Abstract
The number of older adults with HIV-1 disease is increasing but little is known about how age influences behavioral deficits associated with HIV-1 infection. The purpose of this study was to determine in a murine model if aging influenced sickness behavior following central injection of HIV-1 gp120. In initial studies, behavioral deficits induced by acute and repeated intracerebroventricular (ICV) injection of gp120 were greater in aged mice than in adults. Furthermore, repeated ICV injection of gp120 increased hippocampal levels of IL-1β and IL-6 mRNA in aged mice but not in adults. To determine if IL-6, which is elevated in aged brain, affects expression of the gp120- binding target, CCR5, microglia (BV-2 cell line) were incubated with increasing concentrations of IL-6. Cell surface expression of CCR5 was increased by IL-6 in a dose-dependent manner. Additionally, IL-6 increased gp120-dependent chemotaxis. These results suggest that aging increases the sensitivity of mice to behavioral deficits caused by ICV gp120, perhaps by increasing expression of CCR5 and augmenting production of cytokines.
Keywords: aging, behavior, CCR5, gp120, HAD, HIV-1, inflammatory cytokines, mice
1. Introduction
Human immunodeficiency virus type 1 (HIV-1) associated dementia (HAD) is characterized by impaired cognitive function, psychomotor slowing, and altered behavior [13, 16]. It affects 20-30% of HIV-infected persons and is a significant independent risk factor for death due to AIDS [5]. Most of the current knowledge about the cognitive motor effects of HIV infection comes from studies of young adults 20 to 40 years of age. However, 11% of patients with AIDS in the United States are 50 years of age or older (Center for Disease Control). The number of older adults with HIV disease is expected to grow due to the improved longevity of HIV-infected patients prescribed highly active antiretroviral therapy and new infections among older adults. The occurrence and severity of cognitive-motor impairment are likely to be different in older adults infected with HIV-1 than in younger infected persons due to the presence of other dementing conditions that are associated with aging. Indeed, age has been identified as an independent risk factor in the development of HAD [11]. In recent years the incidence of HAD as an AIDS-defining illness has actually increased [4], probably because the HIV protease inhibitors poorly penetrate the CNS, leading to a protected brain reservoir of HIV-1 in older adults.
In the brain, the HIV-1 envelope protein, gp120, in addition to CD4, binds chemokine receptors, CXCR4 and CCR5, that are present on glial cells and neurons [6, 19]. While many cells express both T-tropic (CXCR4) and M-tropic (CCR5) chemokine receptors, most HIV-1 infected cells in the brain are macrophages and microglia [24] suggesting the M-tropic CCR5 is more important in the cognitive and behavioral deficits involved in HAD. HIV-1 infected microglia and uninfected microglia that are stimulated by shed gp120 release reactive oxygen species (ROS) and inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α. These inflammatory molecules are behaviorally active and play an important role in the development of HAD [12]. Therefore, conditions that facilitate microglial cell activation and production of ROS and inflammatory cytokines, independent of HIV-1 infection, are likely to promote and intensify HAD. Interestingly, recent studies indicate that activated microglia, oxidative stress and several inflammatory cytokines are increased in the brains of old but otherwise healthy rodents [9, 14, 17]. The emergence of signs of inflammation in the brain seems to coincide with indications of cognitive aging [3, 17, 25].
A working hypothesis is that the age-associated decline in cognition is due to increased levels of inflammatory cytokines in the brain [10, 27]. Furthermore, aging is proposed to prime microglia, so that they over react to a secondary stimulus from the innate immune system [9, 15]. Accordingly, activation of the innate immune system produced an exaggerated neuroinflammatory response in aged mice that led to a longer and more intense sickness behavior syndrome compared to young adults [9]. Because both aging and HIV-1 disease increase activated microglia, ROS, and inflammatory cytokines, the summative effects of aging and HIV-1 infection on brain inflammation might contribute to HAD in older adults. However, it is not yet known if age exacerbates behavioral deficits and brain inflammation in HIV-1 disease. Therefore, as an initial step to address this question, inflammatory cytokines (IL-1β and IL-6) in the hippocampus—a brain region that is sensitive to the insults of aging—and sickness behavior were assessed in young adult and aged mice after central administration of HIV-1 gp120. Furthermore, because IL-6 is elevated in aged brain [28, 29], its effects on microglial cell expression of CCR5 were determined. The results suggest that aging increases the sensitivity of mice to the behavioral deficits caused by central HIV-1 gp120, perhaps by increasing expression of CCR5 and augmenting production of inflammatory cytokines.
