Abstract
Clinical trials have consistently shown the benefits of beta-blocker treatment in patients with chronic heart failure (HF). As a result, bisoprolol, carvedilol, and metoprolol succinate are now indicated for the treatment of all patients with chronic HF who do not have major contraindications. Bisoprolol is the first beta-blocker shown to improve survival in an outcome trial. In the Cardiac Insufficiency Bisoprolol Study II (CIBIS-II), all-cause mortality and sudden death were reduced in patients treated with bisoprolol compared with those on placebo (11.8% vs 17.3%; p < 0.0001 and 3.6% vs 6.3%, p < 0.002; respectively) regardless of age, NYHA functional class, and co-morbidities. Further studies have shown both the efficacy of bisoprolol on secondary endpoints and patients subgroups as well its high cost effectiveness. More recently, CIBIS-III has shown similar efficacy and safety of the initiation of HF treatment with either bisoprolol or enalapril, with a tendency to a survival advantage with bisoprolol. Nowadays, the role of bisoprolol, as well as that of carvedilol and metoprolol succinate, in HF treatment is firmly established and research is mainly focused on implementation of treatment and better dosing. This article will summarize evidence for the efficacy of bisoprolol in the treatment of HF.
Keywords: bisoprolol, heart failur, beta-blockers
Introduction
Heart failure (HF) is a disease of epidemic proportions. Its prevalence ranges from 0.4% to 2% in the adult population of Western countries and increases 2- to 3-fold when patients with asymptomatic left ventricular (LV) dysfunction and with normal LV ejection fraction (EF) are included (Cowie et al 1997; Cleland et al 2001; Stewart et al 2001). Despite recent advances, its prognosis remains poor. Half of the patients die within 3–5 years after their first diagnosis and 1-year mortality rate may reach 50% in patients with severe HF (Cowie et al 1997; Cleland et al 2001; Stewart et al 2001; Hunt et al 2005; Swedberg et al 2005)
Randomized controlled trials have allowed the selection of therapies able to improve quality of life and outcomes in patients with chronic HF. Hence guidelines now recommend the administration of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs) and, in New York Heart Association (NYHA) class III to IV patients, aldosterone antagonists, to improve prognosis of the patients with HF (Hunt et al 2005; Swedberg et al 2005). Diuretics are indicated for symptomatic treatment of fluid overload when present and manifest as pulmonary congestion or peripheral edema. Digoxin is also indicated to improve symptoms in patients with NYHA class III and IV HF (Swedberg et al 2005).
Beta-blockers are therefore the mainstay of current medical treatment of HF. Bisoprolol was the first beta-blocker shown to have beneficial effects on outcomes in the Cardiac Insufficiency Bisoprolol Study II (CIBIS-II) (CIBIS-II Investigators and Committees 1999). The aim of this article is to review its main pharmacological characteristics with respect to its use in the patients with HF.
Pathophysiological mechanisms in HF
The introduction of beta-blockers in the treatment of HF has represented a major breakthrough in the treatment and interpretation of this syndrome. When HF was considered as a hemodynamic disorder, sympathetic activation was regarded as a favorable response increasing myocardial contractility and cardiac output. However, concomitant studies had shown the independent prognostic role of sympathetic activation in HF as well its long-term deleterious effects on myocardial function and outcome. Increased cardiac sympathetic drive was shown to be associated with increased myocardial energy expenditure and possibly ischemia of the failing heart. Subsequently, beta-1 adrenergic receptors (ARs) stimulation was shown to be a powerful mechanism leading to accelerated cell death, through apoptosis (Communal et al 1998), and to major changes in the qualitative characteristics of myocardial cells with reduced contractility and abnormal intracellular calcium handling by the sarcoplasmic reticulum (Lowes et al 2002). The role of sympathetic stimulation in all these quantitative and qualitative changes in myocardial characteristics was indirectly shown by their reversal with beta-blocker treatment (Bristow 2000; Metra et al 2000b).
Beta-blocker therapy in HF: historical notes
Controlled clinical trials reconciled pathophysiological findings, showing the deleterious effects of long-term sympathetic activation, with clinical findings. Relatively small, single-center trials showed the beneficial effects of beta-blockers on clinical symptoms and, to an even greater extent, on myocardial function. All the major changes associated with LV remodeling: LV dilatation, acquisition of a spherical shape, and mitral regurgitation, were reduced by the long-term administration of beta-blockers with a concomitant, highly significant, improvement in LVEF (Bristow 2000; Lechat et al 1998; Metra et al 1994, 2000b, 2007). The magnitude of these changes is actually greater than that described with ACE inhibitors. Randomized controlled trials, having mortality as primary endpoint, showed the beneficial effects of beta-blockers on mortality and hospitalizations, with reductions in both sudden cardiac deaths (SCD), HF deaths, cardiovascular hospitalizations, and HF hospitalizations (CIBIS II Investigators and Committees 1999; MERIT-HF Study Group 1999; Packer et al 2001). This has led to the indication for beta-blocker therapy for all patients with chronic HF who do not have major contraindications (Hunt et al 2005; McMurray et al 2005; Swedberg et al 2005). Based on the results of early post-infarction trials as well as of the more recent Carvedilol Post-Infarct Survival Control in LV Dysfunction (CAPRICORN) trial (The CAPRICORN Investigators 2001), beta-blockers are also recommended in patients with LV systolic dysfunction following myocardial infarction, regardless of whether they are symptomatic or not for HF (Hunt et al 2005; Swedberg et al 2005).
Bisoprolol has been extensively studied in the patients with chronic HF. In the following sections of this article we will describe its main pharamacokinetic and pharmacodynamic characteristics with respect to the treatment of HF.
