Abstract
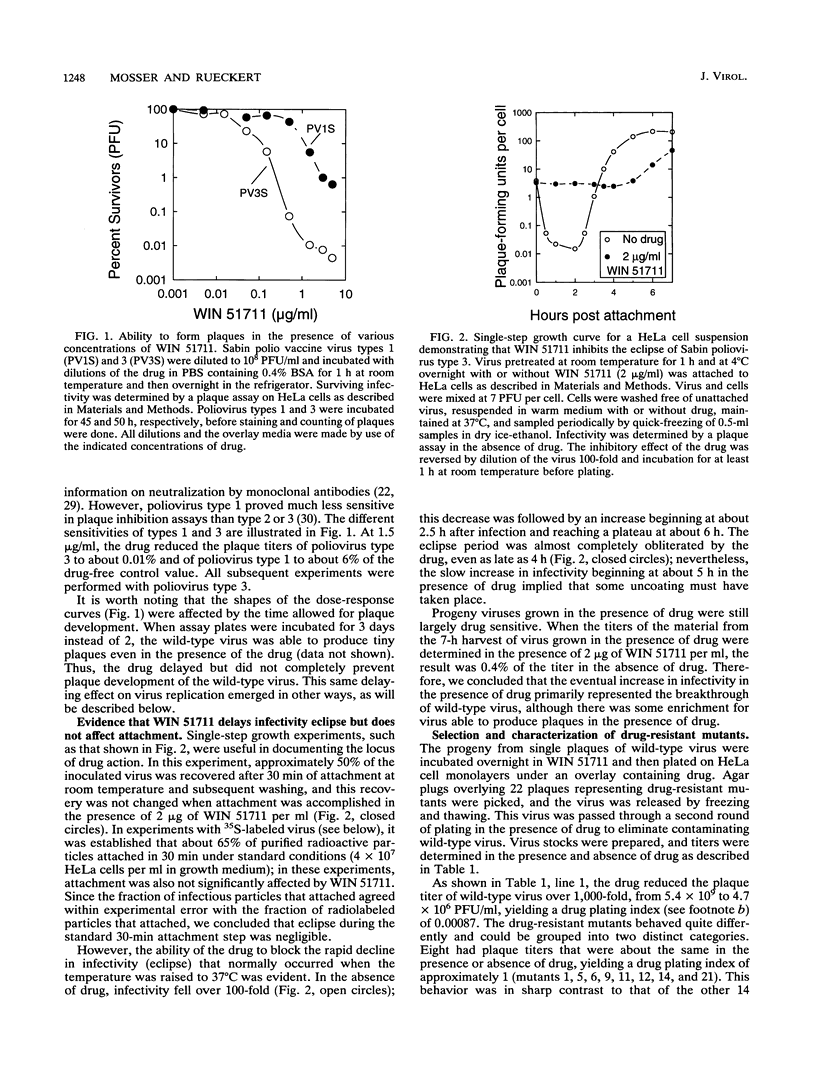
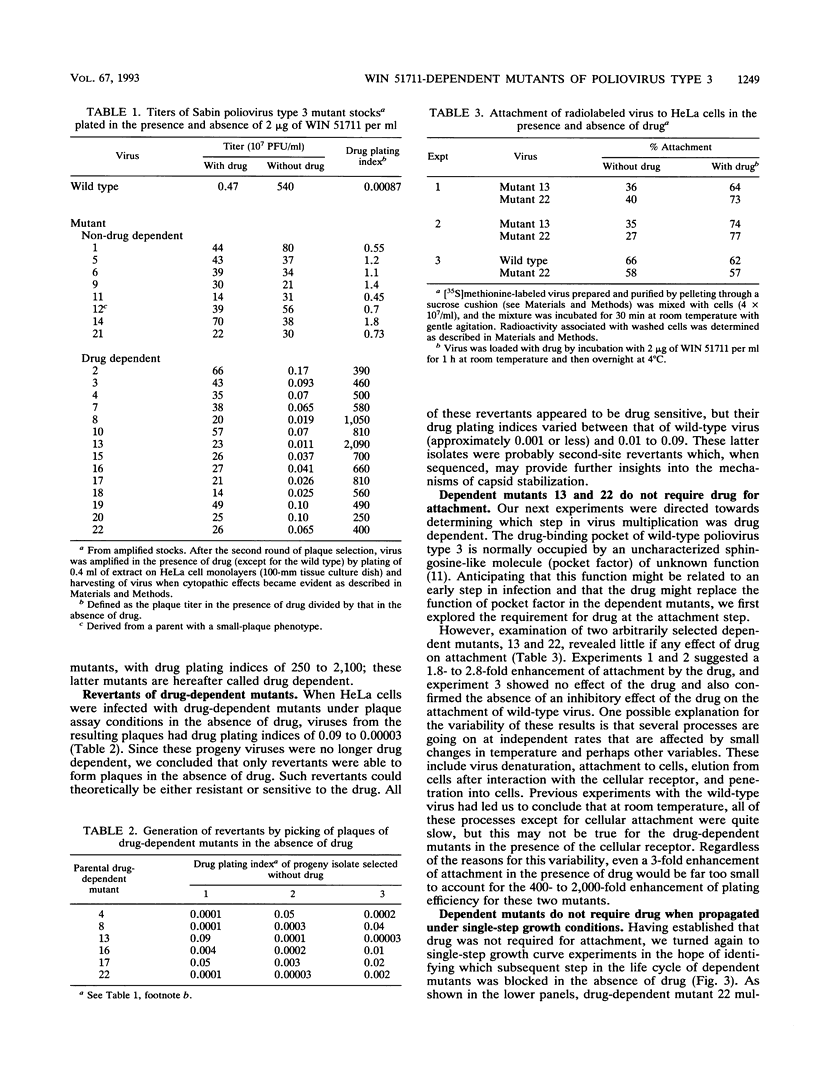
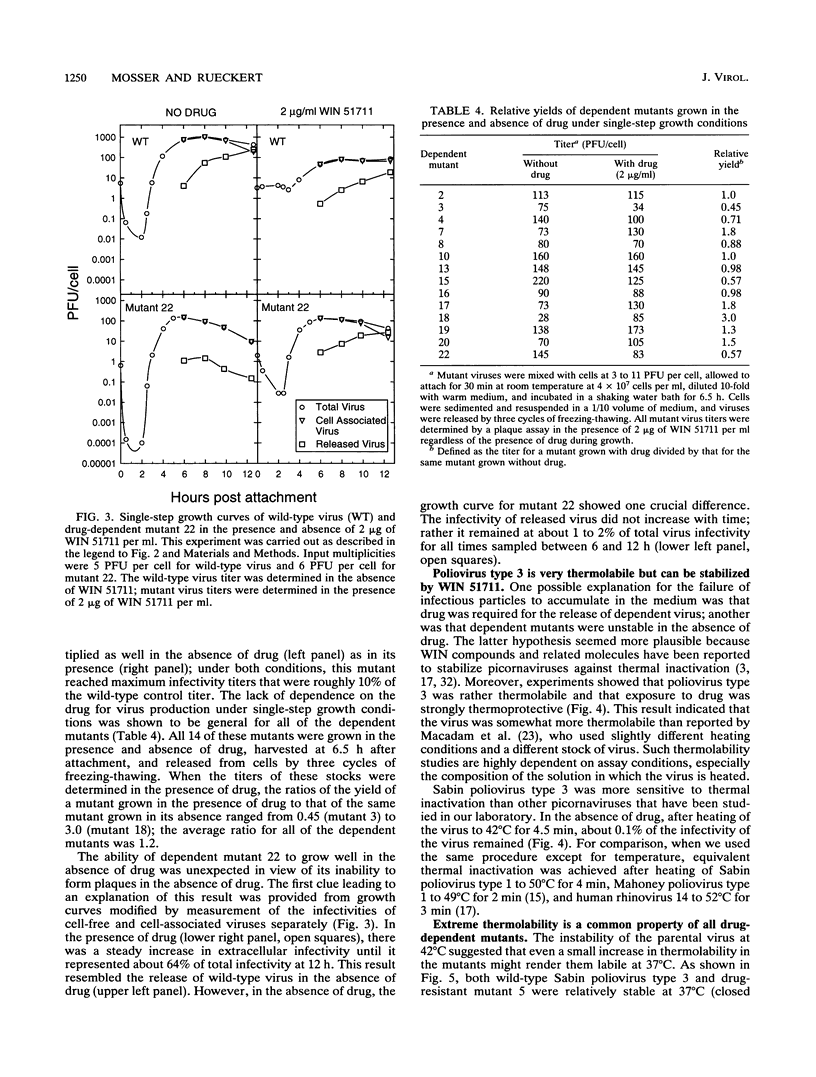
Twenty-two spontaneous mutants of the Sabin strain of poliovirus type 3 were selected for drug resistance by plating on HeLa cell monolayers in the presence of WIN 51711, an uncoating inhibitor. When replated in the presence and absence of drug, two classes of mutants were observed; mutants displayed either a drug-dependent or a non-drug-dependent phenotype, in the proportion 14:8. Non-drug-dependent mutants plaqued with equal efficiency in the presence or absence of drug. By contrast, drug-dependent mutants made no plaques in the absence of drug, except for revertants. In single-step growth curve experiments, however, drug-dependent mutants grew as well in the absence of drug as in its presence. This paradoxical behavior of dependent mutants was traced to extreme thermolability at 37 degrees C (12- to 30-s half-life) in the absence of drug. Thermolability was exhibited only after the virus was released from the cell, implying the presence of a cell-associated protective factor, possibly pocket factor. Thus, in the absence of a thermostabilizing drug, drug-dependent mutants decayed too rapidly after release to permit spread in the plaque assay. The thermodecay product was shown to consist of 135S particles lacking VP4.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger J., Minor I., Kremer M. J., Oliveira M. A., Smith T. J., Griffith J. P., Guerin D. M., Krishnaswamy S., Luo M., Rossmann M. G. Structural analysis of a series of antiviral agents complexed with human rhinovirus 14. Proc Natl Acad Sci U S A. 1988 May;85(10):3304–3308. doi: 10.1073/pnas.85.10.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M. The structure of heated poliovirus particles. J Gen Virol. 1971 Jun;11(3):147–156. doi: 10.1099/0022-1317-11-3-147. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., McSharry J. J., Lawrence G. W. Effect of arildone on modifications of poliovirus in vitro. Virology. 1980 Aug;105(1):86–93. doi: 10.1016/0042-6822(80)90158-0. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Condra J. H., Mizutani S., Callahan P. L., Davies M. E., Murcko M. A. Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5449–5453. doi: 10.1073/pnas.85.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sena J., Mandel B. Studies on the in vitro uncoating of poliovirus. II. Characteristics of the membrane-modified particle. Virology. 1977 May 15;78(2):554–566. doi: 10.1016/0042-6822(77)90130-1. [DOI] [PubMed] [Google Scholar]
