Abstract
Using a mixture of observations and climate model outputs and a simple parametrization of leaf-level photosynthesis incorporating known temperature sensitivities, we find no evidence for tropical forests currently existing ‘dangerously close’ to their optimum temperature range. Our model suggests that although reductions in photosynthetic rate at leaf temperatures (TL) above 30°C may occur, these are almost entirely accountable for in terms of reductions in stomatal conductance in response to higher leaf-to-air vapour pressure deficits D. This is as opposed to direct effects of TL on photosynthetic metabolism. We also find that increases in photosynthetic rates associated with increases in ambient [CO2] over forthcoming decades should more than offset any decline in photosynthetic productivity due to higher D or TL or increased autotrophic respiration rates as a consequence of higher tissue temperatures. We also find little direct evidence that tropical forests should not be able to respond to increases in [CO2] and argue that the magnitude and pattern of increases in forest dynamics across Amazonia observed over the last few decades are consistent with a [CO2]-induced stimulation of tree growth.
Keywords: review, photosynthesis, climate change, plant growth
1. Introduction
In an effort to guide thought as to how tropical forests may respond to climate change, there have been several reviews over the last few years, including Chambers & Silver (2004), Clark (2004) and Wright (2005), which have concluded that CO2 is unlikely to have any positive effect on forest productivity. But with other projected changes in the global climate system, especially increasing temperatures, almost certainly to result in some form of tropical forest decline, it seems to us that nearly all these reviews are, at best, conceptually inconsistent. For example, Clark (2004) argues that increasing atmospheric [CO2] should result in ‘little or no enhancement of biomass production rates’, which is equivalent to stating that the growth of tropical forests is currently not carbon limited. Yet, she also cites numerous examples of how higher temperatures might reduce tropical forest productivity through declined rates of net CO2 assimilation or enhanced rates of respiration, which is, of course, equivalent to assuming the exact opposite. Similarly, when discussing CO2, Wright (2005) suggests that ‘current photosynthesis levels meet, or even exceed, the carbon requirements for maintenance and growth’ but when discussing light availability concludes that ‘solar irradiance limits net primary production by closed-canopy forests…because shade limits photosynthetic carbon uptake by most leaves’. Such inconsistencies suggest a need for a coherent and objective framework in which to assess the probable effects of rising temperatures and atmospheric [CO2] on the physiology and growth of forest trees and this is the objective of this paper.
2. Photosynthesis, respiration and plant growth
We start with the simple question: ‘is the growth of tropical trees carbon limited?’, noting the relationship between photosynthesis and growth can be simply expressed as
| (2.1) |
where NP is the rate of net primary production (new growth); GP is the average rate of photosynthesis; and φ is the proportion of assimilated carbon lost through respiration of all organs (including the leaves at night) as well as through other processes such as volatile organic carbon emission (Harley et al. 2004) or exudation of organic acids and other carbohydrates from roots to the soil solution (Jones et al. 2003). From equation (2.1), we can reasonably infer that if NP varies with a positive correlation to environmental factors known to stimulate GP then positive evidence would be obtained that the growth of tropical forest trees is currently carbon limited.
It is extremely difficult to change ambient [CO2] for forest trees in a long-term experimental setting but a second strong environmental driver influencing tropical forest GP is photon irradiance Q. Clarke & Clarke (1994) and Graham et al. (2003) showed that the NP of neotropical trees responds positively to increases in Q on a seasonal or interannual basis. Keeling (2007) used a calibrated crown illumination index to show that tropical tree stem growth rates are positively correlated with tree canopy light exposure. Shading experiments show that the growth of young seedlings and saplings within tropical forests is almost always limited by light (Turner 2001). As far as we know, no mechanism other than a stimulation of photosynthesis by increased Q has been suggested to account for this. For understorey plants in tropical forests, [CO2] also has a strong stimulatory effect on plant growth (Würth et al. 1998), as would be expected from the strong [CO2] dependence of quantum yield (Ehleringer & Björkman 1977) combined with the observation of shaded tropical forest seedlings often only just surviving very close to their growth compensation points (Turner 2001). Thus, the answer to our question seems to be ‘yes’ and a reasonable place to start any analysis of effects of temperature on forest productivity is thus through effects on photosynthesis and respiration—after which we consider direct long-term effects of [CO2].