2. Materials and Methods
2.1. Animals
Adult (3-6 m) and aged (22-24 m) male BALB/c mice from our in-house specific-pathogen-free colony were used. Mice were housed in polypropylene cages and maintained at 23° C under a reverse phase 12 h light-dark cycle with ad libitum access to water and rodent chow. Male juvenile conspecifics (4-5 week old) used in the social exploratory behavior paradigm were maintained under identical conditions. At the end of each study, mice were examined postmortem for gross signs of disease (e.g. splenomeglia and tumors). Data from mice determined to be unhealthy were excluded from analysis. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
2.2. Intracerebroventricular cannulation
The intracerebroventricular (ICV) cannulation was performed as previously described [8]. In brief, mice were deeply anesthetized using ketamine and xylazine (1 mg and 0.1 mg/10 g BW i.p., respectively) and the surgical cutaneous site was shaved and sterilized. Mice were positioned in a sterotaxic instrument so that the plane formed by the frontal and parietal bones was parallel to the table top. An incision, 1 cm in length, was made on the cranium to reveal the bregma and a 26-gauge stainless-steel guide cannula was placed in the lateral cerebral ventricle using the following stereotaxic coordinates: Lateral 1.6 mm, Antero-posterior 1 mm to the bregma, and Vertical -2 mm from the dura mater. Two anchoring cranial screws were inserted adjacent to the cannula and the cannula was secured with cranioplastic cement. A dummy cannula was inserted in the guide cannula to prevent occlusion and infection. Following surgery, mice were injected subcutaneous with torbugesic (1 mg/10 g BW) every 4-8 h for 24 h. Mice were provided a minimum of 7 days to recover before any treatment was administered. Accurate placement of the cannulas was confirmed at the end of the experiment by injecting trypan blue dye and gross physical examination of dye diffusion throughout the ventricles. Data collected from mice found to have a displaced cannula (<5%) were excluded from analysis.
2.3. Behavioral tests
To estimate locomotor activity, mice were kept in their home cage and video recorded during 3 min tests using a camera mounted approximately 65.0 cm directly above the center of the cage floor. On the video records, cages were divided into 6 identical rectangles and a trained observer who was blind to experimental treatments determined the frequency of line crossing. A subject was considered to have crossed a line only if its fore and hind limbs entered a new rectangle.
To assess motivation to engage in social exploratory behavior, a novel juvenile conspecific was introduced into the test subject’s home cage for a 10-min period. Behavior was videotaped, and the duration engaged in social investigation was determined from the video records by a trained observer who was blind to experimental treatments. Social behavior was determined as the amount of time that the experimental subject spent investigating (e.g., anogenital sniffing, trailing) the juvenile and the results are expressed as percent depression in time engaged in social behavior compared with respective baseline controls. The social behavior test was conducted immediately after the locomotor behavior test when appropriate.
2.4. Cytokine mRNA measurement by quantitative real-time PCR
Total RNA was isolated from brain using the Tri Reagent protocol (Sigma, St. Louis, MO). RNA samples were subjected to a DNAse I digestion procedure and then reverse transcribed to cDNA using a RT Retroscript kit (Ambion, Austin, TX). Quantitative real time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol as previously described [9]. In brief, cDNA was amplified by PCR where a target cDNA (IL-6, Mm00446190_m1; IL-1β, Mm00434228_m1; CCR5, Mm01216171_m1) and a reference cDNA (glucose-3 phosphate dehydrogenase, Mm99999915_g1) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). PCR reactions were performed at the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as fold difference.
2.5. Animal experimentation
HIV-1 SF162 (M-tropic) gp120 (Cat. No. 7363; NIH Aids Research & Reference Reagent Program) was dissolved in sterile saline immediately prior to an experiment. Mice were infused ICV with 2 μl of vehicle or gp120 (100 ng) over a 30 sec period using a 28- gauge injection cannula, Hamilton syringe and syringe pump (World Precision Instruments). In a previous study, we found that this dose of gp120 was optimal for generating sickness behavior (data not shown). In the first study, locomotor activity and social exploratory behavior of adult and aged mice were evaluated immediately before ICV infusion of vehicle or gp120 and again 2, 4, 8, and 24 h later (n=8-9). In a subsequent study, adult and aged mice were injected daily with vehicle or gp120 (100 ng) for five consecutive days (n=8-10). Locomotor activity was evaluated before each injection. To determine whether the behavioral effects of gp120 were dependent on its complex three-dimensional structure and not endotoxin contamination, the effects of heat-inactivated gp120 (65° C for 1 h) on behavior were examined. Mice were injected with either heat-inactivated gp120 (100 ng/mice dissolved in 2 μl of saline) or saline (n=6) and behavior was assessed as described above. Twenty-four hours after the last injection, mice were killed by CO2 asphyxiation and hippocampal tissue was collected to determine steady-state levels of inflammatory cytokine mRNA.
2.6. CCR5 expression and chemotaxis in microglia
BV-2 Cell Culture
The murine microglia cell line, BV-2 was maintained in 150 cm2 tissue culture flasks (BD Falcon, NJ) in Dulbecco’s Modified Eagle’s Medium (DMEM; Cambrex, MD) supplemented with 10% fetal bovine serum (Hyclone Logan, UT) and 100 U/ml penicillin/streptomycin (Invitrogen, CA) at 37° C in a humidified incubator under 5% CO2. Confluent cultures were passed by trypsinization. Cells were centrifuged (5 min at 4°C, 250 g) and culture medium was removed. In all experiments, cells were resuspended in DMEM supplemented with 10% FBS and seeded in 6-well plates (BD Falcon, NJ) before being subjected to treatments.