Essential clinical pharmacology
Beta-receptor selectivity
Bisoprolol has high selectivity for beta-1 ARs with a beta-1 to beta-2 antagonist activity ratio of 119. Its selectivity for beta-1 ARs is higher compared with metoprolol, the other selective beta-blockers used in the treatment of HF (beta-1 to beta-2 ratio, 45) (Bristow 2000). This high selectivity might lead to better tolerability in patients with concomitant chronic obstructive pulmonary disease (COPD) as well as with peripheral vascular disease (Metra et al 1999; Sirak et al 2004; Le Jemtel et al 2007)
Pharmacokinetics
Bisoprolol is almost completely absorbed in the enteric tract (90%) with very low liver first pass metabolism (10%). It has low plasma protein binding (30%) with a 1:1 ratio between hepatic metabolism and renal excretion. In normal subjects, its half-life is long (10–11 hours) and is further prolonged in patients with HF (17 ± 5 hours) and/or with severe renal failure allowing once-daily administrations (Leopold et al 1997). Bisoprolol does not interfere with the metabolism of other drugs.
Modes of administration
As with all beta-blockers in patients with HF, bisoprolol should be started with low doses with gradual (1- to 2-week intervals) uptitration to target doses. Treatment is generally started with 1.25 mg once daily with subsequent uptitration to 2.5 mg, 3.75 mg, 5 mg, 7.5 mg, and 10 mg/daily, if tolerated (CIBIS-II Investigators and Committees 1999). Interestingly, target dose of bisoprolol was 5 mg in the first CIBIS trial, in which bisoprolol did not significantly reduce mortality, while it was 10 mg in CIBIS-II, in which bisoprolol reduced mortality (CIBIS-II Investigators and Committees 1994, 1999). Taking into account the differences in the initial as well as in the target doses (carvedilol, 3.125 mg bid to 25–50 mg bid, metoprolol succinate, 12–25 mg to 200 mg daily), similar protocols for initiation of therapy and uptitration must be used also for the other beta-blockers approved for HF treatment (Hunt et al 2005; McMurray et al 2005; Swedberg et al 2005).
The dose of the beta-blockers may be reduced in case of worsening HF or other adverse hemodynamic effects (hypotension, bradycardia). This may occur during the uptritation phase or, less often, while the patient in on stable maintenance doses. Bisoprolol, like any other beta-blocker indicated for HF treatment, should be completely withdrawn only in case of absolute intolerance. In addition to hemodynamic reasons (hypotension, bradycardia), the only absolute contraindication to beta-blocker treatment is asthma sensitive to the administration of beta-2 ARs agonists (Metra et al 1999, 2004; Hunt et al 2005; McMurray et al 2005; Swedberg et al 2005). Patients with bronchial asthma may, however, show better tolerance to bisoprolol, compared with other beta-blockers, because of its greater beta-1 ARs selectivity (Metra et al 2004).
Bisoprolol, like all other beta-blockers, should not be stopped abruptly as this may cause tachycardia, tachyarrhythmias, angina, and worsening HF. If the discontinuation is necessary, the dose should be decreased gradually.
Effects in the patients with HF
Heart rate (HR)
As expected with beta-blocker treatment, bisoprolol administration is associated with a reduction in HR. This has been consistently shown in CIBIS (−16.3 ± 15.3 vs −1.6 ± 13.4 bpm with placebo and bisoprolol, respectively; p < 0.001), as well as in CIBIS II (−9.8 ± 14.7 vs −0.2 ± 13.7 bpm with placebo) (Lechat et al 1997, 2001). In CIBIS, the changes in HR were predictive of survival, with a longer survival in patients showing the greatest decrease in HR (Lechat et al 1997). Similarly, in CIBIS II, both baseline HR and its changes after 2 months of treatment were related to survival (Lechat et al 2001). This analysis also showed that the beneficial effects of beta-blocker therapy (eg, bisoprolol) were independent of the HR changes. Patients on bisoprolol had a better survival, compared with those on placebo, both in the subgroups of patients with lower or higher HR at baseline and in the patients with no change, a decrease, or an increase in HR from baseline (Lechat et al 2001).
A recent analysis of data from the carvedilol or metoprolol European trial (COMET) confirmed these results. COMET included 3029 patients with chronic HF randomized to carvedilol or metoprolol. In this trial, the HR measured at 4 months after the initiation of beta-blocker therapy was related to subsequent mortality. However, neither the HR before the initiation of beta-blocker therapy nor the changes from baseline in HR had any prognostic value (Metra et al 2005). Beta-blockers counteract the deleterious effects of tachycardia in the failing heart so that the HR measured before treatment loses its prognostic significance.
Blood pressure (BP)
In CIBIS-II, bisoprolol reduced BP from baseline (−4.1 ± 16.4 vs −2.3 ± 16.4 mmHg with placebo for systolic BP, and −2.6 ± 10.7 vs −0.9 ± 10.9 mm Hg with placebo, p <0.0001 in both cases) (Lechat et al 1997, 2001). There was no relation between changes in blood pressure and mortality in CIBIS (Lechat et al 1997, 2001). Interestingly, a low BP is predictive of increased mortality, rather than the opposite, in the patients with HF (Metra et al 2005).
LV function
Bisoprolol is a beta-blocker with high selectivity for beta-1 ARs. It has no intrinsic sympathomimetic activity and no membrane stabilizing activity. Lacking of any ancillary property, it may probably be considered as “the purest” beta-1 AR blocker for the treatment of HF. Its hemodynamic effects in patients with HF were studied in single center trials (Nyolczas et al 2000; Dubach et al 2002; Belenkov et al 2003) as well as in the two, large, randomized, placebo-controlled outcome trials (CIBIS Investigators and Committees 1994; CIBIS-II Investigators and Committees 1999). In the study by Dubach et al (2002) the effects of bisoprolol on LV function were studied by nuclear magnetic resonance myocardial tagging in 28 patients with chronic HF randomized to bisoprolol or placebo. One-year treatment with bisoprolol was associated with an increase in LVEF (from 25 ± 7 to 36 ± 9%; p < 0.05) and a non-significant decline in LV end-diastolic and end-systolic volumes (−54 and −62 mL, respectively). No change occurred with placebo.