3. Temperature and photosynthesis
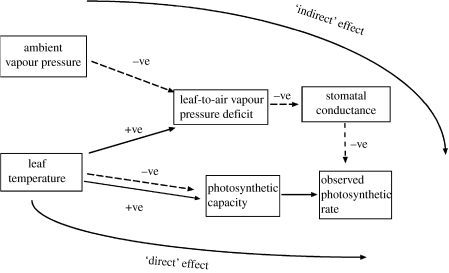
As shown in figure 1, temperature can affect photosynthesis through modulation of the rates of activity of photosynthetic enzymes and the electron transport chain (Sage & Kubien 2007) and, in a more indirect manner, through leaf temperatures defining the magnitude of the leaf-to-air vapour pressure difference D, a key factor influencing stomatal conductances. These two processes are termed here ‘direct’ and ‘indirect’.
Figure 1.
Schematic showing ‘direct’ and ‘indirect’ effects of temperature on leaf photosynthetic metabolism.
(a) Direct (mesophyll) effects
Direct temperature effects on photosynthetic metabolism involve changes in the activity of ribulose-1,5-carboxylase/oxygenase (Rubisco—the main carboxylating enzyme of photosynthesis) as well as processes associated with the regeneration of Rubisco's substrate, ribulose-1,5-bisphosphate (RuBP) through the Calvin cycle.
Temperature effects on Rubisco kinetics are complex with activation energies and Michaelis–Menten constants being affected (von Caemmerer 2000), but these temperature sensitivities are now reasonably well established (Bernacchi et al. 2001) and with an only modest sensitivity of RuBP carboxylation capacity to temperature (Sage & Kubien 2007; see figure 3). This temperature sensitivity varies little with genotype or growth conditions, although there may have been some genetic adaptation of Rubisco specificity to different levels of aridity (Galmes et al. 2005).
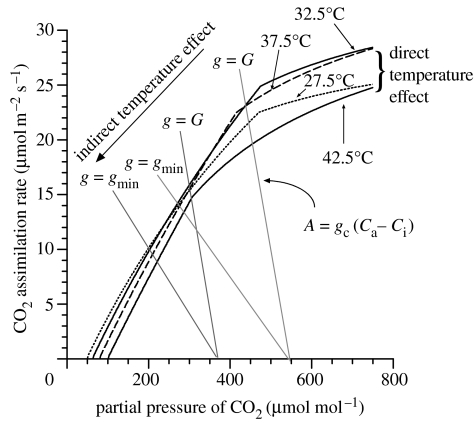
Figure 3.
Modelled response of photosynthesis and stomatal conductance g to the intercellular/chloroplastic partial pressure of CO2 for various leaf temperatures and with operating points at the modelled maximum stomatal conductance G of 0.6 mol m−2 s−1, along with that associated with the maximum leaf-to-air vapour pressure observed within the model for 2000 and 2040 with [CO2]=380 and 550 μmol mol−1, respectively (denoted gmin). The lines intersecting the x-axis represent the ‘stomatal supply’ functions (Farquhar & Sharkey 1982) for g=G and g=gmin at [CO2]=380 μmol ml−1 and [CO2]=550 μmol ml−1.
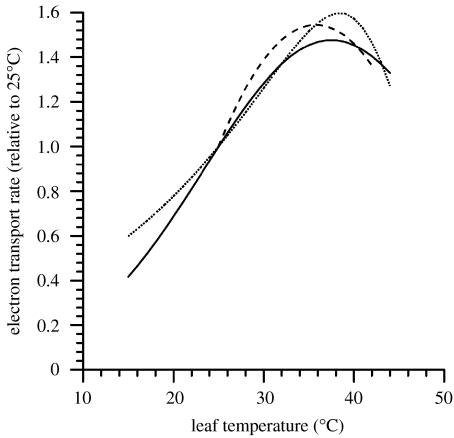
The maximum rate of RuBP regeneration, usually considered to be limited by the maximum rate of electron transport Jmax, is generally more sensitive to temperature than RuBP carboxylation capacity with its temperature sensitivity also varying substantially with growth conditions and/or genotype (June et al. 2004). Typical response curves for Jmax are shown in figure 2, with data from a modelling study of ecosystem flux data from forest near Manaus (Mercado et al. 2006), leaf-level measurements from the same tower (Tribuzy 2005) and from soya bean leaves in the laboratory (June et al. 2004). The latter also showed that inhibition of Jmax at supraoptimal TL is fully reversible. Although the mechanism by which this reversibility occurs is unknown, the decline in Jmax at high TL is associated with an increase in the cyclic flow of electrons around photosystem (PS) I possibly serving as an important mechanism for the protection of both PS II and lipid membranes under high-temperature conditions (Sharkey & Schrader 2006). The considerable variation in the temperature sensitivity of Jmax with both species and growth conditions (June et al. 2004) contrasts with the relatively constant temperature sensitivity of RuBP carboxylation/oxygenation. One general ‘rule of thumb’ then is that enzyme-mediated processes tend to be invariant in their temperature responses, but that, due to potential changes in fluidity and lipid composition (Sung et al. 2003), membrane-mediated processes may exhibit a considerable flexibility in temperature sensitivity according to growth conditions and genotype.
