Abstract
Intravenous enzyme replacement therapy (ERT) with purified glucocerebrosidase (GLA) leads to significant improvement of the clinical manifestations in patients with Type 1 Gaucher disease. However, the high doses required, slow response and inability to recover most of the infused enzyme in the target tissues may be attributed to losses occurring during transit en route to the lysosome. Pre-incubation of GLA with isofagomine (IFG), a slow-binding inhibitor, significantly increased stability of the enzyme to heat, neutral pH and denaturing agents in vitro. Preincubation of GLA with isofagomine prior to uptake by cultured cells results in increased intracellular enzyme activity accompanied by an increase in enzyme protein suggesting that reduced denaturation of GLA in the presence of isofagomine leads to a decrease in the degradation of the enzyme after internalization. Pre-incubation of GLA with slow-binding inhibitors before infusion may improve the effectiveness of ERT for Gaucher disease.
Keywords: pharmacological chaperone, Gaucher disease, lysosomal storage disorders, glucocerebrosidase, molecular chaperone, molecular mechanisms of pharmacological action, enzyme replacement therapy
Introduction
Gaucher disease is the most prevalent metabolic storage disorder of humans. It is caused by insufficient activity of the lysosomal enzyme glucocerebrosidase (GLA, EC 3.2.1.45)[1]. The consequent accumulation of glucocerebroside leads to anemia, thrombocytopenia, hepatosplenomegaly and skeletal abnormalities. Neurological deterioration occurs in some patients. The disease is classified clinically into type 1 (non-neuronopathic), type 2 (acute neuronopathic) and type 3 (chronic neuronopathic)[2]. Enzyme replacement therapy (ERT) by intravenous infusion of purified GLA is an effective treatment for patients with type 1 disease (reviews[3; 4; 5]). More than 5000 patients currently receiving this treatment experience marked clinical improvement. However, response is often slow, the dose required for treatment is high compared to the total body burden of glycolipid and animal studies have shown that delivery of active enzyme through the circulation to target lysosomes is inefficient[6]. The enzyme recovered from mouse liver and spleen had only 50–70% of the catalytic capacity of the infused enzyme[7]. A similar phenomenon was also observed following intracerebral administration. Only about 50% of the administered activity was recovered from rat brain immediately after perfusion[8]. These studies suggest that a significant amount of the enzyme may have been denatured during its transit through plasma, extracellular and intracellular fluids and endosomes prior to reaching the target lysosomal membrane[7; 9]. Thus, a modification of the delivery system to protect exogenous GLA from denaturing conditions may improve the effectiveness of ERT.
The inefficient delivery of GLA to target tissues may be caused in part by high susceptibility of exogenous GLA to denaturation in plasma and tissue fluids. Owing to the hydrophobic and membrane-associated property of native GLA, the purified enzyme used for clinical administration is solubilized with detergents and treated with solvents to remove most of the associated lipid before formulation of the enzyme as an aqueous solution. These steps may lead to an altered conformation compared with native lysosomal (endogenous) GLA leading to greater susceptibility to the action of proteases or denaturation during transit from the site of injection. Some active-site inhibitors induce conformational stabilization of enzymes that protect the active site and stabilize enzymes[10], a strategy previously exploited during purification of various active proteins. We hypothesized that preincubation of GLA with such an inhibitor prior to infusion may reduce denaturation of the enzyme in extracellular fluids and improve the effectiveness of ERT. We chose slow-binding inhibitors, a special class of reversible active-site inhibitors, as candidates for this purpose. Unlike most reversible inhibitors that dissociate immediately after dilution, slow-binding inhibitors are released slowly from the enzyme upon dilution[11; 12; 13]. These inhibitors may also display very high affinity i.e. low dissociation constants in the nanomolar range and thus can be used at very low concentrations prior to infusion minimizing the risk to the patient. Since the enzyme/inhibitor (EI) complex will be diluted by extracellular fluids after infusion the slow dissociation of the enzyme and inhibitor will allow prolonged protection of the enzyme in the extracellular fluids but release the enzyme from inhibition when the lysosomal target is reached. In this study, we investigated our hypothesis in vitro and in tissue cultured cells by using isofagomine (IFG), an active-site inhibitor of GLA which has been shown to be a slow-binding inhibitor of almond β-glucosidase[13].