An improvement in LV function has been shown also with the other beta-blockers having beneficial effects on mortality in HF (Metra et al 1994; Hall et al 1995; Lechat et al 1998; Lowes et al 2002). In a randomized comparison study, carvedilol has been associated with a greater improvement in LVEF, compared with metoprolol tartrate (Metra et al 2000a). However, no comparison study with metoprolol succinate, as well as bisoprolol, has been concluded to our knowledge.
In a retrospective analysis of CIBIS, the changes in LV fractional shortening were related to subsequent prognosis both in the bisoprolol and the placebo groups. Patients who had a fractional shortening change >0.014 (median value) had a better survival than the others. The change in LV fractional shortening, the changes in HR, and bisoprolol administration were independent predictors of survival (Lechat et al 1997). These results were consistent with other studies showing that the changes in LVEF after beta-blocker therapy predict subsequent outcome (Metra et al 2003).
Neurohormonal parameters and inflammatory markers
Pousset et al (1996) assessed HR variability (HRV) in 54 patients enrolled from CIBIS. Patients receiving bisoprolol showed an increase in HRV parameters related to parasympathetic activity (24-hour rMSSD, p = 0.04; 24-hour pNN50, p = 0.04; daytime SDNN, p = 0.05 and daytime high-frequency power, p = 0.03). These authors also analyzed scatterplots of R-R intervals consistently showing an increase in parameters related to parasympathetic tone with a decrease in parameters related to sympathetic tone (Copie et al 1996). All these changes are known to be associated with an improvement in prognosis (Metra et al 2006).
Belenkov et al (2003) and colleagues showed that bisoprolol treatment can also decrease other neorohormonal parameters such as plasma rennin activity (p < 0.05), plasma levels of norepinephrine (p < 0.05), angiotensin II, and aldosterone (p < 0.05).
Similarly to other beta-blockers, also the administration of bisoprolol has been associated with favorable effects on markers of inflammatory activity (tumor necrosis factor [TNF]-alpha, TNF-receptors, interleukins) both in animal models and clinical studies (Ohtsuka et al 2001; von Haehling et al 2005; Ichihara et al 2006).
Quality of life
Beneficial effects on outcome are the main reason why beta-blocker therapy is now indicated for all the patients with HF who do not have major contraindications. However, an improvement in symptoms and quality of life remains the second important objective of treatment (Hunt et al 2005; Swedberg et al 2005). The effects of beta-blockers on these endpoints generally remain less significant (Metra et al 1998).
In Lechat’s meta-analysis (1998), the effects of beta-blockers on NYHA class were less significant, compared with those on mortality, hospitalizations, and LVEF, and disappeared with the addition or removal of only 1 moderate-size study. Bolger and Al-Nasser (2003) have assessed quality of life and parameters related to exercise capacity in over 20 controlled trials with carvedilol, metoprolol, or bisoprolol administration to patients with HF. For quality of life measurements, the effects of beta-blockers were often similar to placebo.
In CIBIS 21% of patients in the bisoprolol group improved their NYHA class vs 15% of those on placebo (p < 0.03). The percentage of patients showing deterioration in their NYHA class was similar between bisoprolol and placebo. Importantly, the rate of withdrawal of treatment caused by side-effects was similar in the placebo and bisoprolol groups (82 patients, 26%, in the placebo group vs 75, 23% in the bisoprolol group; NS) (CIBIS Investigators and Committees 1994).
Baxter et al (2002) investigated the tolerability of bisoprolol treatment in elderly patients with CHF. The rate of withdrawal from beta-blocker therapy was twice than previously reported in clinical trials performed in younger patients. Quality of life, assessed through a score, was improved after bisoprolol, suggesting better perceived health status and a reduction in anxiety and depression (Baxter et al 2002).
Outcome
The Cardiac Insufficiency Bisoprolol Study (CIBIS) was a placebo-controlled, randomized, double-blind, multicenter trial assessing the effect of bisoprolol on outcome and its tolerability in 641 patients with symptomatic HF (NYHA class III or IV) caused by LV systolic dysfunction (LVEF <40%) (CIBIS Investigators and Committees 1994). Bisoprolol did not significantly reduce mortality. Sixty-seven deaths (20.9%) occurred in the placebo group compared with 53 deaths (16.6%) in the bisoprolol group (p = 0.22). Beneficial effects of bisoprolol were found with respect to other endpoints. HF hospitalization rate was lower in the patients assigned to bisoprolol, compared with placebo (61 vs 90, p < 0.01), and NYHA functional classification improved in 21% of patients on bisoprolol compared with 15% on placebo (p = 0.03). Beta-blocker therapy was well tolerated (CIBIS Investigators and Committees 1994).
The CIBIS trial was the first large-scale trial testing the effects of beta-blockade on mortality alone. A few years earlier, the Metoprolol Dilated Cardiomyopathy (MDC) trial had assessed the effects of metoprolol tartrate on the combined endpoint of mortality and heart transplantation with similar results (Waagstein et al 1993). Both CIBIS and MDC were underpowered, with respect of the size pf their study group, to detect a meaningful effect on outcome. In CIBIS, the mortality of the patients enrolled was much lower than expected (5% vs 36%) and this further decreased the power of the study. Second, the target dose of bisoprolol in CIBIS (5 mg/day) might have been too low to reach adequate beta-blockade in a sufficient number of patients. In contrast, target dose of bisoprolol in CIBIS-II was 10.0 mg and thus higher doses were administered during the maintenance phase, with 564 patients, 42.5%, receiving 10 mg of bisoprolol; 152 (11%) receiving 7.5 mg, and 176 (13%) receiving 5.0 mg daily (CIBIS-II Investigators and Committees 1999).