Figure 2.
The temperature sensitivity of electron transport as deduced from the studies of June et al. (2004) with soya bean, and Tribuzy (2005) and Mercado et al. (2006) for Amazon forest trees. Dashed line, Tribuzy; dotted line, Mercado; solid line, June.
Although at lower [CO2] a reduction in the activity of the membrane-bound Rubisco activase at high TL may also limit photosynthesis (Sage & Kubien 2007), where reductions in enzyme activity occur they are usually irreversible and associated with enzyme denaturation at TL>45°C. At such temperatures, irreversible destruction of the thylakoids may also occur, though the temperature at which this occurs depends upon the temperature at which leaves have developed (Berry & Björkman 1980).
(b) Indirect (stomatal) effects
As evaporative demand D increases, stomata tend to close to reduce the rate of water loss through transpiration. Associated with this stomatal closure is a reduction in CO2 assimilation rate A due to a reduction in the rate of supply of CO2 to the chloroplast (Farquhar & Sharkey 1982). For rainforest environments, the absolute humidity of the air tends to remain more or less constant over a day (e.g. Shuttleworth et al. 1985) and so it is diurnal fluctuations in TL that drive the variations in D. This changing D as TL varies over the day gives rise to an apparent temperature dependence of A that is actually associated with stomatal responses to variations in D (Koch et al. 1994). This is the indirect temperature response in figure 1.
4. Tropical forest photosynthetic response to climate change
To quantify the importance of the above, we have developed a simple model of leaf-level photosynthesis, described in full in the electronic supplementary material. In brief, the model consists of standard equations of photosynthesis (Farquhar et al. 1980) interfaced with a hybrid of the stomatal models of Jarvis & Davies (1998) and Buckley et al. (2003), includes a surface energy balance, and is run for 2000 and 2040 using observational and model output for the region of Manaus in central Amazonia. For 2000, it is run for a current day [CO2] of 380 μmol mol−1. For 2040, it is run at [CO2] of 380 and 550 μmol mol−1. The latter is considered a likely [CO2] to be occurring in 2040. The difference between model predictions for 2040 and 2000 with [CO2]=380 μmol mol−1 gives an indication of the direct effect of predicted climate change on A. The difference between 380 and 550 μmol mol−1 in 2040 illustrates the extent to which climate change effects on A will be modified by increases in [CO2]. For all three [CO2]/climate assumptions, the indirect temperature effect is quantified by comparing model predictions with g always set to its maximum value, G=0.6 mol m−2 s−1 (see electronic supplementary material), with predictions applying equation (E7), which allow stomata to respond to changes in D.
Using supply and demand functions (Farquhar & Sharkey 1982), figure 3 shows A as a function of intercellular/chloroplastic [CO2], C, for Q=1500 μmol m−2 s−1. The different demand functions represent the direct temperature effects on A. The indirect (stomatal) supply functions are shown for both g=G and for g at the maximum D occurring in the simulations. Over a wide range of TL, the direct temperature effect is relatively small, with indirect effects, those being associated with reductions in C as g declines in response to increasing D, being much more significant.
The modelled annual rates of CO2 fixation (GP in equation (2.1)), and maximum simulated TL and D are shown in table 1. The difference in GP between g≡G and g from equation (E5) for the 2000 climate (288 versus 207 mol C m−2 a−1) suggests an indirect temperature effect currently reducing GP by approximately 30%. Maximum TL and D are also much greater when g is allowed to vary (37.9 versus 34.2°C and 33.0 versus 20.8 mmol mol−1, respectively) due to higher sensible heat fluxes associated with stomatal closure at high D.
Table 1.