Methods
Glucocerebrosidase assay
Activity of GLA was determined fluorimetrically at pH 5.9 using 4-methylumbellferyl-β-glucopyranoside as substrate as previously described[14].
Slow release of IFG from EI Complex
GLA (Imiglucerase, as a gift from Genzyme Corporation, Cambridge, MA) diluted to 0.16 U/ml in saline was preincubated with IFG (obtained from Amicus Therapeutics, Inc, Cranbury, NJ) in saline from 0–2μM for 30 min at room temperature (RT). The reaction was started by further 20-fold dilution of the enzyme-inhibitor (EI) complex with substrate solution pre-warmed to 37°C, and the released 4-methylumbelliferone was determined at various times after dilution as described above.
In vitro stabilization of GLA
GLA (8 U/ml) was preincubated with IFG at a final concentration of 0–5 μM as above. The EI complex was diluted 50-fold with various solutions below. For experiments assessing the stability of the enzyme to heat-inactivation, the complex was diluted with saline and heated at 54°C, aliquots removed at various times and enzymatic activity assayed immediately at 37°C. To assess the stability at various pH’s or in buffered plasma, the complex was diluted with either citric acid/phosphate buffer (pH 5.0), or phosphate-buffered saline (PBS) (pH 7.4 or 8.0) each containing 0.1% bovine serum albumin (BSA) or human plasma (pH 7.4, buffered containing 20 mM HEPES) and was then incubated at 37°C prior to immediate enzyme assay as above. To assess the stability to sodium dodecyl sulfate (SDS), the complex was diluted with 0.1 % or 0.2% SDS in saline containing 0.1% BSA and incubated at RT for 30 min.
In vitro uptake and binding studies
The mouse macrophage cell line J774E, expressing mannose-specific endocytic receptors[15] were obtained from Dr. Phillip Stahl, Washington University, St. Louis, MO and maintained in RPMI medium with 10% fetal bovine serum (FBS) and 10 μg/ml of 2-amino-6-mercaptopurine (Sigma, St. Louis, MO). GLA was preincubated with 5 μM IFG for 30 min at RT. The complex was diluted with pre-warmed growth medium to achieve a 50-fold dilution and added to the cells. After incubation, medium was removed by aspiration, cells were washed with PBS and then treated with 0.125% trypsin in 1.1 mM EDTA at 37°C for 10 min to eliminate extracellular GLA. Cells were harvested, rinsed with PBS (pH 7.4) and assayed for enzyme activity.
Stability of internalized enzyme was determined by loading macrophages with enzyme for 1 hr, washing three times with PBS, and then further incubation without enzyme at 37°C for the indicated time points, at which time cells were harvested for enzyme assay and Western blot.
To assess internalization of membrane-bound enzyme, cells were pre-incubated with enzyme in medium containing 25 mM HEPES, pH 7.4 at 4°C for 2 hrs, washed with PBS and incubated in medium without enzyme at 37°C for 45 min. Internalized GLA was analyzed by enzyme assay and Western blot.
Western blot analysis
Cell supernatants obtained following by brief sonication in a citric acid/sodium phosphate buffer (pH 6.0) containing 0.2% Triton X-100 and 1% sodium taurocholate, and centrifugation at 20,000g for 30 min at 4°C were used for analysis. Protein concentration was determined with the BCA protein assay reagent (Pierce, Rockford, IL)[16]. Lysates were denatured with LDS sample buffer (Invitrogen, Carlsbad, CA) and heated at 70°C for 10 min. Total protein loaded in each lane was 25 μg. Separation was performed using 10% Bis-Tris NuPAGE gels (Invitrogen) and electrophoretically transferred[17] to PVDF membranes. Membranes were blocked with 5% skim milk then incubated with rabbit polyclonal antibody to human GLA (a gift from the Genzyme Corporation, Cambridge, MA) for 2 hrs at RT. The signal was detected by use of the SuperSignal West Femto kit (Pierce). As loading control, actin was detected with a monoclonal antibody to β-actin (Sigma). The amount of GLA-specific protein was determined by densitometry using AlphaEase software (Alpha Innotech Corporation, San Leandro, CA).