CCR5 Cell Surface Expression
To examine CCR5 expression, BV-2 cells (1×106 cells/well) were incubated for 16 h in 5% CO2 at 37° C. DMEM was carefully removed and cells were replenished with fresh DMEM containing 0, 10, 25, or 50 ng/ml recombinant murine IL-6 (Biosource, Camarillo, CA) for 24 h. The cells were harvested by trypsinization and washed twice with cold Hank’s Balanced Salt Solutions (HBSS, Cambrex, MD). Cells were incubated with 10 μl of a fluorescein-conjugated rat anti-mouse CCR5 monoclonal antibody (BD Pharmingen, Camarillo, CA) for 1 h at 4° C, then washed and resuspended in 0.7 ml HBSS for analysis. FITC-conjugated rat IgG2b (BD Pharmingen, Camarillo, CA) was used as an isotype-matched control. Samples were analyzed by flow cytometry using Cytomation MoFlo™ MLS.
Chemotaxis
The chemotactic response of BV-2 cells to RANTES, the natural ligand of CCR5, as well as to gp120 was evaluated using a 48-well modified Boyden chamber (Neuro Probe, Inc., Gaithersburg, MD). BV-2 cells were treated with recombinant murine IL-6 (0, 10, 25, and 50 ng/ml; Biosource, Camarillo, CA) for 24 h.Cells were harvested with trypsin (5 ml), centrifuged and resuspended in DMEM (2×106 cells/ml) containing 10% FBS. Varying doses of gp120 and RANTES in a total volume of 30 μl were loaded into the wells of the lower chamber, and then covered with a gelatinized polycarbonate filter (5 μm pore size, Nucleopore, MA) and incubated for 5 min at 37° C in a water-saturated atmosphere of 5% CO2. Cells were vortexed and then 50 μl of cells were pipetted into the upper chamber. The chamber was incubated for 2 h at 37° C in a water-saturated atmosphere of 5% CO2. The membrane was removed and stained using a Diff-Quik Kit (Cat. No. B4132-1A; Dade-Behring, Deerfield, IL) and then washed with deiodinized water. Labeled cells were counted microscopically at 40x magnification within five randomly selected microscope fields. Data were normalized by calculating the migration index, which was defined as the ratio of cells migrating in the presence and absence of RANTES and gp120.
2.7. Statistical analysis
Data analysis was completed using the Statistical Analysis System (SAS Inst., Cary, NC). All data were subjected to the Shapiro-Wilk test to ensure normality and to ANOVA using the General Linear Models procedure of SAS. Post hoc Student’s t test using Fisher adjustment of least square means was employed to determine if treatment means were significantly different from one another (p<0.05). All data are presented as means ± standard error of mean.
3. Results
3.1. Aging prolongs gp120-induced behavioral deficits and neuroinflammation
To determine if sickness behavior induced by gp120 is affected by aging, locomotor activity and social behavior of adult and aged mice were assessed before and after a single ICV injection of gp120. Analysis of social behavior showed a significant age x gp120 interaction (p<0.01; Figure 1A). ICV injection of gp120 depressed social behavior in aged but not in adult mice. In aged mice, duration of social exploration returned to that of the aged mice injected with saline by 24 h. Analysis of locomotor activity also revealed a significant age x gp120 interaction (p<0.01; Figure 1B). Similar to what was observed for social behavior, locomotor activity was depressed only in aged mice beginning 4 h after ICV injection of gp120. Locomotor activity remained depressed at 24 h, suggesting this test was a more sensitive measure of sickness behavior than social behavior.
Figure 1. Aging prolonged gp120-induced deficits in social behavior and locomotor activity.