Model estimates for annual net CO2 assimilation, maximum leaf temperature and maximum leaf-to-air vapour pressure difference for a leaf growing at the top of the canopy near Manaus for 2000 and 2040 in the absence of soil water deficits. (For 2040, simulations have been done both with the assumed [CO2] for 2000 (380 μmol mol−1) and for a more likely [CO2] around that time of 550 μmol mol−1. Two model assumptions for stomatal conductance g have been invoked: first, with g≡0.6 mol m−2 s−1 (minimal stomatal limitation); and secondly, and more realistically, with g responding to variations in leaf-to-air vapour pressure deficit and linking with leaf biochemistry according to equation (E5). The most likely values are shown in italics.)
| 2000 climate | 2040 climate | |||
|---|---|---|---|---|
| model run | output parameter | [CO2]=380 μmol mol−1 | [CO2]=380 μmol mol−1 | [CO2]=550 μmol mol−1 |
| g≡0.6 mol m−2 s−1 | annual net CO2 assimilation (mol C m−2 a−1) | 287.6 | 294.8 | 379.3 |
| maximum leaf temperature (°C) | 34.2 | 35.8 | 35.8 | |
| maximum leaf-to-air vapour pressure difference (mmol mol−1) | 20.8 | 26.8 | 26.8 | |
| interactive g from equation (E5) | annual net CO2 assimilation (mol C m−2 a−1) | 207.4 | 188.7 | 271.1 |
| maximum leaf temperature (°C) | 37.9 | 39.7 | 39.7 | |
| maximum leaf-to-air vapour pressure difference (mmol mol−1) | 33.0 | 40.7 | 40.8 | |
Comparing GP at [CO2]=380 μmol mol−1 and g≡G for 2000 and 2400 suggests a direct temperature effect of climate change of less than 2% (295 versus 288 mol C m−2 a−1) with the slight increase attributable to higher Q in 2040. However, when stomatal interactions with the environment are included, GP is reduced from 207 to 187 mol C m−2 a−1. This 10% reduction is due to higher TL and D under the 2040 climate. Nevertheless, once higher [CO2] in 2040 is also taken into account, the indirect temperature effect reduction in GP is more than negated by the increased availability of CO2. Similar results have also been reported for a fully coupled simulation in a global circulation model (Bounoua et al. 1999) and changes in g in direct response to higher [CO2] for our model are considered in the electronic supplementary material.
We conclude that temperature rises of the order of 1.5°C in Amazonia over the next 35 years or so are unlikely to have a significant direct effect on GP. Lower g due to higher D may reduce GP below the value that would otherwise occur, but this effect will be more than offset by higher [CO2]. It is also important to recognize that current day indirect responses to temperature may bear little relationship to indirect effects of higher temperatures in the future. This is because increased temperatures associated with climate change will be accompanied by increases in sea temperatures, and therefore transiently increased evaporation from the oceans. Thus, on average, higher ambient humidities will occur and changes in D as global temperatures increase will be smaller than currently observed as temperatures vary on a daily or seasonal basis. Nevertheless, this effect may be offset by large-scale variations in precipitation patterns. Indeed, we have used 2040 for the simulation of future climatic effects on GP, because the Hadley Centre model used predicts significantly more rapid drying in the Amazon than most other general circulation models (GCMs), also with greater reductions in atmospheric humidity (Li et al. 2006). This leads to biome shifts after this time. Most other GCMs should therefore predict less severe increases in D than is the case here, and an even greater stimulation of GP in 2040. Nevertheless, many of these precipitation estimates might also be substantially modified once detailed land-use change effects are taken into account (Moore et al. 2007).
5. Plant respiration and temperature
Expressed as a proportion of GP, plant respiration is φ in equation (2.1). It has long been known that for tropical forests φ tends to be higher than other ecosystems, typically ranging from 0.60 to 0.85 (Lloyd & Farquhar 1996). The reasons for this are unclear as available evidence suggests that, due to long-term acclimation, plants growing in warmer ecosystems should not necessarily have higher φ than their cooler counterparts (Atkin et al. 2005). One explanation, also consistent with a high proportion of tropical forest autotrophic respiration being below ground, is that tropical trees, growing on relatively infertile soils, need to invest a high proportion of their acquired carbon in the acquisition of phosphorus through mychorrizal associations and via high rates of organic acid exudation (Lloyd et al. 2001). The suggestion of Chambers & Silver (2004) that much of this tropical tree respiration is simply ‘wasteful’ is without foundation.
Consistent with the idea that the growth of tropical trees may be carbon limited, enhanced respiration losses have been invoked as one explanation for tropical tree growth reductions associated with longer-term warming trends (Clark 2007; Feeley et al. 2007), even though this is also at odds with the high levels of carbohydrate reserves generally found in tropical trees (Würth et al. 2005) indicating that carbohydrate availability is not limiting for growth (Wright 2005). But, in any case, the extreme apparent sensitivity of growth to temperature suggested by Clark (2007) and Feeley et al. (2007) requires a tropical tree respiration Q10>5 (see electronic supplementary material). This is in clear contradiction to observation (Meir et al. 2008) and other/additional explanations may exist. For example, the indirect detrimental effects of high temperatures on A demonstrated above may be linked to the growth reductions of tropical trees observed in warmer and drier years.