Results
IFG is a slow-release inhibitor of human GLA
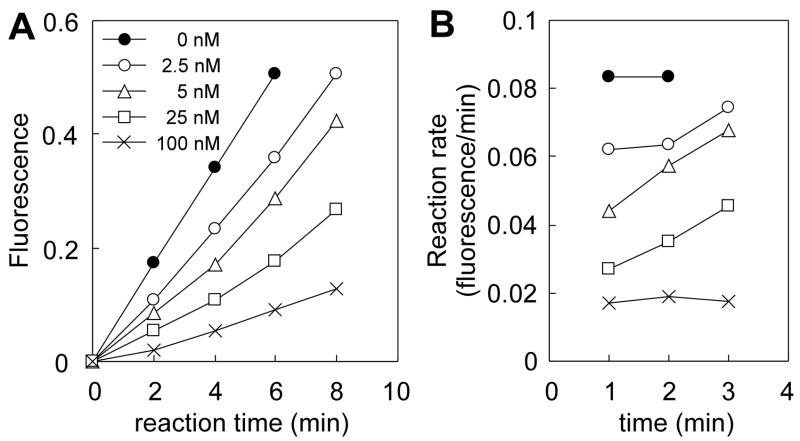
The slow release of IFG from human GLA, was demonstrated by preincubation of GLAwith IFG (GLA-IFG) and following the kinetics of the return to full activity after dilution into assay buffer. A time-dependent increase in reaction rate of the enzyme was observed (Fig 1 A and B) that was inversely related to IFG concentration. Following dilution of GLA-IFG at the lowest concentration tested (2.5 and 5 nM IFG final), the hydrolysis rate approaches that of the uninhibited enzyme after approximately 6 min indicating slow release of the inhibitor from the enzyme.
Figure 1.
Slow release of IFG from enzyme-inhibitor complex upon dilution. (A) Progress curves for the hydrolysis of substrate. GLA was preincubated with 0 to 2 μM IFG, and then rapidly diluted 20x into substrate solution to achieve the final concentrations of IFG shown. (B) Reaction rates calculated from (A).
IFG protects GLA from denaturation in vitro
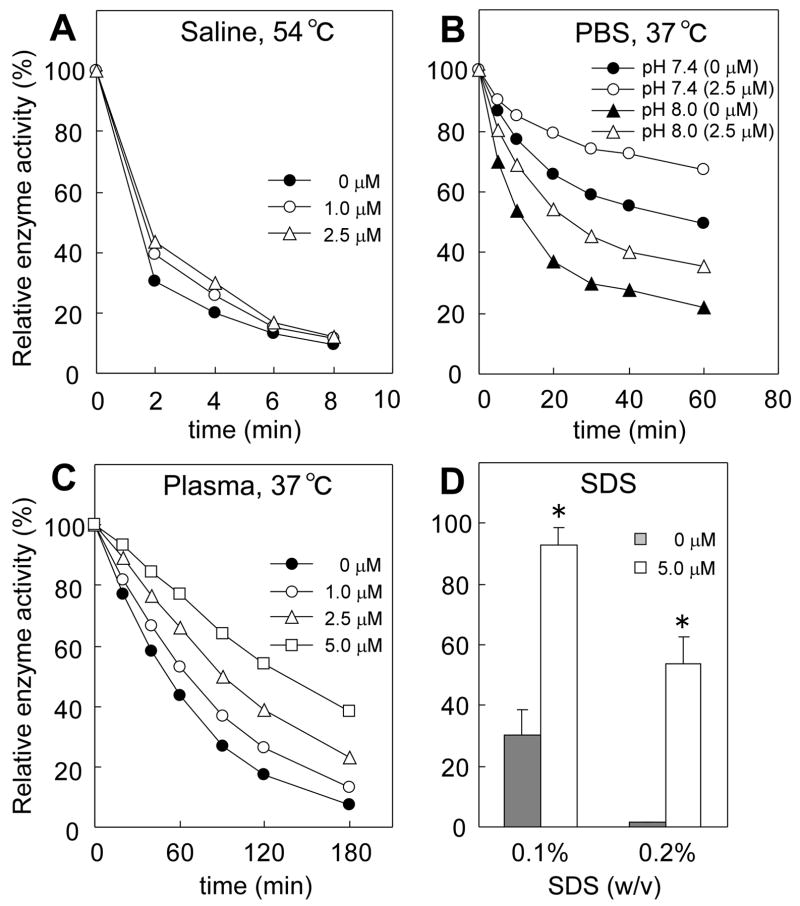
GLA-IFG was preincubated at room temperature for 30 min, then the EI mixture was diluted 50-fold under various conditions of heat, pH and in the presence of denaturants. Stability was assessed in parallel by enzyme assay and Western blots. GLA rapidly lost activity to less than 10% of the original value within 8 min when heated at 54 C in unbuffered saline. Preincubation of GLA with IFG (1 – 2.5 μM) increased the stability of the enzyme in a dose-dependent manner (Fig 2A). At pH 5.0, GLA was stable with no loss of enzyme activity during 1 h incubation at 37 C (data not shown), however, at neutral and mildly alkaline pH (pH 7.4 and 8.0) in PBS, it lost its activity rapidly with time (Fig 2B). IFG-preincubation (2.5 μM) significantly increased the stability of the enzyme at both neutral and alkaline pH. The loss of activity of untreated enzyme