soc. B. 2004;359:311–329. doi: 10.1098/rstb.2003.1433. doi:10.1098/rstb.2003.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi Y, Baldocchi D.D, Jarvis P.G. The carbon balance of tropical, temperate and boreal forests. Plant Cell Environ. 1999;22:715–740. doi:10.1046/j.1365-3040.1999.00453.x [Google Scholar]
- McWilliam A.-L. C, Roberts J.M, Cabral O.M.R, Leitao M.V.B.R, de Costa A.C.L, Maitelli G.T, Zamparoni C.A.G.P. Leaf area index and above ground biomass of terra firme rain forest and adjacent clearings in Amazonia. Funct. Ecol. 1993;7:310–317. doi:10.2307/2390210 [Google Scholar]
- Meir P. University of Edinburgh; Edinburgh, UK: 1996. The exchange of carbon dioxide by tropical rain forest; p. 208. [Google Scholar]
- Meir P, Grace J. Scaling relationships for woody tissue respiration in two tropical rain forests. Plant Cell Environ. 2002;25:963–973. doi:10.1046/j.1365-3040.2002.00877.x [Google Scholar]
- Meir P, Grace J. The response by tropical forest ecosystems to drought. In: Malhi Y, Phillips O.L, editors. Tropical forests and global atmospheric change. Oxford University Press; Oxford, UK: 2005. pp. 71–80. [Google Scholar]
- Meir P, Grace J, Miranda A.C. Leaf respiration in two tropical rainforests: constraints on physiology by phosphorus, nitrogen and temperature. Funct. Ecol. 2001;15:378–387. doi:10.1046/j.1365-2435.2001.00534.x [Google Scholar]
- Meir P, Cox P, Grace J. The influence of terrestrial ecosystems on climate. Trends Ecol. Evol. 2006;21:254–260. doi: 10.1016/j.tree.2006.03.005. doi:10.1016/j.tree.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Metcalfe D.B, et al. Factors controlling spatio-temporal variation in carbon dioxide efflux from surface litter, roots, and soil organic matter at four rain forest sites in the eastern Amazon. J. Geophys. Res. 2007;112:G04001. doi:10.1029/2007JG000443 [Google Scholar]
- Metcalfe, D. B. et al In preparation. Leaf respiration after four years of an experimentally-imposed drought in an eastern Amazonian rainforest.
- Miranda E.J, Vourlitis G.L, Filho N.P, Priante P.C, Campelo J.H, Jr, Suli G.S, Fritzen C.L, Lobo F.de.A, Shiraiwa S. Seasonal variation in the leaf gas exchange of tropical forest trees in the rain forest–savanna transition of the southern Amazon Basin. J. Trop. Ecol. 2005;21:451–460. doi:10.1017/S0266467405002427 [Google Scholar]
- Nepstad D, Lefebvre P, da Silva U.L, Tomasella J, Schlesinger P, Solórzano L, Moutinho P, Ray D, Benito J.G. Amazon drought and its implications for forest flammability and tree growth: a basin-wide analysis. Glob. Change Biol. 2004;10:704–717. doi:10.1111/j.1529-8817.2003.00772.x [Google Scholar]
- Ometto J, Nobre A.D, Rocha H.R, Artaxo P, Martinelli L.A. Amazonia and the modern carbon cycle: lessons learned. Oecologia. 2005;143:483–500. doi: 10.1007/s00442-005-0034-3. doi:10.1007/s00442-005-0034-3 [DOI] [PubMed] [Google Scholar]
- Palace M, Keller M, Asner G.P, Silva J.N.M, Passos C. Necromass in undisturbed and logged forests in the Brazilian Amazon. Forest Ecol. Manage. 2007;238:309–318. doi:10.1016/j.foreco.2006.10.026 [Google Scholar]
- Penning de Vries F.W.T. The cost of maintenance processes in plant cells. Ann. Bot. 1975;39:77–92. [Google Scholar]
- Pennington R.T, Prado D.E, Pendry C.A. Neotropical seasonally dry forests and quaternary vegetation changes. J. Biogeogr. 2000;27:261–273. doi:10.1046/j.1365-2699.2000.00397.x [Google Scholar]
- Reichstein M, et al. Determinants of terrestrial ecosystem carbon balance inferred from European eddy covariance flux sites. Geophys. Res. Lett. 2007;34:L01402. doi:10.1029/2006GL027880 [Google Scholar]
- Ribas-Carbo M, et al. Effects of water stress on respiration in soybean leaves. Plant Physiol. 2005;139:466–473. doi: 10.1104/pp.105.065565. doi:10.1104/pp.105.065565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A.H, et al. Carbon balance and vegetation dynamics in an old-growth Amazonian forest. Ecol. Appl. 2004;14:S55–S71. doi:10.1890/02-6006 [Google Scholar]