In the longer term, there is no reason to believe that tropical trees should not be able to acclimate their respiration to increasing temperatures (Atkin et al. 2005) and, even if enhanced respiratory losses do occur in the future, they should be more than offset by the capability for increased GP as [CO2] increases simultaneously (table 1 and electronic supplementary material).
6. Other temperature-related factors
Although we have focused on photosynthesis and respiration, other physiological processes may also be important. For example, reproductive processes such as flowering and fruit set may be especially sensitive to high temperatures (e.g. Sato et al. 2006). Another process that may become increasingly important as tropical forests warm may be the ability of plants to emit isoprene, a process thought to help maintain membrane stability under moderately high temperatures (Sharkey & Schrader 2006).
7. Increasing [CO2] and plant growth
Although an increased [CO2] accompanying climate change should more than offset any detrimental effects of higher temperatures and increased D on tropical forest productivity, this does not necessarily mean that increasing [CO2] should also be serving to stimulate growth rates above those which would otherwise occur. Indeed, although increasing [CO2] provides a simple explanation for observed increases in recruitment and growth rates of Amazon forests over the last few decades (Lewis et al. 2004), this also being quantitatively consistent with theoretical predictions (Lloyd & Farquhar 1996), it has also been argued that a direct stimulation of plant productivity by CO2 cannot account for these growth responses observed (Chambers & Silver 2004). The latter study did, however, make conservative assumptions regarding the extent to which CO2 may stimulate tropical forest productivity (a 25% stimulation of NP for an increase in [CO2] from approx. 270 to 700 μmol mol−1), so their result is not that surprising. In any case, it is not at all clear that experiments exposing plants to large-step changes in [CO2], such as in typical CO2 enrichment experiments, provide an adequate analogue for probable growth responses when [CO2] is gradually increasing, such as is presently the case. For example, seedlings can only typically adjust their ratios of root to shoot by a factor of less than 0.2 in response to a doubling of [CO2] (Curtis & Wang 1998), but for mature tropical forests an approximately threefold variation in the ratios of root to shoot exists, probably due to variations in nutrient availability (Maycock & Congdon 2000; Powers et al. 2005). Thus, although it may be the case that nutrients, especially P, are becoming relatively more limiting for tropical forest growth as NP and [CO2] continue to increase (Chambers & Silver 2004), it would also be remarkable if tropical forest trees were not gradually increasing the proportion of biomass allocated below ground to facilitate relatively greater rates of nutrient acquisition. Moreover, numerous mechanisms exist that allow extra phosphorus to be taken up from the soil solution to support increased growth in response to higher [CO2] (Lloyd et al. 2001). Plants, it seems, have ready access to what are often considered ‘unavailable’ phosphorus pools (Parfitt 1979; Johnson & Loeppert 2006), much of which should be available to support slowly increasing [CO2]-mediated increases in growth (cf. Chambers & Silver 2004).
It has also been argued that because tropical tree carbohydrate concentrations, [CH2O], are ‘generally high’, [CH2O] must already be in excess with increasing [CO2] unable to further stimulate plant growth (Körner 2003; Wright 2005). That plants growing under elevated [CO2] often have higher [CH2O] has also been taken as additional evidence for this idea (Körner 2003). Nevertheless, recent advances in our understanding of the signalling of growth responses in plants, in particular interactions between sugar and plant hormone signalling with nutrients and other growth limitations (Rolland et al. 2006), show that higher [CH2O] is, in fact, usually associated with a stimulation of sink activity (i.e. faster rates of growth). Thus, increases in plant tissue [CH2O] with higher [CO2] do not mean that plant growth is not ‘carbon’ limited (Masle et al. 1990) and sugar signalling provides a simple explanation for why increased [CO2] usually causing increases in both [CH2O] and NP. For a sugar sensing mechanism to work, it must be the case that, on average, any change in [CH2O] gives rise to a less than proportional change in NP. Conceptually this bears a strong resemblance to the general economic theory of Keynes (1936)—in particular the notion that in order for an economic system in which savings occur to be able to operate, it is necessary for the long-term ratio of expenditure to income to always be less than unity. In that respect, the need for plants to maintain considerable CH2O reserves as insurance against drought, defoliation by pests or unexpected shading by competitors should not be discounted.