in plasma (pH 7.4) at 37 C was similar to that described for PBS (pH 7.4). IFG (1–5 μM) significantly slowed this loss of activity in a dose-dependent fashion (Fig 2C). The half-life of enzyme activity was increased from 46.9 min to 127.2 min by preincubation with 5 μM IFG. There was no significant change in the intensity of GLA-specific bands detected by Western blot after 3 hrs exposure to PBS (pH 7.4) or plasma (pH 7.4) at 37 C with or without IFG-preincubation (data not shown). This suggests that the loss of activity of GLA in plasma should be attributed to denaturation rather than degradation of the enzyme. Untreated GLA was susceptible to loss of activity in SDS, losing 70% of its initial activity when incubated for 30 min in 0.1% SDS or 99% in 0.2% SDS for 30 min (Fig 2D). In contrast, GLA-IFG provided nearly complete protection from the lower SDS concentration and greatly diminished the inactivation of GLA in 0.2 % SDS.
Figure 2.
Stabilization of GLA by IFG-preincubation in vitro. GLA was preincubated with IFG at room temperature for 30 min. The EI complex was diluted 50-fold, incubated for indicated length of time at indicated temperature, and then assayed for activity. Concentrations indicated are final concentrations of IFG in EI complex. (A) EI complex was diluted in saline and was heated at 54°C. (B) EI complex was diluted with PBS (pH 7.4 and pH 8.0) and heated at 37°C. (C) EI complex was diluted in human plasma and was heated at 37°C. (D) EI complex was diluted in SDS solutions (0.1% and 0.2%) and incubated at room temperature for 30 min. Enzyme activity is expressed as relative activity to untreated enzyme. For (A–C), results are the mean of duplicate assays and are representative of two independent experiments. For (D), data are presented as mean ± SE (n = 3). *P<0.004, comparison of the enzyme with and without IFG preincubated, two-tailed Student’s t test.