- Rodenbeck C, Houweling S, Gloor M, Heimann M. CO2 flux history 1982–2001 inferred from atmospheric data using a global inversion of atmospheric transport. Atmos. Chem. Phys. 2003;3:1919–1964. [Google Scholar]
- Ryan M.G. Growth and maintenance respiration in stems of Pinus contorta and Picea engelmannii. Can. J. Forest Res. 1990;20:48–57. [Google Scholar]
- Saleska S.R, et al. Carbon in Amazon forests: unexpected seasonal fluxes and disturbance-induced losses. Science. 2003;302:1554–1557. doi: 10.1126/science.1091165. doi:10.1126/science.1091165 [DOI] [PubMed] [Google Scholar]
- Schwendenmann L, Veldkamp E, Brenes T, O'Brien J.J, Mackensen J. Spatial and temporal variation in soil CO2 efflux in an old-growth neotropical rain forest, La Selva, Costa Rica. Biogeochemistry. 2003;64:111–128. doi:10.1023/A:1024941614919 [Google Scholar]
- Shinozaki K. A quantitative analysis of plant form—the pipe model theory. Jpn J. Ecol. 1964;14:97–132. [Google Scholar]
- Sotta E.D, et al. Soil CO2 efflux in a tropical forest in the central Amazon. Glob. Change Biol. 2004;10:601–617. doi:10.1111/j.1529-8817.2003.00761.x [Google Scholar]
- Sotta E.D, Veldkamp E, Schwendenmann L, Guimaráes B.R, Paixão R.K, Ruivo M.de.L, Costa A. C. L. da, Meir P. Effects of an induced drought on soil CO2 efflux and soil CO2 production in an eastern Amazonian rainforest, Brazil. Glob. Change Biol. 2007;13:2218–2229. doi:10.1111/j.1365-2486.2007.01416.x [Google Scholar]
- Tian H.Q, Melillo J.M, Kicklighter D.W, Mcguire A.D, Helfrich J.V.K, Moore B, Vorosmarty C.J. Effect of interannual climate variability on carbon storage in Amazonian ecosystems. Nature. 1998;396:664–667. doi:10.1038/25328 [Google Scholar]
- Trumbore S.U.S.A. Carbon respired by terrestrial ecosystems—recent progress and challenges. Glob. Change Biol. 2006;12:141–153. doi:10.1111/j.1365-2486.2006.01067.x [Google Scholar]
- Uppala S.M, et al. The ERA-40 re-analysis. Q. J. R. Meteorol. Soc. 2005;131:2961–3012. doi:10.1256/qj.04.176 [Google Scholar]
- van der Werf G.R, Randerson J.T, Collatz G.J, Giglio L, Kasibhatla P.S, Arellano A.F, Jr, Olsen S.C, Kasischke E.S. Continental-scale partitioning of fire emissions during the 1997 to 2001 El Niño/La Niña period. Science. 2004;303:73–76. doi: 10.1126/science.1090753. doi:10.1126/science.1090753 [DOI] [PubMed] [Google Scholar]
- Vasconcelos S.S, et al. Moisture and substrate availability constrain soil trace gas fluxes in an eastern Amazonian regrowth forest. Glob. Biogeochem. Cycle. 2004;18:GB2009. doi:10.1029/2003GB002210 [Google Scholar]
- Vasconcelos S.S, Zarin D.J, da Rosa M.B.S, Oliveira F.de A, de Carvalho C.J.R. Leaf decomposition in a dry season irrigation experiment in eastern Amazonian forest regrowth. Biotropica. 2007;39:593–600. doi:10.1111/j.1744-7429.2007.00313.x [Google Scholar]
- Vourlitis G.L, et al. The sensitivity of diel CO2 and H2O vapor exchange of a tropical transitional forest to seasonal variation in meteorology and water availability. Earth Interact. 2005;9:1–23. doi:10.1175/EI124.1 [Google Scholar]
- Wright I.J, Reich P.B, Atkin O.K, Lusk C.H, Tjoelker M.G, Westoby M. Irradiance, temperature and rainfall influence leaf dark respiration in woody plants: evidence from comparisons across 20 sites. New Phytol. 2006;169:309–319. doi: 10.1111/j.1469-8137.2005.01590.x. doi:10.1111/j.1469-8137.2005.01590.x [DOI] [PubMed] [Google Scholar]
- Yoneda T. Dynamics of aboveground big woody organs in a foothill Dipterocarp forest, West Sumatra, Indonesia. Ecol. Res. 1990;5:111–130. doi:10.1007/BF02348467 [Google Scholar]
- Zeng N, Mariotti A, Wetzel P. Terrestrial mechanisms of interannual CO2 variability. Glob. Biogeochem. Cycles. 2005;19:GB1016. doi:101029/2004GB002273 [Google Scholar]