It is seedlings, saplings and trees growing under shaded conditions that tend to be the most carbon limited (Wright 2005) and plants adapted to and/or growing in shade are the most responsive to elevated [CO2] (Curtis & Wang 1998; Kerstiens 2001). A strong sensitivity of tropical forest seedling growth to elevated [CO2] has also been demonstrated (Würth et al. 1998), consistent with carbohydrate storage enhancing shade and stress tolerances for tropical forest seedlings (Myers & Kitajima 2007).
It thus seems likely to us that the currently observed accelerating dynamics of Amazon forests can reasonably be attributed to increases in [CO2], mediated at the seedling stage, although other factors such as changing light levels may, of course, also be involved (Wright 2005). Observations of increased growth being followed by increased mortality rates (Lewis et al. 2004) are also both conceptually and quantitatively consistent with ecosystem-level stimulations of GP and NP associated with slowly increasing [CO2] (Lloyd & Farquhar 1996). Although this provides a plausible mechanism for the observed accelerating dynamics of tropical forests, there must be a limit to the maximum size that any forest can attain. Our inability to understand the basis of variations in aboveground carbon stocks for all but the driest Amazon forests (Saatchi et al. 2007) currently limits our understanding of how long any sequestration is likely to continue.
Footnotes
One contribution of 27 to a Theme Issue ‘Climate change and the fate of the Amazon’.
Supplementary Material
Description of photosynthesis model with a note on stomatal responses to [CO2] and the response of tropical forest respiration rates to increasing temperatures.
References
- Atkin O.K, Bruhn D, Hurry V.M, Tjoelker M.G. The hot and the cold: unravelling the variable response of plant respiration to temperature. Funct. Plant Biol. 2005;32:87–105. doi: 10.1071/FP03176. doi:10.1071/FP03176 [DOI] [PubMed] [Google Scholar]
- Bernacchi C.J, Singsaas E.L, Pimental C, Portis A.R, Jr, Long S.P. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 2001;24:253–259. doi:10.1111/j.1365-3040.2001.00668.x [Google Scholar]
- Berry J.A, Björkman O. Photosynthetic response and adoption to temperature in higher plants. Annu. Rev. Plant Physiol. 1980;31:491–543. doi:10.1146/annurev.pp.31.060180.002423 [Google Scholar]
- Bounoua L, et al. Interactions between vegetation and climate: radiative and physiological effects of doubled CO2. J. Clim. 1999;12:309–324. doi:10.1175/1520-0442(1999)012<0309:IBVACR>2.0.CO;2 [Google Scholar]
- Buckley T.N, Mott K.A, Farquhar G.D. A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ. 2003;26:1767–1785. doi:10.1046/j.1365-3040.2003.01094.x [Google Scholar]
- Chambers J.Q, Silver W.L. Some aspects of ecophysiological and biogeochemical responses of tropical forests to atmospheric change. Phil. Trans. R. Soc. B. 2004;359:463–476. doi: 10.1098/rstb.2003.1424. doi:10.1098/rstb.2003.1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A. Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Phil. Trans. R. Soc. B. 2004;359:477–491. doi: 10.1098/rstb.2003.1426. doi:10.1098/rstb.2003.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A. Detecting tropical responses to global climatic and atmospheric change: current challenges and a way forward. Biotropica. 2007;39:4–19. doi:10.1111/j.1744-7429.2006.00227.x [Google Scholar]
- Clarke D.A, Clarke D.B. Climate-induced annual variation in canopy tree growth in a Costa Rican tropical rain forest. J. Ecol. 1994;82:865–872. doi:10.2307/2261450 [Google Scholar]
- Curtis P.S, Wang X. A meta-analysis of elevated CO2 effects on woody plant mass, form and physiology. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. doi:10.1007/s004420050381 [DOI] [PubMed] [Google Scholar]
- Ehleringer J, Björkman O. Quantum yields for CO2 uptake in C3 and C4 plants. Dependence on temperature, CO2 and O2 concentration. Plant Physiol. 1977;59:86–90. doi: 10.1104/pp.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar G.D, Sharkey T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982;33:317–345. doi:10.1146/annurev.pp.33.060182.001533 [Google Scholar]
