Abstract
Acne is a disease of the pilosebaceous unit with involving abnormalities in sebum production, microbial flora changes, abnormal keratinization, and inflammation. There are several therapeutic options like topical and systemic retinoids, antibiotics, and systemic hormonal drugs. The topical retinoids a play very important role in the treatment of acne vulgaris. However, their use is limited due to skin irritation. A new generation product, adapalene is a good choice in the treatment of acne vulgaris with less side effects and high efficacy confirmed by numerous clinical studies.
Keywords: adapalene, acne vulgaris, treatment
Introduction
Acne vulgaris is a chronic, inflammatory disease of the pilosebaceous unit, that affects seborrhoeic areas like face, back, and chest and characterized by comedones, papules, pustules, nodules, cysts, and scars. Almost every individual has some degree of acne during puberty with spontaneous resolution occurring in early adult life. Occasionally, the disease persists into the fourth decade or even remains a lifelong problem. Because of the involvement of the face with considerable cosmetic problems, acne is a major psychosocial problem for many teenagers and young adults (Cunliffe and Simpson 1998; Strauss and Thiboutot 1999; Braun-Falco et al 2001).
The pathogenesis of acne
In the pathogenesis of acne, the most important site is pilosebaceous unit which consists of a hair follicle and several sebaceous glands. These units are found everywhere on the body except the palms and soles. Pilosebaceous density is greatest on the face, upper neck, and chest, in roughly nine times the concentration found elsewhere on the body (Leyden 1995; Habif and Habie 1996).
There are four main interacting factors in the pathogenesis of acne vulgaris:
a) Increased sebum production,
b) Microbial flora changes,
c) Abnormal keratinization,
d) Inflammation (Strasburger 1997; Cunliffe and Simpson 1998; Braun-Falco et al 2001; Korkut and Piskin 2005).
To be able to treat acne, these factors should be targeted. The aim is to reduce or eliminate the primary clinical lesion, microcomedone, which is the precursor of almost all other acne lesions (Cunliffe et al 2003). There are a lot of topical or systemic agents for this purpose.
Treatment
The treatment of acne vulgaris is not curative. The purpose is to reduce discomfort due to inflamed lesions, to improve the appearance, and to prevent scars. Acne management is a long-term treatment and requires patience. The patient should be informed on the issue (Cunliffe and Simpson 1998; Oberomok and Shalita 2002).
Topical preparations constitute the sole treatment in many patients with acne vulgaris and are a part of therapeutic regimen in almost all patients. Topical treatment is enough for comedonal acne. In case of more severe acne, topical treatment can be combined with systemic treatment (Cunliffe and Simpson 1998).
Topical treatment of acne vulgaris has changed over the years. Agents containing sulphur or resorcinol were used in especially first part of 20th century. Salicylic acid which is a keratolytic agent was popular in some time. Nowadays, the most popular topical agents were retinoids, benzoyl peroxide, azelaic acid, and topical antibiotics (Bergfeld 1998).
Topical retinoids
Topical retinoids, derivatives of vitamin A have been used to treat acne for almost three decades. They are the most effective comedolytic agents for the treatment of acne vulgaris by normalizing or even increasing the desquamation process, thereby decreasing the formation and the number of microcomedones. They also promote the clearing of preexisting comedones (Bergfel 1998) and decrease in papulopustular lesions (Ellis et al 1998; Thiboutot et al 2001; Bershad et al 2002). In addition, they have a marked anti-inflammatory effect by inhibiting the activity of leukocytes, the release of pro-inflammatory cytokines and other mediators, and the expression of transcription factors and toll-like receptors involved in immunomodulation. They also help penetration of other active agents. Thus, they should be utilized in nearly every patient with acne and are the preferred agents in maintenance therapy (James et al 2000).
Until recently, tretinoin, which is the active form of a metabolic product of vitamin A, was the only available topical retinoid (Leyden 1998). However, its use has been limited by local irritation after initiation of therapy. This side effect is a minimal problem with the third generation topical retinoids, such as adapalane. Tretinoin is available in a new delivery system (Retin-A Micro) to decrease the irritative effects. The purpose in this delivery system is to provide the drug directly to the follicle by entrapping it in microspheres (Skov et al 1997).