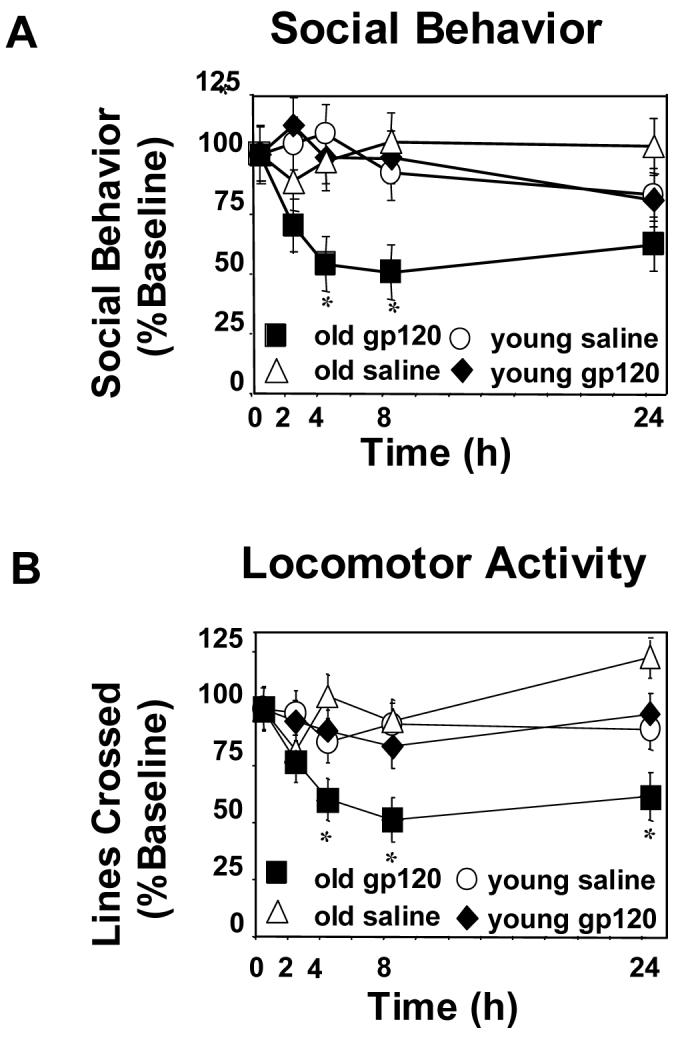
Adult and aged mice were injected i.c.v. with either saline or gp120 (100ng) and (A) social behavior and (B) locomotor activity were measured at 0, 2, 4, 8, and 24 hours after injection. Graphs are means ± SEM (n=8-9). Means with * are significantly different (p<0.05).
To determine if depression in behavior of aged animals is also evident when gp120 is administered chronically, adult and aged mice received an ICV injection of saline or gp120 (100 ng) for 5 consecutive days. Because locomotor activity appeared more sensitive to depression caused by gp120, it was assessed each day just prior to the ICV injection. Figure 2A and 2B show that both young and old mice had reduced locomotor activity after ICV injection of HIV-1 gp120 (both p<0.001). Beginning on day 3, locomotor activity decreased in both adult and aged mice. However, by day 5, adult mice showed a 52% decrease in locomotor activity whereas aged mice showed a 74% decrease (both p<0.001). Importantly, the specificity of gp120 was confirmed, as ICV injection of heat-inactivated gp120 did not induce behavioral deficits in either adult or aged mice (data not shown). Notably, on day 3 when activity decreased in both adult and aged mice, there was no difference between heat-inactivated gp120 mice compared to adult or aged controls (p=.365 and p=.260, respectively). Twenty-four h after the last injection, hippocampal tissue was collected and IL-1β and IL-6 mRNA were quantified by real-time PCR. There was a significant age x gp120 (p<0.001) interaction for both IL-1β and IL-6. In response to gp120, aged mice had increased steady-state cytokine mRNA levels compared to saline-treated animals (p<0.05; Figure 3B). However, there was no significant difference in saline-treated and gp120-treated adult mice. Notably, after ICV injection of gp120, the magnitude of IL-1β and IL-6 mRNA response in the aged brain was almost five times greater than that of adult saline- or gp120-treated animals. Collectively, these data suggest that aging increases the sensitivity to behavioral deficits caused by central gp120 and this heightened sensitivity of aged mice may be due to an increased brain cytokine response.
Figure 2. Aging further depressed deficits in locomotor activity caused by chronic administration of gp120.
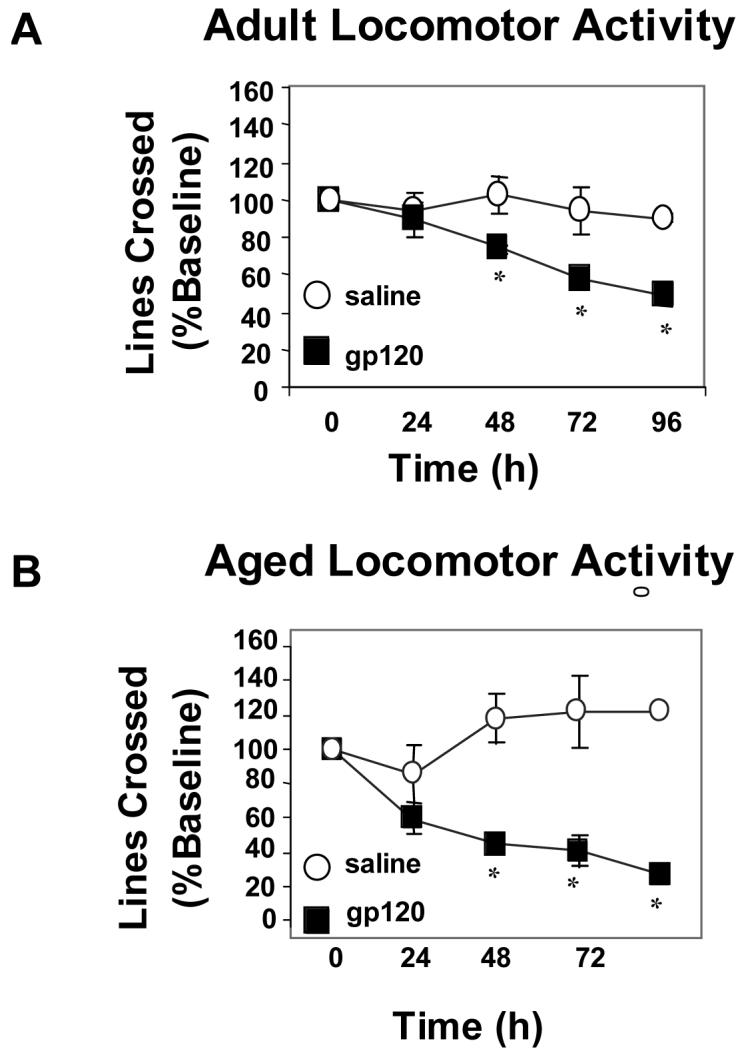
Adult (A) and aged (B) mice received a daily injection of saline or gp120 (100ng) for 5 consecutive days. Locomotor activity was assessed each day just prior to injection. Graphs are means ± SEM (n=8-10). Means with * are significantly different (p<0.05).