- Farquhar G.D, von Caemmerer S, Berry J.A. A biochemical model of the photosynthetic assimilation in C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. doi:10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- Feeley K.J, Wright S.J, Supardi N.N.N, Kassim A.R, Davies S.J. Decelerating growth in tropical forest trees. Ecol. Lett. 2007;10:461–469. doi: 10.1111/j.1461-0248.2007.01033.x. doi:10.1111/j.1461-0248.2007.01033.x [DOI] [PubMed] [Google Scholar]
- Galmes J, Flexas J, Keys A.J, Cifre J, Mitchell R.A.C, Madgwick P.J, Haslam R.P, Medrano H, Parry M.A.J. Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ. 2005;28:571–579. doi:10.1111/j.1365-3040.2005.01300.x [Google Scholar]
- Graham E, Mulkey S, Kitajima K, Phillips N, Wright S.J. Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc. Natl Acad. Sci. USA. 2003;100:572–576. doi: 10.1073/pnas.0133045100. doi:10.1073/pnas.0133045100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley P, et al. Variation in potential for isoprene emissions amongst neotropical forest sites. Glob. Change Biol. 2004;10:630–650. doi:10.1111/j.1529-8817.2003.00760.x [Google Scholar]
- Jarvis A.J, Davies W.J. The coupled response of stomatal conductance to photosynthesis and transpiration. J. Exp. Bot. 1998;49:399–406. doi:10.1093/jexbot/49.suppl_1.399 [Google Scholar]
- Johnson S.E, Loeppert R.H. Role of organic acids in phosphate mobilization from iron oxide. Soil Sci. Soc. Am. J. 2006;70:222–234. doi:10.2136/sssaj2005.0012 [Google Scholar]
- Jones D.L, Dennis P.G, Owen A.G, van Hees P.A.W. Organic acid behaviour in soils—misconceptions and knowledge gaps. Plant Soil. 2003;248:31–41. doi:10.1023/A:1022304332313 [Google Scholar]
- June T, Evans J.R, Farquhar G.D. A simple new equation for the reversible temperature dependence of photosynthetic electron transport: a study on soybean leaf. Funct. Plant Biol. 2004;31:275–283. doi: 10.1071/FP03250. doi:10.1071/FP03250 [DOI] [PubMed] [Google Scholar]
- Keeling, H. 2007 Forest structure and function: identifying the factors which control forest productivity and biomass. PhD thesis, University of Leeds, UK.
- Kerstiens G. Meta-analysis of the interaction between shade tolerance, light environment and growth response of woody species to elevated CO2. Acta Oecol. 2001;22:61–69. doi:10.1016/S1146-609X(00)01096-1 [Google Scholar]
- Keynes J.M. The general theory of employment, interest and money. Macmillan; London, UK: 1936. [Google Scholar]
- Koch G.W, Amthor J.S, Goulden M.L. Diurnal patterns of leaf photosynthesis, conductance and water potential at the top of a lowland rain forest canopy in Cameroon: measurements from the Radeau des Cimes. Tree Physiol. 1994;14:347–360. doi: 10.1093/treephys/14.4.347. [DOI] [PubMed] [Google Scholar]
- Körner Ch. Carbon limitation in trees. J. Ecol. 2003;91:4–17. doi:10.1046/j.1365-2745.2003.00742.x [Google Scholar]
- Lewis S.L, et al. Concerted changes in tropical forest structure and dynamics: evidence from 50 long-term plots. Phil. Trans. R. Soc. B. 2004;359:421–436. doi: 10.1098/rstb.2003.1431. doi:10.1098/rstb.2003.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Fu R, Dickson R.E. Rainfall and its seasonality in the 21st century as assessed by the coupled models for the IPCC AR4. J. Geophys. Res. 2006;111:D02111. doi:10.1029/2005JD006355 [Google Scholar]
- Lloyd J, Farquhar G.D. The CO2 dependence of photosynthesis, plant growth responses to elevated atmospheric CO2 concentrations and their interaction with plant nutrient status. Funct. Ecol. 1996;10:4–32. doi:10.2307/2390258 [Google Scholar]
- Lloyd J, Bird M.I, Veenendaal E, Kruijt B. Should phosphorus availability be constraining moist tropical forest responses to increasing CO2 concentrations? In: Schulze E.-D, Harrison S.P, Heimann M, Holland E.A, Lloyd J, Prentice I.C, Schimel D, editors. Global biogeochemical cycles in the climate system. Academic Press; San Diego, CA: 2001. pp. 96–114. [Google Scholar]
- Masle J, Farquhar G.D, Gifford R.M. Growth and carbon economy of wheat seedlings as affected by soil resistance to penetration and ambient partial pressure of CO2. Aust. J. Plant Physiol. 1990;17:465–487. [Google Scholar]