Adapalene
Adapalene is a synthetic naphthoic acid derivative with retinoid activity. The chemical name of adapalene is 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid. Adapalene is a white to off-white powder which is soluble in tetrahydrofuran, sparingly soluble in ethanol, and practically insoluble in water. The molecular formula is C28H28O3 and molecular weight is 412.52. Adapalene is represented by the structural formula represented on Figure 1.
Figure 1.
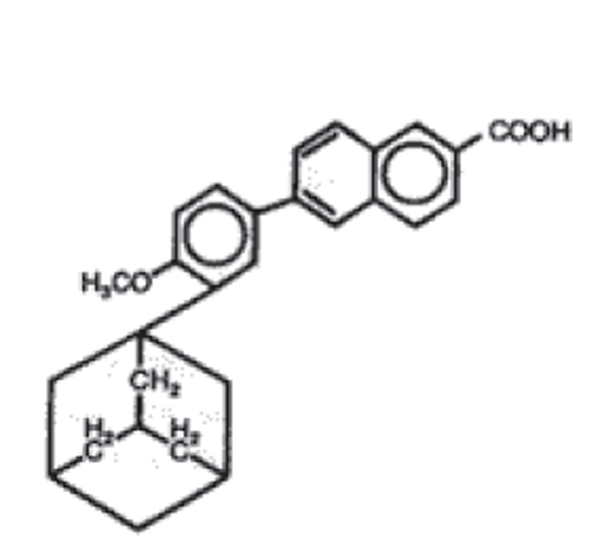
Structural formula of adapalene.
Some of its biologic activities are the same with tretinoin, however it is chemically more stable and lipophilic. By this way, it can reach higher concentrations in pilosebaceous unit. In addition, it has higher affinity towards retinoic acid receptor (RAR) β and γ unlike tretinoin. It is important because epithelial cells have mainly RAR γ. Then, RAR-adapalene complex binds retinoid X receptor (RXR) and this regulates gene transcription by binding specific DNA sites (Leyden 1998; Czernielewski et al 2001). Adapalene modulates cellular keratinization and inflammatory process. This anti-inflammatory effect is due to inhibition of the lipooxygenase activity and also to oxidative metabolism of arachidonic acid. These mechanisms may be the reason for decreased risk of irritation with adapalene. Adapalene has a very low percutaneous absorption once the drug has penetrated the stratum corneum, so that it becomes entrapped in the epidermis and hair follicle, which are targeted areas (Millikan 2000).
Absorption of adapalene through human skin is low. Only trace amounts (0.25 ng/ml) of parent substance have been found in the plasma of acne patients following chronic topical application of adapalene in controlled trials. Excretion appears to be primarily by the biliary route. Erythema, peeling, dryness and burning are the most frequent encountered side effects.
Clinical studies
Over the past five years, numerous clinical trials have been conducted on comparing the efficacy and tolerability of adapalene and tretinoin in the treatment of acne vulgaris. A meta-analysis of five large studies with more than 900 patients over 12 weeks demonstrated that adapalene 0.1% gel is as effective as tretinoin 0.025% gel (Cunliffe et al 1998). After 12 weeks, both agents were equally effective but adapalene had a faster onset of action and less irritation. However, the comparison of adapalene 0.1% gel and tretinoin 0.1% microsphere gel in a double-blind study demonstrated more rapid comedone reduction with the tretinoin gel than with adapalene, but again, there was less irritation in patients using adapalene (Nyirady et al 2001). Grosshans et al (1998) compared 0.1% adapalene and 0.025% tretinoin on 105 patients for 3 months and Ellis et al (1998) compared 0.1% adapalene and 0.025% tretinoin on 297 patients for 3 months. In both of these studies, there was no difference between these drugs in terms of efficacy. In another study, Cunliffe et al (1997) compared 0.1% adapalene and 0.025% tretinoin on 323 patients for 3 months. They found that adapalene caused more decrease in total and noninflammatory lesions than tretinoin. However, there was no significant difference in terms of inflammatory lesions. Korkut and Piskin (2005) demonstrated that adapalane is more effective in noninflammatory lesions than inflammatory lesions.
Adapalene 0.1% gel has been studied in 80 patients against isotretinoin 0.05% gel, which is the cis-isomer of retinoic acid, to compare their effectiveness and tolerance by Ioannides et al (2002). Both lesion counts and global assessment showed a better degree of efficacy with adapalene than isotretinoin, although the difference between two drugs was not significant. Although isotretinoin is less irritating than tretinoin, adapalene is significantly less irritating than isotretinoin.