- De Sena J., Torian B. Studies on the in vitro uncoating of poliovirus. III. Roles of membrane-modifying and -stabilizing factors in the generation of subviral particles. Virology. 1980 Jul 15;104(1):149–163. doi: 10.1016/0042-6822(80)90373-6. [DOI] [PubMed] [Google Scholar]
- Dorval B. L., Chow M., Klibanov A. M. Stabilization of poliovirus against heat inactivation. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1177–1183. doi: 10.1016/0006-291x(89)92234-1. [DOI] [PubMed] [Google Scholar]
- Drees O., Borna C. Uber die Spaltung physikalisch intakter Poliovirus-Teilchen in Nucleinsäure und leere Proteinhüllen durch Wärmebehandlung. Z Naturforsch B. 1965 Sep;20(9):870–878. [PubMed] [Google Scholar]
- Everaert L., Vrijsen R., Boeyé A. Eclipse products of poliovirus after cold-synchronized infection of HeLa cells. Virology. 1989 Jul;171(1):76–82. doi: 10.1016/0042-6822(89)90512-6. [DOI] [PubMed] [Google Scholar]
- Filman D. J., Syed R., Chow M., Macadam A. J., Minor P. D., Hogle J. M. Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. EMBO J. 1989 May;8(5):1567–1579. doi: 10.1002/j.1460-2075.1989.tb03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. P., Otto M. J., McKinlay M. A. Prevention of rhinovirus and poliovirus uncoating by WIN 51711, a new antiviral drug. Antimicrob Agents Chemother. 1986 Jul;30(1):110–116. doi: 10.1128/aac.30.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberger M., Pevear D., Diana G. D., Kuechler E., Blaas D. Stabilization of human rhinovirus serotype 2 against pH-induced conformational change by antiviral compounds. J Gen Virol. 1991 Feb;72(Pt 2):431–433. doi: 10.1099/0022-1317-72-2-431. [DOI] [PubMed] [Google Scholar]