CIBIS-II gave an answer to the questions left unresolved by the previous trial, namely, the size of the study population of CIBIS-II was large enough to detect meaningful differences in outcome between patients randomized to bisoprolol or placebo. The results of CIBIS-II were confirmed and expanded by further trials with metoprolol succinate and carvedilol having mortality as primary end-point (MERIT-HF Study Group 1999; Packer et al 2001).
CIBIS-II was a multicenter, double-blind, randomized placebo controlled trial enrolling 2647 patients with symptomatic HF (NYHA class III-IV) and a LVEF ≤35%, on standard therapy with diuretic and ACE inhibitors. This trial was prematurely stopped, after a mean follow-up of 1.3 years, for the significant reduction in mortality in the patients randomized to bisoprolol, compared with those on placebo. One hundred and fifty-six patients (11.8%) died in the bisoprolol group, compared with 228 patients (17.3%) in the placebo group (p < 0.0001). The estimated annual mortality rate was 8.8% in the bisoprolol group and 13.2% in the placebo group (hazard ratio [HR]; 95% confidence intervals [CI], 0.66; 0.54–0.81) (CIBIS-II Investigators and Committees 1999).
Mortality reduction was mainly caused by a reduction in SCD (48 patients on the bisoprolol vs 83 patients on placebo, HR; 95% CI, 0.56, 0.39–0.80; p = 0.0011). Pump failure deaths were reduced but this result did not reach statistical significance because of the lower number of events (36 vs 47 patients, HR; 95% CI, 0.74; 0.48–1.14; p = 0.17). This finding, as well as the relatively low 1-year mortality of the studied patients, is consistent with the enrollment of patients with relatively mild HF having SCD as the main cause of mortality.
Bisoprolol also had important effects on cardiovascular morbidity. All-cause hospitalizations were lower in the patients on bisoprolol (440 patients, 33%) compared with those on placebo (513 patients, 39%, HR; 95% CI, 0.80, 0.71–0.91; p = 0.0006). Hospital admission for worsening HF were also reduced in the bisoprolol compared with the placebo group (12% vs 18%, HR; 95% CI, 0.64, 0.53–0.79; p = 0.0001). Hospitalizations for ventricular arrhythmias and hypotension were also reduced by bisoprolol administration. In contrast, hospitalizations for bradycardia and, unexpectedly, for stroke (31 vs 16, p = 0.04), were more frequent in the patients randomized to bisoprolol and hospitalizations for angina, myocardial infarction, cardiogenic shock, and coronary revascularizations were not different in the bisoprolol, compared with the placebo group. This finding, as well as the increase in the hospitalizations for stroke, is rather unexpected and likely related to the relatively small number of events. It is, however, consistent with the lower effect on vascular events shown by another selective beta-blocker (metoprolol tartrate), compared with carvedilol, in COMET (Remme et al 2007).
Subgroup analyses of CIBIS-II showed that the beneficial effects of bisoprolol on mortality and hospitalizations were independent from cause of HF, HF severity, and bisoprolol dose. Patients assuming higher doses of the study drug had less severe HF at baseline and showed a reduction in mortality of greater magnitude with bisoprolol, compared with the patients who could tolerate only low doses (Simon et al 2003).
The beneficial effects of bisoprolol on outcome were maintained also in high risk patients. In spite of their expected increase in the overall risk of death and hospitalization, patients with diabetes, renal insufficiency, NYHA class IV symptoms, and the elderly showed a similar reduction in mortality and morbidity as the other patients enrolled in CIBIS-II. Similarly, also the patients taking either digitalis, amiodarone, or aldosterone antagonists as co-medication had similar benefits from bisoprolol therapy as patients not on these drugs (Erdmann et al 2001).
Lastly, but importantly, CIBIS-II showed the excellent tolerability of bisoprolol. These results were in contrast with the widely held belief (at the time of the study) that beta-blocker therapy is not well tolerated, if not even contraindicated, in patients with HF. The number of treatment withdrawals was actually the same in the patients on bisoprolol and on placebo.
A meta-analysis was performed, including the results on CIBIS and CIBIS-II, for a total of 3288 patients. It was confirmed that bisoprolol administration is associated with a highly significant reduction of overall death (p = 0.0003), cardiovascular death and hospitalizations (p = 0.0001) (Leizorovicz et al 2002).
Which drug first for the treatment of heart failure? The CIBIS-III trial
Despite the high significance of results obtained with beta-blocker therapy, all the data had been obtained with the administration of beta-blockers on top of standard therapy, including ACE inhibitors; hence the recent guidelines indications (Hunt et al 2005; Swedberg et al 2005). However, there are reasons to believe that initiation of treatment with beta-blockers, rather than ACE inhibitors, may be beneficial as well. First, sympathetic activation may precede activation of the renin-angiotensin system in HF (Francis et al 1990). Second, beta-blockers may reduce renin-angiotensin activation to a greater extent than what achieved by ACE inhibitors with respect to sympathetic activation (Campbell et al 2001). Third, SCD is the most important cause of death in the early course of HF (MERIT-HF Study Group 1999) and beta-blockers, differently from ACE inhibitors, have a significant effects protective effect on SCD (CIBIS-II Investigators and Committees 1999; MERIT-HF Study Group 1999; Bristow 2000; Packer 2001)
Only two relatively small studies had assessed the effects of initiating treatment of HF with beta-blockers, rather than ACE inhibitors (Remme et al 2004; Leier 2004; Sliwa et al 2004). In the carvedilol and ACE inhibitor remodeling mild heart failure evaluation (CARMEN) trial, the administration of the combination of carvedilol and enalapril was associated with the greatest reduction in LV end-systolic volume whereas treatment with carvedilol alone had a weaker effect and enalapril alone had no effect (Remme et al 2004). This study was the first showing the greater beneficial effects of combined administration of a beta-blocker and an ACE inhibitor, compared with either agent alone. The lack of effects of enalapril on LV remodeling was, however, somehow unexpected. It was likely related to the relatively high percentage of patients who were on enalapril before entry into the study and who might have developed tolerance to the effects of ACE inhibition.