IFG leads to higher uptake of GLA by cultured macrophages
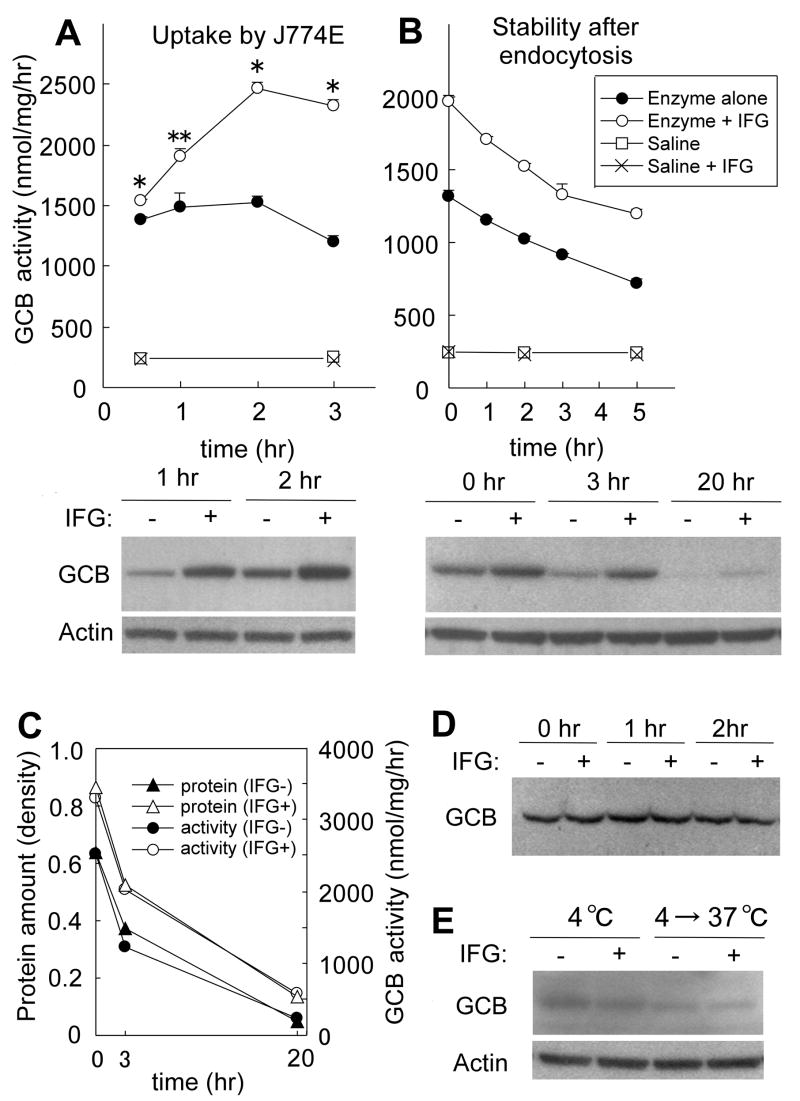
Following 30 min incubation of J774E macrophages with exogenous GLA or GLA-IFG, intracellular activity increased to more than 6 times the endogenous GLA level. Intracellular activity peaked at 2 hrs incubation and started to decrease thereafter (Fig 3A). At all times, intracellular activity of cells loaded with GLA-IFG was markedly higher than that of cells loaded with untreated GLA (Fig 3A, upper). Endocytosed GLA-specific protein was also analyzed by Western blot. Cells loaded with GLA-IFG contained a significantly higher amount of GLA-specific protein than cells loaded with untreated enzyme (Fig 3A, lower). Enzyme activity and GLA-specific protein level of endogenous GLA in control cells were not altered by IFG present in the media (Fig 3A, upper).
Figure 3.
Effect of IFG on enzyme uptake by macrophages. (A) Uptake of GLA with or without IFG-preincubation. Intracellular enzyme was assessed by enzyme activity (upper) and Western blot analysis (lower). Data are presented as mean ± SE (n = 3). *P < 0.005, **P<0.05, activity of the cells loaded with IFG-preincubated enzyme compared to that with untreated enzyme, t test. (B) Stability of endocytosed GLA with or without IFG-preincubation. Intracellular enzyme was assessed by enzyme activity (upper) and Western blot analysis (lower). Data are presented as mean ± SE (n = 3). (C) Comparison of activity and protein level of endocytosed GLA through 20 hrs after removal of enzyme from medium. Protein level was quantified by densitometry. Enzyme activity was obtained by subtracting the endogenous GLA activity in control cells from the activity in enzyme-loaded cells. (D) Effect of IFG on stability of the enzyme in the culture medium. GLA with or without IFG-preincubation was incubated in the medium at 37°C, and the enzyme amount was assessed by Western blotting. (E) Effect of IFG on binding and internalization of the enzyme to the cells. Binding was conducted at 4°C for 2 hrs. To assess internalization of surface-bound protein, the cells were transferred to 37°C and incubated for 45 min.