Figure 3. Aging increased hippocampal IL-6 and IL-1β mRNA.
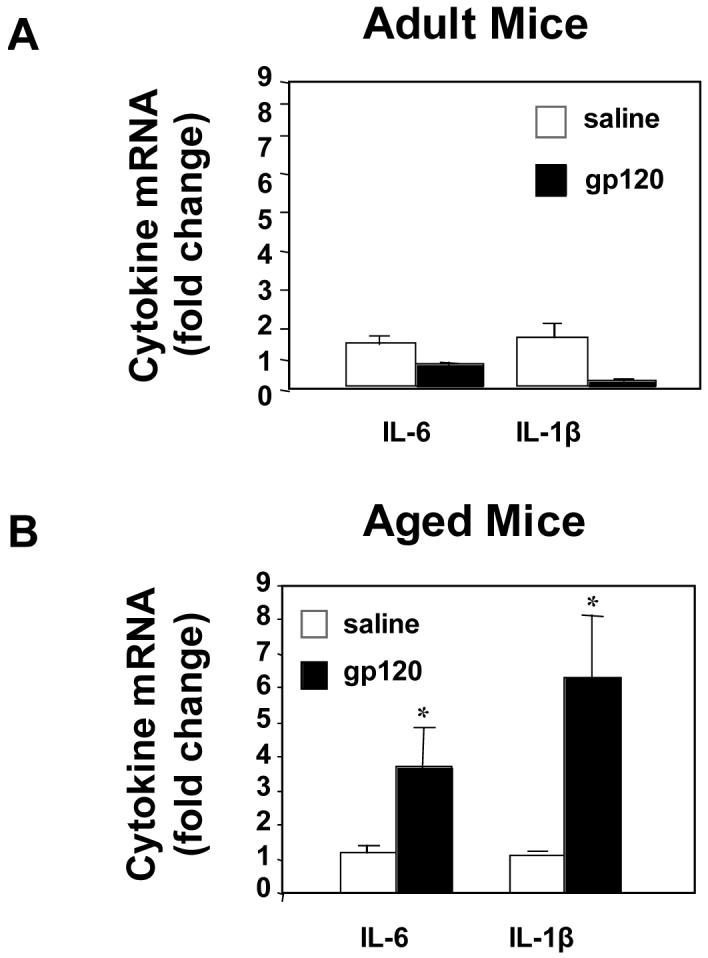
Adult (A) and aged (B) mice received a daily injection of saline or gp120 (100ng) for 5 consecutive days. 24 h after the last injection, hippocampal tissue was collected and cytokine mRNA were quantified by real-time PCR. Bars represent means ± SEM (n=8-10). Means with * are significantly different (p<0.05).
3.2. Expression of CCR5 on microglia is induced by IL-6
To investigate why aged mice are more sensitive to gp120 than adults, we next assessed if IL-6, a pro-inflammatory cytokine that has been shown to be elevated in the normal healthy aged brain [29], increases expression of the macrophage-specific receptor, CCR5, a well-known binding target of gp120 [12]. BV-2 cells were incubated with recombinant IL-6 and cell surface expression of CCR5 was determined by flow cytometry. Exposure to IL-6 increased the number of BV-2 cells expressing CCR5 (p<0.01; Figure 4A). While 2% of naïve BV-2 cells expressed CCR5 on the surface, treatment of BV-2 cells with IL-6 for 24 h increased CCR5 cell surface expression in a dose-dependent manner, with approximately 10% of BV-2 cells expressing CCR5, in the presence of 50 ng/ml of IL-6 (p<0.01; Figure 4B).
Figure 4. Expression of CCR5 on microglia is induced by IL-6.