The study by Sliwa et al (2004) compared the effects of initiating HF treatment with a beta-blocker rather than an ACE inhibitor. Its design was therefore very similar to CIBIS-III. In the study by Sliwa et al (2004) initiation of therapy with a beta-blocker was associated with a greater effect on LVEF and volumes. However, this study was not blinded.
The hypothesis of the CIBIS-III trial was that initiation of treatment of HF with the beta-1-selective beta-blocker bisoprolol (to which enalapril is subsequently added) is as effective and safe as a treatment regimen based on the initiation with the ACE inhibitor enalapril (to which bisoprolol is subsequently added). Thus, the primary endpoint of the study was showing that initiation of therapy with bisoprolol, followed by combination therapy with enalapril after 6 months, was comparable (non-inferior) to initiation of therapy with enalapril, followed by combination therapy with bisoprolol, after 6 months, with respect to the prevention of all-cause death and all-cause hospitalizations (Willenheimer et al 2005).
CIBIS-III was an investigator-initiated, multi-center, prospective, randomized, open-label, blinded endpoint evaluation trial with an independent steering committee, data safety monitoring board, masked endpoint committee and clinical trial data center. The study included patients aged ≥65 years, with mild to moderate HF (NYHA class II to III), low LVEF (≤35%), and stable clinical conditions in the previous ≥7 days. Patients were assigned to initiation of treatment with either bisoprolol, titrated at bi-weekly intervals, from 1.25 to a target dose of 10 mg/day, or enalapril, titrated from 5 mg/day to a target dose of 20 mg/day. After 6 months of monotherapy, combined administration of enalapril and bisoprolol was started and therapy was continued for an additional 6–18 months. As already pointed out, CIBIS-III was designed as a non-inferiority trial of bisoprolol first treatment vs enalapril first treatment (Willenheimer et al 2005).
CIBIS-III started in October 2002 and was terminated in May 2005 with the inclusion of 1010 patients from 128 centers from 20 different countries. Follow-up was completed for 445/505 patients in the bisoprolol-first group and 446/505 patients in the enalapril-first group. Mean follow-up duration was 1.22 ± 0.42 years. Mean patients’ age was 72.4 ± 5.8 years, 68.9% of patients were males, and mean LVEF was 28.8%.
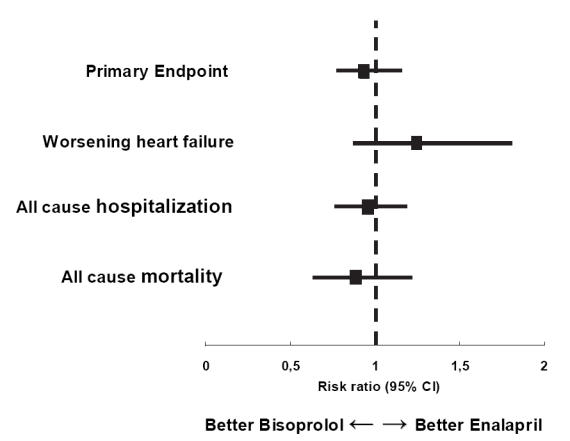
The trial was successful with respect to its primary endpoint, ie, bisoprolol-first was not inferior to enalapril-first treatment. In the intention-to-treat sample, the primary endpoint of all-cause deaths or hospitalizations occurred in 178 patients in the bisoprolol-first group and 186 patients in the enalapril-first group (absolute difference −1.6%, 95% CI, 7.6%–4.4%, HR 0.94; 95% CI 0.77–1.16; non-inferiority for bisoprolol-first vs enalapril-first treatment, p = 0.019). In the per-protocol sample, the primary endpoint was achieved in 163 patients in the bisoprolol-first group and 165 patients in enalapril-first group (absolute difference −0.7%, 95% CI −0.66 to 5.1%, HR 0.97%; 95% CI 0.78 to 1.21; non inferiority for bisoprolol-first vs enalapril-first treatment, p = 0.046).
There were 65 deaths in the bisoprolol-first group, as compared with 73 in the enalapril-first group (HR 0.88; 95% CI 0.63–1.22; between-group difference, p = 0.44), and cardiovascular deaths were not significantly different in the two groups (55 in bisoprolol-first treatment vs 56 in enalapril-first treatment (HR 0.97; 95% CI 0.67–1.40; between-group difference, p = 0.86).
One hundred and fifty-one patients in the bisoprolol-first group were hospitalized, as compared with 157 in the enalapril-first group (HR 0.95; 95% CI 0.76–1.19; between-group difference, p = 0.66). Sixty-three patients in the bisoprolol-first group and 51 in the enalapril-first group had a hospitalization for worsening HF (HR 1.25; 95% CI 0.87–1.81; between-group difference, p = 0.23). Other analyses regarded patients’ follow-up during either the mono-therapy phase (first 6 months) or the first year since randomization (Willenheimer et al 2005).
When the results obtained at the end of the monotherapy phase (first 6 months) were analyzed, 109 patients in the bisoprolol-first treatment vs 108 patients in the enalapril-first treatment group reached the primary endpoint (HR 1.02, 95% CI 0.78–1.33; p = 0.90); 23 vs 32 died (HR 0.72, 95% CI 0.42–1.24; p = 0.24); 99 vs 92 were hospitalized (HR 1.08, 95% CI 0.81–1.43; p = 0.59).