Stability of the endocytosed enzyme was assessed by loading J774E cells with GLA (with or without preincubation with IFG) for 1 hr as above followed by incubation in culture media without enzyme for various time periods. The loss of intracellular activity of GLA with and without IFG-preincubation was similar with half-life of 6.93 and 5.75 hrs respectively (Fig 3B, upper) indicating IFG did not significantly alter enzyme stability after endocytosis. However, GLA activity of the cells that were loaded with GLA-IFG was much higher than cells loaded with untreated GLA throughout the 5 hrs observation period. Western blot analysis showed that the cells loaded with GLA-IFG contain significantly higher amount of GLA than untreated enzyme for up to 20 hrs after removal of enzyme (Fig 3B, lower). The decrease of the GLA-specific protein quantified by densitometry of the Western blot paralleled that of enzyme activity (Fig 3C) suggesting that the loss of activity after endocytosis is mainly due to degradation of the enzyme. In the culture medium at 37 C and neutral pH the activity of untreated GLA was lost rapidly with time. IFG alleviated this loss of activity resulting in more than a doubling of the half-life of enzyme activity (from 33.8 min to 72.8 min). The amounts of GLA-specific protein remaining in the media were similar between GLA and GLA-IFG during 2 hrs of incubation (Fig 3D) suggesting that IFG stabilized GLA against denaturation in the culture medium and that degradation did not occur.
Efficiencies of GLA binding and internalization into cells were also assessed to clarify whether these processes were affected by IFG resulting in higher uptake of GLA-IFG. Plasma membrane bound enzyme was determined by binding at 4 C and analyzing GLA-specific protein by Western blot. There was no difference in GLA-specific protein at 4 C between GLA-IFG and untreated enzyme (Fig 3E). To assess the effect of IFG on internalization of GLA, cells incubated with the untreated enzyme or the GLA-IFG at 4 C were transferred to 37 C to allow internalization of surface-bound GLA to proceed. There was no significant difference in the amounts of intracellular GLA-specific protein between the cells loaded with GLA-IFG and untreated enzyme (Fig 3E). Results showed both the binding and internalization steps of the enzyme were not altered by IFG-preincubation.
Discussion
IFG effectively stabilizes GLA in vitro and appears to protect the enzyme against denaturation by heat, neutral pH and denaturant. Preincubation with IFG led to substantially increased uptake of GLA by cultured macrophages. The results suggest the potential utility of slow-binding inhibitors in improving ERT for Gaucher disease.
In ERT, the administered GLA is subjected to considerable stresses during transit through the circulation and tissue fluids. Body temperature and the neutral pH of blood may rapidly denature the enzyme. In addition to these factors, GLA could be more susceptible to denaturation since it may already be misfolded as a result of purification steps such as solubilization by detergents, or removal of protective lipid such as phosphatidylserine[18]. In our study, GLA lost activity rapidly after exposure to neutral pH at 37 C as might be encountered in human plasma. Binding of IFG to GLA increased the stability of the enzyme in these conditions. A recent crystal structure study has shown that IFG binds to the GLA active site and induces critical alterations in loops at the mouth of the active site[19]. Although the mechanism by which IFG protect exogenous enzyme from denaturation needs elucidation, it seems that interaction of IFG with key active-site residues of GLA locks the active-site conformation, reduces its flexibility and thus prevents the disruption of the active site caused by various external stresses.