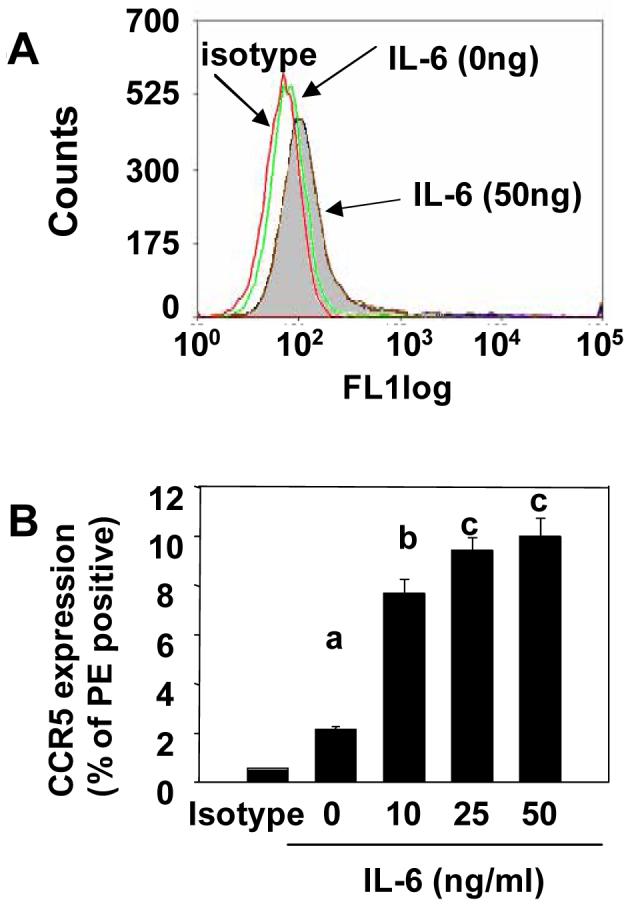
Exposure to IL-6 increased the number of BV-2 cells expressing CCR5 (A). Treatment of BV-2 cells with IL-6 for 24 h increased CCR5 cell surface expression in a dose-dependent manner (B). Bars represent means ± SEM. Means with different letters (a, b, or c) are significantly different (p<0.05) from each other.
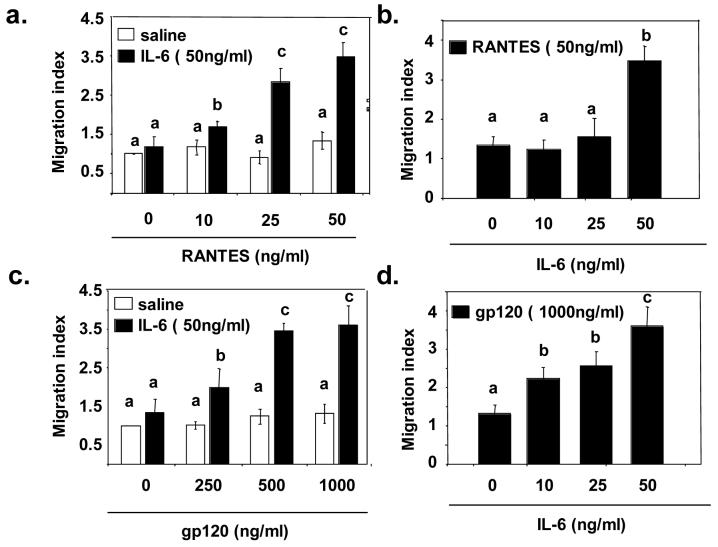
3.3. Microglial cell chemotaxis toward gp120 is enhanced by IL-6
To assess the functional significance of the IL-6-induced up regulation of CCR5, the chemotactic response of BV-2 cells to RANTES and gp120 was assessed in a cell migration assay. In an initial study, BV-2 cells were incubated with 0 or 50 ng/ml IL-6 and their chemotactic response to increasing concentrations of RANTES or gp120 was determined. Consistent with the finding that IL-6 increased CCR5 expression (Figure 4), pre-treatment with IL-6 increased the chemotactic response of BV-2 cells to RANTES (p<0.001, Figure 5a) and gp120 (p<0.001; Figure 5c). In a following study, the effects of exposing BV-2 cells to increasing concentrations of IL-6 on the subsequent chemotactic response to RANTES or gp120 were assessed. Pre-treatment with IL-6 increased the number of migrating BV-2 cells in a dose-dependent manner when either RANTES (p<0.01; Figure 5b) or gp120 (p<0.001; Figure 5d) were used as chemoattractants. Taken together these data indicate that IL-6 increased microglial cell expression of CCR5 and the chemotactic response to RANTES and gp120. Thus, the heightened sensitivity of aged mice to behavioral depression caused by gp120 may be related to cytokine driven increases in the expression of the macrophage-specific receptor, CCR5, and the subsequent release of proinflammatory cytokines.
Figure 5. Microglial cell chemotaxis toward macrophage-tropic gp120 is enhanced by IL-6.