In the study comparing tazarotene applied every other day and adapalene applied daily by Guenther (2003), both drugs had comparable efficacy and tolerability. Dosik et al (2005) performed a study to compare the ability of epidermis to tolerate adapalene 0.1% cream and gel and tazarotene 0.05% and 0.1% creams on 26 subjects for a period of three weeks. The mean 21-day cumulative irritancy indices for adapalene 0.1% cream and gel were significantly lower than those for tazarotene 0.05% and 0.1% creams and not notably higher than that of negative control.
A multicenter, randomized, double-blind study by Thiboutot et al (2006a) on 653 patients demonstrated that adapalene 0.3% gel was significantly superior to adapalene 0.1% gel and well-tolerated. In another study, the efficacy and safety of adapalene 0.3% gel were compared with adapalene 0.1% gel and vehicle on 214 subjects for 12 weeks. The results of this study demonstrated that adapalene gel 0.3% was superior to adapalene 0.1% gel and vehicle in moderate to moderately severe acne while retaining a similar study and tolerability profile to adapalene 0.1% gel (Pariser et al 2005).
Benzoyl peroxide and adapalene are among the most effective topical agents used in the treatment of acne vulgaris. Despite the fact that there are a lot of studies with benzoyl peroxide and adapalene alone, there are only a few studies comparing these two drugs. do Nascimento et al (2003) compared the efficacy and safety of benzoyl peroxide 4% gel used twice daily with adapalene 0.1% gel used once daily on 178 patients for 11 weeks. They found benzoyl peroxide more effective than adapalane on noninflammatory and inflammatory lesions at weeks 2 and 5, and they found both drugs safe. Korkut and Piskin (2005) have compared the efficacy and safety of 5% benzoyl peroxide, 0.1% adapalene, and their combination. The study revealed that all three therapeutic protocols were effective in treating noninflammatory and inflammatory lesions and that there were no significant difference between the groups in terms of efficacy or side effects. Adapalene and benzoyl peroxide are effective and well tolerated agents for acne vulgaris; combination therapy has no superiority over adapalene or benzoyl peroxide alone. There are a few studies that compare the side effects of benzoyl peroxide and adapalene. Brand et al (2003) demonstrated that 0.1% adapalene and 5% benzoyl peroxide combination was safe and well-tolerated.
Thiboutot et al (2005) compared the efficacy and safety of the combination of adapalene 0.1% gel and doxycycline with doxycycline alone for severe acne vulgaris. This study demonstrated that the combination of adapalene and an oral antibiotic provide a superior and faster benefit than antibiotic alone and should be considered in the initiation treatment.
Adapalene is also useful in maintenance therapy. Thiboutot et al (2006b) performed a study on 253 subjects to assess the maintenance effect of adapalene 0.1% gel and gel vehicle in subjects successfully treated in a previous 12 week study of adapalene-doxycycline combination. The study demonstrated a clinical benefit of continued treatment with adapalene 0.1% gel as a maintenance therapy. In another study by Zhang et al (2004), a total of 300 acne subjects entered the multicentre, randomized, investigator-blinded study comparing the efficacy and safety of adapalene 0.1% gel plus clindamycin 1% solution versus clindamycin 1% solution alone. In the second part of the study (weeks 12–24) completed by 241 subjects, the efficacy and safety of adapalene 0.1% gel alone as a maintenance therapy were investigated. This study confirmed the importance of a maintenance therapy after a successful initial treatment and underlined the benefit of a combination therapy with a topical retinoid such as adapalane and a topical antibiotic in the treatment of inflammatory acne.
Adapalene treatment has a theoretical risk for retinoid embryopathy. However, manufacturer reports that only trace amounts of adapalene are absorbed into the skin. In the manufacturer’s studies on pregnant animals using doses 120–150 times the human topical dose did not show an increased risk of adverse outcome or malformations. There have not been performed human studies to date, so the risk is undetermined for adapalene usage in pregnancy. However, because only trace amounts of the drug absorb into skin, it seems unlikely the drug induces malformations.
In summary, numerous clinical studies demonstrating that adapalene treatment is a good choice for topical treatment of acne vulgaris with less side effects and high efficacy.