- Maycock C.R, Congdon R.A. Fine root biomass and soil N and P in North Queensland rain forests. Biotropica. 2000;32:185–190. [Google Scholar]
- Meir P, Metcalfe D.B, Costa A.C.L, Fisher R.A. The fate of assimilated carbon during drought: impacts on respiration in Amazon rainforests. Phil. Trans. R. Soc. B. 2008;363:1849–1855. doi: 10.1098/rstb.2007.0021. doi:10.1098/rstb.2007.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado L, Lloyd J, Carswell F, Mahli Y, Meir P, Nobre A.D. Modelling Amazonian forest eddy covariance data: a comparison of big leaf versus sun/shade models for the C-14 tower at Manaus I. Canopy photosynthesis. Acta Amazon. 2006;36:69–82. [Google Scholar]
- Moore N, Arima E, Walker R, da Silva R.R. Uncertainty and the changing hydroclimatology of the Amazon. Geophys. Res. Lett. 2007;34:L14707. doi:10.1029/2007GL030157 [Google Scholar]
- Myers J.A, Kitajima K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007;95:383–395. doi:10.1111/j.1365-2745.2006.01207.x [Google Scholar]
- Parfitt R.L. The availability of P from phosphate-goethite bridging complexes. Desorbtion and uptake by ryegrass. Plant Soil. 1979;53:55–65. doi:10.1007/BF02181879 [Google Scholar]
- Powers J.S, Tresder K.K, Lerdau M.T. Fine roots, arbuscular mycorrhizal hyphae and soil nutrients in four neotropical rain forests: patterns across large geographic distances. New Phytol. 2005;165:913–921. doi: 10.1111/j.1469-8137.2004.01279.x. doi:10.1111/j.1469-8137.2004.01279.x [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signalling in plants: conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. doi:10.1146/annurev.arplant.57.032905.105441 [DOI] [PubMed] [Google Scholar]
- Saatchi S.S, Houghton R.A, Dos Santos Alvala R.C, Soare J.V, Yu Y. Distribution of aboveground live biomass in the Amazon basin. Glob. Change Biol. 2007;13:816–837. [Google Scholar]
- Sage R.F, Kubien D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007;30:1086–1106. doi: 10.1111/j.1365-3040.2007.01682.x. doi:10.1111/j.1365-3040.2007.01682.x [DOI] [PubMed] [Google Scholar]
- Sato S, Kamiyama M, Iwata T, Makita N, Furukawa H, Ikeda H. Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot. 2006;97:731–738. doi: 10.1093/aob/mcl037. doi:10.1093/aob/mcl037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T.D, Schrader S.M. High temperature sense. In: Rao K.V.M, Raghavendra A.S, Reddy K.J, editors. Physiology and molecular biology of stress tolerance in plants. Springer; Dordrecht, The Netherlands: 2006. pp. 101–129. [Google Scholar]
- Shuttleworth W.J, et al. Daily variations in temperature and humidity within and above Amazonian forest. Weather. 1985;40:102–108. [Google Scholar]
- Sung D.-Y, Kaplan F, Lee K.-J, Guy C.L. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003;8:179–187. doi: 10.1016/S1360-1385(03)00047-5. doi:10.1016/S1360-1385(03)00047-5 [DOI] [PubMed] [Google Scholar]
- Tribuzy, E. S. 2005 Variacóes da temperaratura foliar do dossel e o suu effeito na taxa assimilatória de CO2 na Amazônia Central. PhD thesis, Universidade de São Paulo, Brazil.
- Turner D. The ecology of trees in the tropical rain forest. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- von Caemmerer S. Biochemical models of leaf photosynthesis. CSIRO; Collingwood, Australia: 2001. [Google Scholar]
- Wright S.J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005;20:553–560. doi: 10.1016/j.tree.2005.07.009. doi:10.1016/j.tree.2005.07.009 [DOI] [PubMed] [Google Scholar]
- Würth M.K.R, Winter K, Körner Ch. In situ responses to elevated CO2 in tropical forest understorey plants. Funct. Ecol. 1998;12:886–895. doi:10.1046/j.1365-2435.1998.00278.x [Google Scholar]
- Würth M.K.R, Peláez-Riedl S, Wright S.J, Körner Chr. Nonstructural carbohydrate pools in a tropical forest. Oecologia. 2005;143:11–24. doi: 10.1007/s00442-004-1773-2. doi:10.1007/s00442-004-1773-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of photosynthesis model with a note on stomatal responses to [CO2] and the response of tropical forest respiration rates to increasing temperatures.