Pre-treatment with IL-6 increased the chemotactic response of BV-2 cells to RANTES (A) and M-tropic gp120 (C). Pretreatment with IL-6 increased the number of migrating BV-2 cells in a dose-dependent manner when either RANTES (B) or M-tropic gp120 (D) were used as chemoattractants. Bars represent means ± SEM. Means with different letters (a, b, c, d, or e) are significantly different (p<0.05) from each other.
4. Discussion
The number of older adults with HIV disease and the incidence of HAD as an AIDS-defining illness has increased but little is known about how aging affects the behavioral complications associated with HIV-1 infection. Therefore, we sought to determine if aging affected sickness behavior following central administration of the HIV-1 envelope protein, gp120. The study was important in showing that aged mice are more sensitive to behavioral deficits induced by centrally administered HIV-1 gp120. Aged mice experienced a greater than 50% depression in social behavior beginning 4 h after a single injection of gp120 that was still evident 20 h later (i.e., 24 h post injection). Behavior of young adults, however, was not depressed by acute central administration of gp120. In a subsequent study, repeated ICV injection of gp120 depressed behavior in both young adult and aged mice, but the depression was exaggerated in the aged. Furthermore, the gp120-induced depression in behavior in aged mice was associated with increased steady-state levels of IL-1β and IL-6 mRNA in the hippocampus. Thus, gp120-induced sickness behavior may be facilitated in the aged due to enhanced production of behaviorally active inflammatory cytokines.
HIV-1 infection activates microglia, which in turn produce reactive oxygen species and inflammatory cytokines [12, 13]. HIV-1-infected patients develop slowness in thinking, have difficulties with attention and memory, and can become febrile, anorectic and cachectic [21]. Because the behavioral deficits evident in HIV-1 infection are largely due to the production of inflammatory molecules in the brain, conditions that facilitate microglial cell activation and production of cytokines, independent of HIV-1 infection, are likely to promote and intensify HAD or other behavioral complications. Therefore, we hypothesized that behavioral deficits induced by HIV-1 gp120 would be greater in aged mice than adults because we previously reported with this model that aging alone induced signs of oxidative stress and production of inflammatory cytokines in the brain [9, 17]. A working hypothesis is that cognitive aging is due in part to the emergence of age-associated neuroinflammation [10, 25, 27]. The fact that aged mice injected with gp120 displayed increased sickness behavior is consistent with age being a risk factor for HAD [23]. While using a murine model to investigate HAD is limiting, ICV injection of gp120 has been used previously in rodents to induce behavioral deficits that are reminiscent of HIV-1 infection [1]. It should be noted that these behavioral effects are specific to M-tropic gp120 since administration of T-tropic gp120, as well as repeated injection of heat-inactivated gp120 did not depress locomotor activity in either adult or aged mice (unreported observation).
There is good evidence that aging also alters the brain’s sensitivity to inflammatory stimuli. For example, activation of the peripheral innate immune system in aged mice resulted in an exaggerated inflammatory cytokine response in the brain and a more severe sickness behavior syndrome [9]. Another study found that aged rats inoculated with Escherichia coli had higher steady-state levels of IL-1β mRNA in hippocampus and deficits in hippocampal-dependent learning and memory compared to adults [2]. The expression of MHC class II, a marker of activated microglia, is increased in the brains of aged but otherwise healthy humans, nonhuman primates, and rodents [15, 18, 20, 22]. We recently reported with this model that MHC class II mRNA levels were 2-fold higher in brains of aged mice than adults [9]. Thus, aging may prime microglia so that these cells overreact to additional stimuli [15]. In the current study, aged mice had higher levels of IL-1β and IL-6 mRNA independent of gp120 treatment. In response to ICV administration of gp120, inflammatory cytokine mRNAs were markedly increased in hippocampus of aged mice but not in adults. The increased production of inflammatory cytokines in aged mice is likely responsible for the increased sickness behavior. By extension this might be important to older HIV-infected patients because the number of HIV-infected cells and the amount of viral antigen in the CNS does not correlate well with clinical signs of HAD, but the number of activated microglia [7] and increased steady-state levels of TNF-α mRNA in microglia does [26]. Interestingly, adult mice given repeated injections of gp120 had depressed motor activity after three days, however, inflammatory cytokine mRNAs were not elevated. Thus, it is possible that gp120 depressed behavior via another pathway independent of microglia and IL-1β and IL-6. In support of this notion, in another study inflammatory cytokine production was not induced by HIV-1 gp120 in primary microglia isolated from neonatal mice (unreported observation).