- Heinz B. A., Rueckert R. R., Shepard D. A., Dutko F. J., McKinlay M. A., Fancher M., Rossmann M. G., Badger J., Smith T. J. Genetic and molecular analyses of spontaneous mutants of human rhinovirus 14 that are resistant to an antiviral compound. J Virol. 1989 Jun;63(6):2476–2485. doi: 10.1128/jvi.63.6.2476-2485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Katagiri S., Fukuda M., Fukushi K., Watanabe Y. Kinetic studies on the thermal degradation of purified poliovirus. Biken J. 1965 Sep;8(3):143–153. [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Ismail-Cassim N., Chezzi C., Newman J. F. Inhibition of the uncoating of bovine enterovirus by short chain fatty acids. J Gen Virol. 1990 Oct;71(Pt 10):2283–2289. doi: 10.1099/0022-1317-71-10-2283. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Freistadt M. S., Racaniello V. R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990 Oct;64(10):4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadam A. J., Ferguson G., Arnold C., Minor P. D. An assembly defect as a result of an attenuating mutation in the capsid proteins of the poliovirus type 3 vaccine strain. J Virol. 1991 Oct;65(10):5225–5231. doi: 10.1128/jvi.65.10.5225-5231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor S., Mayor H. D. Internal components released from rhinovirus and poliovirus by heat. J Gen Virol. 1971 Feb;10(2):203–207. doi: 10.1099/0022-1317-10-2-203. [DOI] [PubMed] [Google Scholar]
- McKinlay M. A. WIN 51711, a new systematically active broad-spectrum antipicornavirus agent. J Antimicrob Chemother. 1985 Sep;16(3):284–286. doi: 10.1093/jac/16.3.284. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Caliguiri L. A., Eggers H. J. Inhibition of uncoating of poliovirus by arildone, a new antiviral drug. Virology. 1979 Sep;97(2):307–315. doi: 10.1016/0042-6822(79)90342-8. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Otto M. J., Fox M. P., Fancher M. J., Kuhrt M. F., Diana G. D., McKinlay M. A. In vitro activity of WIN 51711, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1985 Jun;27(6):883–886. doi: 10.1128/aac.27.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Fancher M. J., Felock P. J., Rossmann M. G., Miller M. S., Diana G., Treasurywala A. M., McKinlay M. A., Dutko F. J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989 May;63(5):2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombaut B., Andries K., Boeyé A. A comparison of WIN 51711 and R 78206 as stabilizers of poliovirus virions and procapsids. J Gen Virol. 1991 Sep;72(Pt 9):2153–2157. doi: 10.1099/0022-1317-72-9-2153. [DOI] [PubMed] [Google Scholar]