Theoretically, preincubation of any enzyme with an inhibitor at 10-times the dissociation constant will result in greater than 90% occupancy of the enzyme active site by the inhibitor. If the enzyme is then administered to a patient by infusion of a small volume relative to the total plasma volume, dissociation will occur resulting in release of the free (fully active) enzyme. In our studies we modeled our dilution on the commonly used infusion volume (100 ml) of enzyme diluted by a total plasma volume of 5 liters (a 50-fold dilution). Further dilution will occur before the enzyme arrives at the target organelle. The slow release of the inhibitor makes this a feasible approach to stabilizing the enzyme during this transit phase.
IFG-preincubation led to significantly higher intracellular GLA activity compared to untreated enzyme. This higher enzyme activity is proportional to the higher amount of GLA protein taken up. We speculate that the greater amount of GLA following exposure to IFG-preincubated GLA compared to the untreated enzyme might be the result of reduced denaturation of the enzyme in the culture medium. Endocytosed proteins are subjected to proteolytic degradation to various degrees. As denatured GLA may be more susceptible to degradation than the intact enzyme, a reduction in the proportion of denatured enzyme prior to internalization could reduce the amount of enzyme subjected to degradation following internalization. Thus, the denaturation state of exogenous enzyme may affect the rate of loss of intracellular enzyme after endocytosis. The finding that IFG did not increase the level of endocytosed GLA when membranes were preloaded with enzyme under conditions where little denaturation occurs, such as at 4 C (Fig 3E), supports this speculation.
In our initial experiments, we assessed the potential stabilization effect of N-octadecylglucosylamine, a well-characterized slow-binding inhibitor of GLA ([12]). Similar to IFG, N-octadecylglucosylamine alleviated loss of enzyme activity caused by heat inactivation suggesting that other slow-binding inhibitors may have a similar protective effect. The slow-releasing property of these inhibitors may be important in their utility in ERT offering prolonged protection of the enzyme during transit in the circulation or in tissue fluids. Further studies need to be conducted to screen and compare other slow-binding inhibitors and to verify their ability to improve enzyme delivery in animal models.
To date ERT is the safest and most effective treatment for patients with type 1 Gaucher disease although recent studies have shown that chemical chaperones[20; 21; 22] and substrate reduction therapy[23] may be viable as alternative treatment strategies. Our study demonstrates that by preincubation of GLA with IFG, the level of active GLA that reached the targeted destination within the lysosomes was increased. Stabilization of exogenous GLA by inhibitors may provide an improved ERT for patients with Gaucher disease by reducing the dose and frequency of infusions. This approach may also be applied to a variety of other enzyme or protein deficiencies in which the administered protein must be delivered in an active form.
Acknowledgments
This work was funded by the Intramural Program of the National Institute of Neurological Disorders and Stroke, Bethesda MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brady RO, Kanfer JN, Shapiro D. Metabolism of Glucocerebrosides. Ii. Evidence of an Enzymatic Deficiency in Gaucher's Disease. Biochem Biophys Res Commun. 1965;18:221–5. doi: 10.1016/0006-291x(65)90743-6. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E, Grabowski GA. Gaucher disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Diseases. McGraw-Hill; New York: 2001. pp. 3635–3668. [Google Scholar]
- 3.Brady RO. Enzyme replacement for lysosomal diseases. Annu Rev Med. 2006;57:283–96. doi: 10.1146/annurev.med.57.110104.115650. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb NJ, Charrow J, Andersson HC, Kaplan P, Kolodny EH, Mistry P, Pastores G, Rosenbloom BE, Scott CR, Wappner RS, Zimran A. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. Am J Med. 2002;113:112–9. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- 5.Desnick RJ. Enzyme replacement and enhancement therapies for lysosomal diseases. J Inherit Metab Dis. 2004;27:385–410. doi: 10.1023/B:BOLI.0000031101.12838.c6. [DOI] [PubMed] [Google Scholar]