In a post hoc analysis of patients’ outcome during their first year from randomization, 155 in the bisoprolol-first group vs 165 patients in the enalapril-first group reached the primary endpoint (HR 0.94, 95% CI 0.76–1.17, p = 0.59) and 42 vs 60 patients died (HR 0.69, 95% CI 0.46–1.02; p = 0.065).
Another analysis regarded the effects of treatment on SCD rate. During the first 6 months of monotherapy, 8 of 23 deaths in the bisoprolol-first group were sudden, compared with 16 of 32 in the enalapril-first group (HR 0.50, p = 0.107). During the first year, 16 of 42 deaths in the bisoprolol-first group were sudden vs 29 of 60 deaths in the enalapril-first group, representing a significant 46% reduction (HR, 0.54, p = 0.049). The incidence of other causes of death was, in contrast, similar between the two treatment groups. Alongside the early improvement in SCD rates with bisoprolol treatment, there was an increase in early HF hospitalization rates. During the first 6 months, 39 patients in the bisoprolol-first group vs 25 patients in the enalapril-first group were hospitalized for worsening HF (Willenheimer 2006).
The two different therapeutic strategies were similar with respect to the adverse events. The two drugs had similar effects on blood pressure either during the mono-therapy phase or the phase of concomitant treatment. Bisoprolol, but not enalapril, decreased HR, with similar changes during the phase of associated therapy.
Subgroup analysis showed no interaction with any variable except for LVEF. Amongst the patients with LVEF <28% (median value), bisoprolol-first was significantly better then enalapril-first treatment (HR 0.61, 95% CI 0.44–0.85, p = 0.003) whereas an opposite trend was seen among the patients with LVEF ≥28% (HR 1.23, 95% CI 0.94–1.61, p = 0.13). This interaction was, however, caused mainly by differences in non-cardiovascular hospitalizations during monotherapy phase.
Differences between the two regimens may have been partially related to differences in the percentage of patients reaching target doses in the two arms of the trial. A higher percentage of patients who were started with bisoprolol reached the target dose of 10 mg/day, (69.1%) compared with 53.8% of the patients in the enalapril-first group. Similarly, target dose of 20 mg/day of enalapril was reached in 76.7% of the enalapril-first patients, compared with 67.4% of the patients in the bisoprolol-first group (Willenheimer et al 2005).
CIBIS III has shown that there is no difference with respect to efficacy and safety between initiation of treatment with either bisoprolol or enalapril in patients with NHYA class II or III HF and low LVEF. It also suggested a greater benefit on mortality and SCD by initiating treatment with bisoprolol, rather than enalapril. The main criticism to this trial is that it is based on a rather artificial design in which patients continue monotherapy for 6 months before receiving combined therapy. In clinical practice, patients either start both treatments simultaneously or, as recommended by guidelines (Hunt et al 2005; Swedberg et al 2005), start with the ACE inhibitor and then receive combined therapy after a short time (Cleland et al 2005; Dickstein 2006; Willenheimer et al 2006).
Tolerability
Controlled trials have shown that treatment with bisoprolol is well tolerated. In CIBIS trials, withdrawal rates for lack of tolerance to bisoprolol were similar to placebo. Contraindications to bisoprolol initiation are the same as with all other beta-blockers and are clearly summarized in guidelines. They include sinus bradycardia, atrio-ventricular block, bronchial asthma sensitive to beta-agonist administration (Hunt et al 2005; Swedberg et al 2005; McMurray et al 2005, Metra et al 1999). A recent episode of HF decompensation is a contraindication to the initiation of bisoprolol treatment, although recent data have shown that beta-blockers can be initiated during the same hospitalization caused by acute HF and this allows higher doses to be reached and better long-term outcome (Gattis et al 2004).
Bisoprolol treatment, as well as treatment with any other beta-blocker, still needs implementation in clinical practice. Beta-blocker therapy is still underused and underdosed. Galatius and colleagues showed as bisoprolol mean dose after two months attendance in patients with CHF was only 33% of target dose (3.1 ± 2.6 mg) and 41% at discharge compared with 27% of target dose (13.4 ± 14.0 mg) and 32% at discharge in carvedilol group. Thirty-nine and 40% of the bisoprolol and carvediolol treated patients, respectively, had stopped beta-blocker therapy at discharge and only a minority reached target dose (Galatius et al 2004).
Conclusions
The past few years have seen a revolution in our attitude towards beta-blockers in HF patients. Years ago, these agents were contraindicated. Now we know that they are associated with a highly significant reduction in mortality and hospitalization rates in patients with HF. This paradigm shift is the result of a better understanding of HF pathophysiology and of the results of randomized controlled clinical trials. Despite the continuous rise in their prescription rates, beta-blockers remain underused and underdosed. Education and further implementation of guidelines regarding beta-blocker therapy are therefore warranted.
Figure 1.
Primary and secondary endpoint in Cardiac Insufficiency BIsoprolol Study III.