HIV-1 gp120 binds chemokine receptors (CCR5 and CXCR4) and plays a key role in commencing the cascade that ultimately leads to HIV encephalitis and HAD [12, 13]. In the brain, CCR5 is constitutively expressed by various CNS cell types, including neurons, microglia, and astrocytes [6, 19]. CCR5 mediates neuronal cell death upon binding gp120, which may lead to HAD [12]. Because IL-6 expression increases in the aged brain [28, 29] [30], we thought an age-related increase in CCR5 might underlie the exaggerated sickness behavior induced by gp120. Notably, because microglia are the main HIV reservoir in the central nervous system and most likely play the major role in the development of HIV dementia, we thought the expression of the predominant coreceptor used for infection of microglia, CCR5, would be increased and thus explain the sickness response seen in aged animals. In this sense, IL-6 might prime microglia and elicit a greater response to HIV-1 gp120. In support of this hypothesis, treatment of BV-2 cells with IL-6 increased CCR5 expression and migration towards RANTES, its natural ligand, as well as towards M-tropic gp120. However, attempts to discern an effect of age on CCR5 mRNA expression in hippocampus by real time PCR have thus far been equivocal.
In conclusion, the present study demonstrates that aged mice are more sensitive than adult mice to depressed behavior induced by central administration of HIV-1 gp120, perhaps due to a greater neuroinflammatory response. These findings are important because the severity of cognitive-motor impairment in older HIV-1-infected persons is increased compared to young infected persons [11]. Although it is not clear what occurs during aging to facilitate HAD, it seems that reducing neuroinflammation could be important for minimizing behavioral complications in older HIV-1 infected individuals. Zinck et al. [31] recently described a promising method of reducing neuroinflammation. In their study, SIV-infected pigtailed macaques treated with minocycline showed reduced severity of encephalitis, suppressed viral load in the brain, and decreased expression of CNS inflammatory markers. These findings suggest that minocycline, a safe and readily available antibiotic should be further investigated as an anti-HIV therapeutic as well as minimizing behavioral complications seen in HAD patients.
5. Acknowledgements
We thank Dr. Linda Van Eldik, Feinberg School of Medicine, Dept Cell and Molecular Biology, Northwestern University, Evanston, IL for the kind gift of the BV-2 cell line. This research was supported by NIH grants AG16710 and MH069148 to R. W. J.
5.1. Disclosure statement
The authors declared no actual or potential competing interests. The experimental procedures involving animals were consistent with PHS guidelines and approved by the campus IACUC.
This research was supported by NIH grants AG16710 and MH069148 to R. W. J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- [1].Barak O, Weidenfeld J, Goshen I, Ben-Hur T, Taylor AN, Yirmiya R. Intracerebral HIV-1 glycoprotein 120 produces sickness behavior and pituitary-adrenal activation in rats: Role of prostaglandins. Brain Behav Immun. 2002;16(6):720–35. doi: 10.1016/s0889-1591(02)00025-9. [DOI] [PubMed] [Google Scholar]
- [2].Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [3].Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23(9):3807–19. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. Aids. 1999;13(10):1249–53. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- [5].Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, Abramson I, Thal LJ, Atkinson JH, Wallace MR, Grant I, San Diego HIV Neurobehavioral Research Center Group Neurocognitive impairment is an independent risk factor for death in HIV infection. Arch Neurol. 1997;54(4):416–24. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- [6].Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 2003;136(12):84–93. doi: 10.1016/s0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- [7].Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38(5):755–62. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- [8].Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005;169(12):97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- [9].Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19(10):1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- [10].Godbout JP, Johnson RW. Interleukin-6 in the aging brain. J Neuroimmunol. 2004;147(12):141–4. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- [11].Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology. 1992;42(8):1472–6. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- [12].Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- [13].Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–92. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- [14].Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25(3):294–7. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- [15].Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–12. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- [16].Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis. 1988;158(5):1079–83. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- [17].Richwine AF, Godbout JP, Berg BM, Chen J, Escobar J, Millard DK, Johnson RW. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain Behav Immun. 2005;19(6):512–20. doi: 10.1016/j.bbi.2004.12.005. [DOI] [PubMed] [Google Scholar]
- [18].Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9(4):339–49. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- [19].Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997;151(5):1341–51. [PMC free article] [PubMed] [Google Scholar]
- [20].Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19(1):47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- [21].Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14(4):403–18. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- [22].Streit WJ, Sparks DL. Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med. 1997;75(2):130–8. doi: 10.1007/s001090050097. [DOI] [PubMed] [Google Scholar]
- [23].Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–7. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vallat AV, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith TW, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152(1):167–78. [PMC free article] [PubMed] [Google Scholar]
- [25].Verbitsky M, Yonan AL, Malleret G, Kandel ER, Gilliam TC, Pavlidis P. Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice. Learn Mem. 2004;11(3):253–60. doi: 10.1101/lm.68204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74(12):1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- [27].Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to- toe inflammatory paradigm. J Am Geriatr Soc. 2002;50(12):2041–56. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- [28].Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182(1):135–41. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- [29].Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93(12):139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- [30].--- Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J Neuroimmunol. 2001;117(12):87–96. doi: 10.1016/s0165-5728(01)00316-2. [DOI] [PubMed] [Google Scholar]
- [31].Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. Jama. 2005;293(16):2003–11. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
Web References
- CDC AIDS among persons aged greater than or equal to 50 years-United States, 1991-1996. MMWR. 1998;47:21–27. [PubMed] [Google Scholar]