- Rombaut B., Boeyé A. In vitro assembly of poliovirus 14 S subunits: disoxaril stabilization as a model for the antigenicity conferring activity of infected cell extracts. Virology. 1991 Feb;180(2):788–792. doi: 10.1016/0042-6822(91)90092-p. [DOI] [PubMed] [Google Scholar]
- Rombaut B., Brioen P., Boeyé A. Disoxaril stabilization and immunogenicity of poliovirus procapsids. J Gen Virol. 1990 May;71(Pt 5):1081–1086. doi: 10.1099/0022-1317-71-5-1081. [DOI] [PubMed] [Google Scholar]
- Rombaut B., Vrijsen R., Boeyé A. Stabilization by host cell components and Mg2+ of the neutralization epitopes of poliovirus. J Gen Virol. 1985 Feb;66(Pt 2):303–307. doi: 10.1099/0022-1317-66-2-303. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Johnson J. E. Icosahedral RNA virus structure. Annu Rev Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Kremer M. J., Luo M., Vriend G., Arnold E., Kamer G., Rossmann M. G., McKinlay M. A., Diana G. D., Otto M. J. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986 Sep 19;233(4770):1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. Stabilization of poliovirus by cations. Tex Rep Biol Med. 1961;19:683–700. [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Identification of detergents as components of wastewater sludge that modify the thermal stability of reovirus and enteroviruses. Appl Environ Microbiol. 1978 Dec;36(6):889–897. doi: 10.1128/aem.36.6.889-897.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetz K., Kucinski T. Influence of different ionic and pH environments on structural alterations of poliovirus and their possible relation to virus uncoating. J Gen Virol. 1991 Oct;72(Pt 10):2541–2544. doi: 10.1099/0022-1317-72-10-2541. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Jacobson D. H., Martin A., Wychowski C., Girard M., Filman D. J., Hogle J. M. Three-dimensional structure of a mouse-adapted type 2/type 1 poliovirus chimera. EMBO J. 1991 Sep;10(9):2331–2341. doi: 10.1002/j.1460-2075.1991.tb07772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichhardt H., Otto M. J., McKinlay M. A., Willingmann P., Habermehl K. O. Inhibition of poliovirus uncoating by disoxaril (WIN 51711). Virology. 1987 Sep;160(1):281–285. doi: 10.1016/0042-6822(87)90075-4. [DOI] [PubMed] [Google Scholar]