References
- Baxter AJ, Spensley A, Hildreth, et al. Beta-blockers in older persons with HF: tolerability and impact on quality of life. Heart. 2002;88:611–14. doi: 10.1136/heart.88.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkov IuN, Skvortsov AA, Mareev VIu, et al. Clinical, hemodynamic and neuorohumoral effects of long-term therapy of patients with severe chronic heart failure with beta-adrenoblocker bisoprolol. Kardiologiia. 2003;43:10–12. [PubMed] [Google Scholar]
- Bolger AP, Al-Nasser F. Beta-blockers for chronic HF: surviving longer but feeling better? Int Cardiol. 2003;92:1–8. doi: 10.1016/s0167-5273(03)00050-0. [DOI] [PubMed] [Google Scholar]
- Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–69. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study: a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- Campbell DJ, Aggarwal A, Esler M, et al. Beta-blockers, angiotensin II, and ACE inhibitors in patients with heart failure. Lancet. 2001;358:1609–10. doi: 10.1016/S0140-6736(01)06660-0. [DOI] [PubMed] [Google Scholar]
- CIBIS Investigators and Committees 1994. A Randomized Trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS) Circulation. 90:1765–73. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Khand A, Clark A. The heart failure epidemic: exactly how big is it? Eur Heart J. 2001;22:623–6. doi: 10.1053/euhj.2000.2493. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Coletta AP, Lammiman M, et al. Clinical trials update from the European Society of Cardiology meeting 2005: CARE-HF extension study, ESSENTIAL, CIBIS-III, S-ICD, ISSUE-2, STRIDE-2, SOFA, IMAGINE, PREAMI, SIRIUS-II and ACTIVE. Eur J Heart Fail. 2005;7:1070–5. doi: 10.1016/j.ejheart.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Pimentel DR, et al. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–34. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- Copie X, Pousset F, Lechat P, et al. Effects of ²-blockade with bisoprolol on heart rate variability in advanced heart failure: Analysis of scatterplots or R-R intervals at selected heart rates. Am Heart J. 1996;132:369–75. doi: 10.1016/s0002-8703(96)90435-4. [DOI] [PubMed] [Google Scholar]
- Cowie MR, Mosterd A, Wood DA, et al. The epidemiology of heart failure. Eur Heart J. 1997;18:208–25. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- Dickstein K. Clinical trials update from the European Society of Cardiology Meeting 2005: CIBIS-III. Response to correspondence from R. Willenheimer et al. Eur J Heart Fail. 2006;8:221–2. doi: 10.1016/j.ejheart.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Dubach P, Myers J, Bonetti P, et al. Effects of bisoprolol fumarate on left ventricular size, function, and exercise capacity in patents with heart failure: analysis with magnetic resonance myocardial tagging. Am Heart J. 2002;143:676–83. doi: 10.1067/mhj.2002.121269. [DOI] [PubMed] [Google Scholar]
- Erdmann E, Lechat P, Verkenne P, et al. Results from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patients groups with chronic heart failure. Eur J Heart Fail. 2001;3:469–79. doi: 10.1016/s1388-9842(01)00174-x. [DOI] [PubMed] [Google Scholar]
- Francis GS, Benedict C, Johnstone, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–9. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- Galatius S, Gustafsson F, Atar D, et al. Tolerability of beta-blocker initiation and titration with bisoprolol and carvedilol in congestive HF- A Randomized comparison. Cardiology. 2004;102:160–5. doi: 10.1159/000080485. [DOI] [PubMed] [Google Scholar]
- Gattis WA, O’Connor CM, Gallup DS, et al. IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534–41. doi: 10.1016/j.jacc.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Hall SA, Cigarroa CG, Marcoux L, et al. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25:1154–61. doi: 10.1016/0735-1097(94)00543-y. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA. Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Ichihara S, Yamada Y, Ichihara G, et al. Attenuation of oxidative stress and cardiac dysfunction by bisoprolol in an animal model of dilated cardiomyopathy. Biochem Biophys Res Commun. 2006;350:105–13. doi: 10.1016/j.bbrc.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Lechat P, Escolano S, Golmard JL, et al. Prognostic value of bisoprolol-induced hemodynamic effects in heart failure during the Cardiac Insufficiency BIsoprolol Study (CIBIS) Circulation. 1997;96:2197–205. doi: 10.1161/01.cir.96.7.2197. [DOI] [PubMed] [Google Scholar]
- Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation. 2001;103:1428–33. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- Lechat P, Packer M, Chalon S, et al. Clinical effects of beta-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation. 1998;98:1184–91. doi: 10.1161/01.cir.98.12.1184. [DOI] [PubMed] [Google Scholar]
- Leier CV. Dismantling mandates in the treatment of heart failure. J Am Coll Cardiol. 2004;44:1831–3. doi: 10.1016/j.jacc.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Leizorovicz A, Lechat P, Cucherat M. Bisoprolol for treatment of chronic heart failure: A meta-analysis on individual data of two placebo-controlled studies-CIBIS and CIBIS II. Am Heart J. 2002;143:301–7. doi: 10.1067/mhj.2002.120768. [DOI] [PubMed] [Google Scholar]
- Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol. 2007;49:171–80. doi: 10.1016/j.jacc.2006.08.046. [DOI] [PubMed] [Google Scholar]
- Leopold G, Kutz K. Bisoprolol: pharmacokinetic profile. Rev Contemp Pharmacother. 1997;8:35–43. [Google Scholar]
- Lowes BD, Gilbert EM, Abraham WT, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–65. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- McMurray J, Cohen-Solal A, Dietz R, et al. Practical recommendations for the use of ACE inhibitors, beta-blockers, aldosterone antagonists and angiotensin receptor blockers in heart failure: putting guidelines into practice. Eur J Heart Fail. 2005;7:710–21. doi: 10.1016/j.ejheart.2005.07.002. [DOI] [PubMed] [Google Scholar]