References
- Bergfeld WF. The evolving role of retinoids in the management of cutaneous conditions. Clinician. 1998;16:1–32. [Google Scholar]
- Bershad S, Kranjac Singer GK, Parente JE, et al. Successful treatment of acne vulgaris using a new method: results of a randomized vehicle-controlled trial of short-contact therapy with 0.1% tazarotene gel. Arch Dermatol. 2002;138:481–9. doi: 10.1001/archderm.138.4.481. [DOI] [PubMed] [Google Scholar]
- Brand B, Gilbert R, Baker MD, et al. Cumulative irritancy comparision of adapalene gel 0.1% versus other retinoid products when applied in combination with topical antimicrobial agents. J Am Acad Dermatol. 2003;49:S227–32. doi: 10.1067/s0190-9622(03)01151-4. [DOI] [PubMed] [Google Scholar]
- Braun-Falco O, Plewig G, Wolff HH, et al. Dermatology. 2. Berlin: Springer-Verlag; 2001. [Google Scholar]
- Cunliffe WJ, Caputo R, Dreno B, et al. Clinical efficacy and safety comparision of adapalene gel and tretinoin gel in the treatment of acne vulgaris: Europe and U.S. multicenter trials. J Am Acad Dermatol. 1997;36:S126–34. doi: 10.1016/s0190-9622(97)70056-2. [DOI] [PubMed] [Google Scholar]
- Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and theurapeutic strategies. Dermatology. 2003;206:11–6. doi: 10.1159/000067825. [DOI] [PubMed] [Google Scholar]
- Cunliffe WJ, Poncet M, Loesche C, et al. A comparison of the efficacy and tolerability of adapalene 0.1% gel versus tretinoin 0.025% gel in patients with acne vulgaris: a meta-analysis of five randomized trials. Br J Dermatol. 1998;139(Suppl 52):48–56. doi: 10.1046/j.1365-2133.1998.1390s2048.x. [DOI] [PubMed] [Google Scholar]
- Cunliffe WJ, Simpson NB. Disorders of sebaceous glands. In: Champion RH, Burton JL, Burns DA, Brethnach SM, editors. Rook/Wilkinson/Ebling Textbook of dermatology. 6. Milan: Blackwell Science Ltd; 1998. pp. 1927–84. [Google Scholar]
- Czernielewski J, Michel S, Bouclier M, et al. Adapalene biochemistry and the evaluation of a new topical retinoid for treatment of acne. J Eur Acad Dermatol Venereol. 2001;15(Suppl 3):5–12. doi: 10.1046/j.0926-9959.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- do Nascimento LV, Guedes ACM, Magalhães GM, et al. Single-blind comparative clinical study of the efficacy and safety of benzoyl peroxide 4% gel (BID) and adapalene 0.1% gel (QD) in the treatment of acne vulgaris for 11 weeks. J Dermatol Treat. 2003;14:166–71. doi: 10.1080/09546630310007088. [DOI] [PubMed] [Google Scholar]
- Dosik JS, Homer K, Arsonnaud S. Cumulative irritation potential of adapalene 0.1% cream and gel compared with tazarotene cream 0.05% and 0.1% Cutis. 2005;75:289–93. [PubMed] [Google Scholar]
- Ellis CN, Millikan LE, Smith EB, et al. Comparision of adalapene 0.1% solution and tretinoin 0.025% gel in topical treatment of acne vulgaris. Br J Dermatol. 1998;139(Suppl 52):41–7. doi: 10.1046/j.1365-2133.1998.1390s2041.x. [DOI] [PubMed] [Google Scholar]
- Grosshans E, Marks R, Mascaro JM, et al. Evaluation of clinical efficacy and safety of adapalene 0.1% gel versus tretinoin 0.025% gel in the treatment of acne vulgaris, with particular reference to the onset of action and impact on quality of life. Br J Dermatol. 1998;139(Suppl 52):26–33. doi: 10.1046/j.1365-2133.1998.1390s2026.x. [DOI] [PubMed] [Google Scholar]
- Guenther LC. Optimizing treatment with topical tazarotene. Am J Clin Dermatol. 2003;4:197–202. doi: 10.2165/00128071-200304030-00006. [DOI] [PubMed] [Google Scholar]
- Habif TP, Habie TP. Clinical dermatology: A color guide to diagnosis and therapy. Philadelphia: Mosby Co; 1996. [Google Scholar]
- Ioannides D, Rigopoulos D, Katsambas A. Topical adapalene gel 0.1% vs. isotretinoin gel 0.05% in the treatment of acne vulgaris: a randomized open-label clinical study. Br J Dermatol. 2002;147:523–27. doi: 10.1046/j.1365-2133.2002.04873.x. [DOI] [PubMed] [Google Scholar]