- 6.Murray GJ, Jin FS. Immunoelectron microscopic localization of mannose-terminal glucocerebrosidase in lysosomes of rat liver Kupffer cells. J Histochem Cytochem. 1995;43:149–58. doi: 10.1177/43.2.7822772. [DOI] [PubMed] [Google Scholar]
- 7.Xu YH, Ponce E, Sun Y, Leonova T, Bove K, Witte D, Grabowski GA. Turnover and distribution of intravenously administered mannose-terminated human acid beta-glucosidase in murine and human tissues. Pediatr Res. 1996;39:313–22. doi: 10.1203/00006450-199602000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Zirzow GC, Sanchez OA, Murray GJ, Brady RO, Oldfield EH. Delivery, distribution, and neuronal uptake of exogenous mannose-terminal glucocerebrosidase in the intact rat brain. Neurochem Res. 1999;24:301–5. doi: 10.1023/a:1022578424693. [DOI] [PubMed] [Google Scholar]
- 9.Grabowski GA. Delivery of lysosomal enzymes for therapeutic use: glucocerebrosidase as an example. Expert Opin Drug Deliv. 2006;3:771–82. doi: 10.1517/17425247.3.6.771. [DOI] [PubMed] [Google Scholar]
- 10.Pace CN, McGrath T. Substrate stabilization of lysozyme to thermal and guanidine hydrochloride denaturation. J Biol Chem. 1980;255:3862–5. [PubMed] [Google Scholar]
- 11.Szedlacsek SE, Duggleby RG. Kinetics of slow and tight-binding inhibitors. Methods Enzymol. 1995;249:144–80. doi: 10.1016/0076-6879(95)49034-5. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg P, Merrill AH, Liotta DC, Grabowski GA. Human acid beta-glucosidase: use of sphingosyl and N-alkyl-glucosylamine inhibitors to investigate the properties of the active site. Biochim Biophys Acta. 1990;1039:12–20. doi: 10.1016/0167-4838(90)90220-a. [DOI] [PubMed] [Google Scholar]
- 13.Lohse A, Hardlei T, Jensen A, Plesner IW, Bols M. Investigation of the slow inhibition of almond beta-glucosidase and yeast isomaltase by 1-azasugar inhibitors: evidence for the 'direct binding' model. Biochem J. 2000;349:211–5. doi: 10.1042/0264-6021:3490211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K. Enzymic diagnosis of sphingolipidoses. Methods Enzymol. 1978;50:456–88. doi: 10.1016/0076-6879(78)50049-9. [DOI] [PubMed] [Google Scholar]
- 15.Diment S, Leech MS, Stahl PD. Generation of macrophage variants with 5-azacytidine: selection for mannose receptor expression. J Leukoc Biol. 1987;42:485–90. doi: 10.1002/jlb.42.5.485. [DOI] [PubMed] [Google Scholar]
- 16.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 17.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dale GL, Villacorte DG, Beutler E. Solubilization of glucocerebrosidase from human placenta and demonstration of a phospholipid requirement for its catalytic activity. Biochem Biophys Res Commun. 1976;71:1048–53. doi: 10.1016/0006-291x(76)90760-9. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman RL, Wustman BA, Huertas P, Powe AC, Jr, Pine CW, Khanna R, Schlossmacher MG, Ringe D, Petsko GA. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007;3:101–7. doi: 10.1038/nchembio850. [DOI] [PubMed] [Google Scholar]
- 20.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S beta -glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci U S A. 2002;99:15428–33. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steet RA, Chung S, Wustman B, Powe A, Do H, Kornfeld SA. The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms. Proc Natl Acad Sci U S A. 2006;103:13813–8. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang HH, Asano N, Ishii S, Ichikawa Y, Fan JQ. Hydrophilic iminosugar active-site-specific chaperones increase residual glucocerebrosidase activity in fibroblasts from Gaucher patients. Febs J. 2006;273:4082–92. doi: 10.1111/j.1742-4658.2006.05410.x. [DOI] [PubMed] [Google Scholar]
- 23.Lachmann RH, Platt FM. Substrate reduction therapy for glycosphingolipid storage disorders. Expert Opin Investig Drugs. 2001;10:455–66. doi: 10.1517/13543784.10.3.455. [DOI] [PubMed] [Google Scholar]