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- Metra M, Dei Cas L, Cleland JG. Pharmacokinetic and pharmacodynamic characteristics of beta-blockers: When differences may matter. J Card Fail. 2006;12:177–81. doi: 10.1016/j.cardfail.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Metra M, Dei Cas L, Di Lenarda A, et al. Beta-blockers in heart failure: are pharmacological differences clinically important? Heart Fail Rev. 2004;9:123–30. doi: 10.1023/B:HREV.0000046367.99002.a4. [DOI] [PubMed] [Google Scholar]
- Metra M, Giubbini R, Nodari S, et al. Differential effects of ²-blockers in patients with heart failure. A prospective, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000a;102:546–551. doi: 10.1161/01.cir.102.5.546. [DOI] [PubMed] [Google Scholar]
- Metra M, Nardi M, Giubbini R, et al. Effects of short- and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994;24:1678–87. doi: 10.1016/0735-1097(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Metra M, Nodari S, Bordonali T, et al. Beta-blocker therapy of heart failure: an update. Expert Opin Pharmacother. 2007;8:289–98. doi: 10.1517/14656566.8.3.289. [DOI] [PubMed] [Google Scholar]
- Metra M, Nodari S, D’Aloia A, et al. Beta blockers in heart failure: issues in the management of individual patients. Heart Fail Rev. 1999;4:65–77. [Google Scholar]
- Metra M, Nodari S, D’Aloia A, et al. Effects of neurohormonal antagonism on symptoms and quality of life in heart failure. Eur Heart J. 1998;19(Suppl B):B25–35. [PubMed] [Google Scholar]
- Metra M, Nodari S, D’Aloia A, et al. A rationale for the use of beta-blockers as standard treatment for heart failure. Am Heart J. 2000b;139:511–21. doi: 10.1016/s0002-8703(00)90096-6. [DOI] [PubMed] [Google Scholar]
- Metra M, Nodari S, Parrinello G, et al. Marked improvement in left ventricular ejection fraction during long-term beta-blockade in patients with chronic heart failure: clinical correlates and prognostic significance. Am Heart J. 2003;145:292–9. doi: 10.1067/mhj.2003.105. [DOI] [PubMed] [Google Scholar]
- Metra M, Torp-Pedersen C, Swedberg K, et al. Influence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J. 2005;26:2259–68. doi: 10.1093/eurheartj/ehi386. [DOI] [PubMed] [Google Scholar]
- Nyolczas N, Dékàny M, Fiòk J, et al. Prediction of the effect of bisoprolol in dilated cardiomyopathy. Cardiovasc Drugs Ther. 2000;14:543–50. doi: 10.1023/a:1007849409096. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Hamada M, Hiasa G, et al. Effect of beta-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:412–7. doi: 10.1016/s0735-1097(00)01121-9. [DOI] [PubMed] [Google Scholar]
- Packer M, Coats A, Fowler M, et al. Effect of carvedilol on survival in severe chronic heart failure (COPERNICUS trial) N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- Pousset F, Copie X, Lechat P, et al. Effects of bisoprolol on heart variability in heart failure. Am J Cardiol. 1996;7:612–7. doi: 10.1016/s0002-9149(97)89316-2. [DOI] [PubMed] [Google Scholar]
- Remme WJ, Riegger G, Hildebrandt P, et al. The benefits of early combination treatment of carvedilol and an ACE-inhibitor in mild heart failure and left ventricular systolic dysfunction. The carvedilol and ACE-inhibitor remodelling mild heart failure evaluation trial (CARMEN) Cardiovasc Drugs Ther. 2004;18:57–66. doi: 10.1023/B:CARD.0000025756.32499.6f. [DOI] [PubMed] [Google Scholar]
- Remme WH, Torp-Pedersen C, Cleland JGF, et al. Carvedilol protects better against vascular events than metoprolol in heart failure – Results from COMET. J Am Coll Cardiol. 2007;49:963–71. doi: 10.1016/j.jacc.2006.10.059. [DOI] [PubMed] [Google Scholar]
- Simon T, Mary-Krause M, Funch-Brentano C. Bisoprolol dose-response relationship in patients with congestive heart failure: a subgroup analysis in the cardiac insufficiency bisoprolol study (CIBIS II) Eur Heart J. 2003;24:552–9. doi: 10.1016/s0195-668x(02)00743-1. [DOI] [PubMed] [Google Scholar]
- Sirak TE, Jelic S, Le Jemtel TH. Therapeutic update: non-selective beta- and alpha-adrenergic blockade in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol. 2004;44:497–502. doi: 10.1016/j.jacc.2004.03.063. [DOI] [PubMed] [Google Scholar]
- Sliwa K, Norton GR, Kone N, et al. Impact of initiating carvedilol before angiotensin-converting enzyme inhibitor therapy on cardiac function in newly diagnosed heart failure. J Am Coll Cardiol. 2004;44:1825–30. doi: 10.1016/j.jacc.2004.05.087. [DOI] [PubMed] [Google Scholar]
- Stewart S, MacIntyre K, Hole DJ, et al. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–22. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Cleland J, Dargie H, et al. Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005) Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- The CAPRICORN Investigators. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- von Haehling S, Genth-Zotz S, Bolger AP, et al. Effect of noradrenaline and isoproterenol on lipopolysaccharide-induced tumor necrosis factor-alpha production in whole blood from patients with chronic heart failure and the role of beta-adrenergic receptors. Am J Cardiol. 2005;95:885–9. doi: 10.1016/j.amjcard.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Waagstein F, Bristow MR, Swedberg K, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet. 1993;342:1441–46. doi: 10.1016/0140-6736(93)92930-r. [DOI] [PubMed] [Google Scholar]
- Willenheimer R, Van Veldhuisen DJ, Silke B, et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence. Results of the Randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005;112:2426–35. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]
- Willenheimer R, Krum H, van Veldhuisen DJ, et al. Comment on “Clinical trials update from the European Society of Cardiology meeting 2005: CIBIS-III, by JGF Cleland and others”. Eur J Heart Fail. 2006;8:219–20. doi: 10.1016/j.ejheart.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Willenheimer R. Hotline session at the World Congress of Cardiology/European Congress of Cardiology) in Barcelona. 2006 [Google Scholar]