- James WD, Berger TG, Elston DM. Acne. Andrews’ diseases of the skin Clinical Dermatology. 10. Philadelphia: WB Saunders Company; 2000. [Google Scholar]
- Kligman AM. The growing importance of topical retinoids in clinical dermatology: a retrospective and prospective analysis. J Am Acad Dermatol. 1998;39:S2–7. doi: 10.1016/s0190-9622(98)70437-2. [DOI] [PubMed] [Google Scholar]
- Korkut C, Piskin S. Benzoyl peroxide, adapalene, and their combination in the treatment of acne vulgaris. J Dermatol. 2005;32:169–73. doi: 10.1111/j.1346-8138.2005.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Leyden JJ. New understandings of the pathogenesis of acne. J Am Acad Dermatol. 1995;32:S15–25. doi: 10.1016/0190-9622(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Leyden JJ. Topical treatment of acne vulgaris: retinoids and cutaneous irritation. J Am Acad Dermatol. 1998;38:S1–4. doi: 10.1016/s0190-9622(98)70138-0. [DOI] [PubMed] [Google Scholar]
- Millikan LE. Adapalene: an update on newer comparative studies between the various retinoids. Int J Dermatol. 2000;39:784–8. doi: 10.1046/j.1365-4362.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Nyirady J, Grossman RM, Nighland M, et al. A comparative trial of two retinoids commonly used in the treatment of acne vulgaris. J Dermatol Treat. 2001;12:149–57. doi: 10.1080/09546630152607880. [DOI] [PubMed] [Google Scholar]
- Oberemok SS, Shalita AR. Acne vulgaris, II: treatment. Cutis. 2002;70:111–4. [PubMed] [Google Scholar]
- Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: A randomized, multicenter, investigator-blinded, controlled comparision study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76:145–51. [PubMed] [Google Scholar]
- Skov MJ, Quigley JW, Bucks DA. Topical delivery system for tretinoin: research and clinical implications. J Pharm Sci. 1997;86:1138–43. doi: 10.1021/js9604568. [DOI] [PubMed] [Google Scholar]
- Strasburger VC. Acne. What every pediatrician should know about treatment? Pediatr Clin North Am. 1997;44:1505–23. doi: 10.1016/s0031-3955(05)70571-x. [DOI] [PubMed] [Google Scholar]
- Strauss JS, Thiboutot DM. Diseases of sebaceous glands. In: Freedberg MI, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, Fitzpatrick TB, editors. Dermatology in general medicine. 5. New York: McGraw Hill Co; 1999. pp. 769–84. [Google Scholar]
- Thiboutot D, Gold MH, Jarratt MT, et al. Randomized controlled trial of the tolerability, safety, and efficacy of adapalene gel 0.1% and tretinoin microsphere gel 0.1% for the treatment of acne vulgaris. Cutis. 2001;68(4 Suppl):10–9. [PubMed] [Google Scholar]
- Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: A multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006a;54:242–50. doi: 10.1016/j.jaad.2004.10.879. [DOI] [PubMed] [Google Scholar]
- Thiboutot DM, Shalita AR, Yamauchi PS, et al. Adapalene gel, 0.1%, as maintenance therapy for acne vulgaris; a randomized, controlled, investigator-blind follow-up of a recent combination study. Arch Dermatol. 2006b;142:597–602. doi: 10.1001/archderm.142.5.597. [DOI] [PubMed] [Google Scholar]
- Thiboutot DM, Shalita AR, Yamauchi PS, et al. Combination therapy with adapalene gel 0.1% and doxycycline for severe acne vulgaris: a multicenter, investigator-blind, randomized, controlled study. Skinmed. 2005;4:138–46. doi: 10.1111/j.1540-9740.2005.04279.x. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Li LF, Tu YT, et al. A successful maintenance approach in inflammatory acne with adapalane gel 0.1% after an initial treatment in combination with clindamycin topical solution 1% or after monotherapy with clindamycin topical solution 1% J Dermatol Treat. 2004;15:372–8. doi: 10.1080/09546630410021702. [DOI] [PubMed] [Google Scholar]


