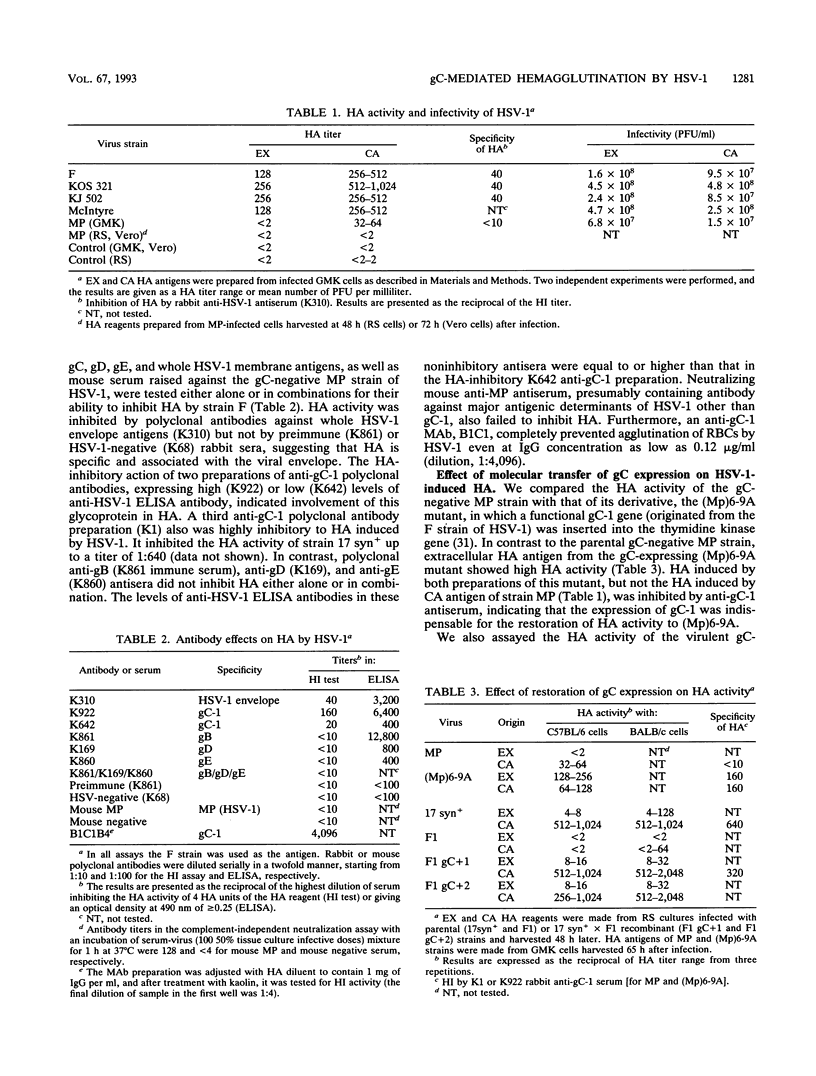
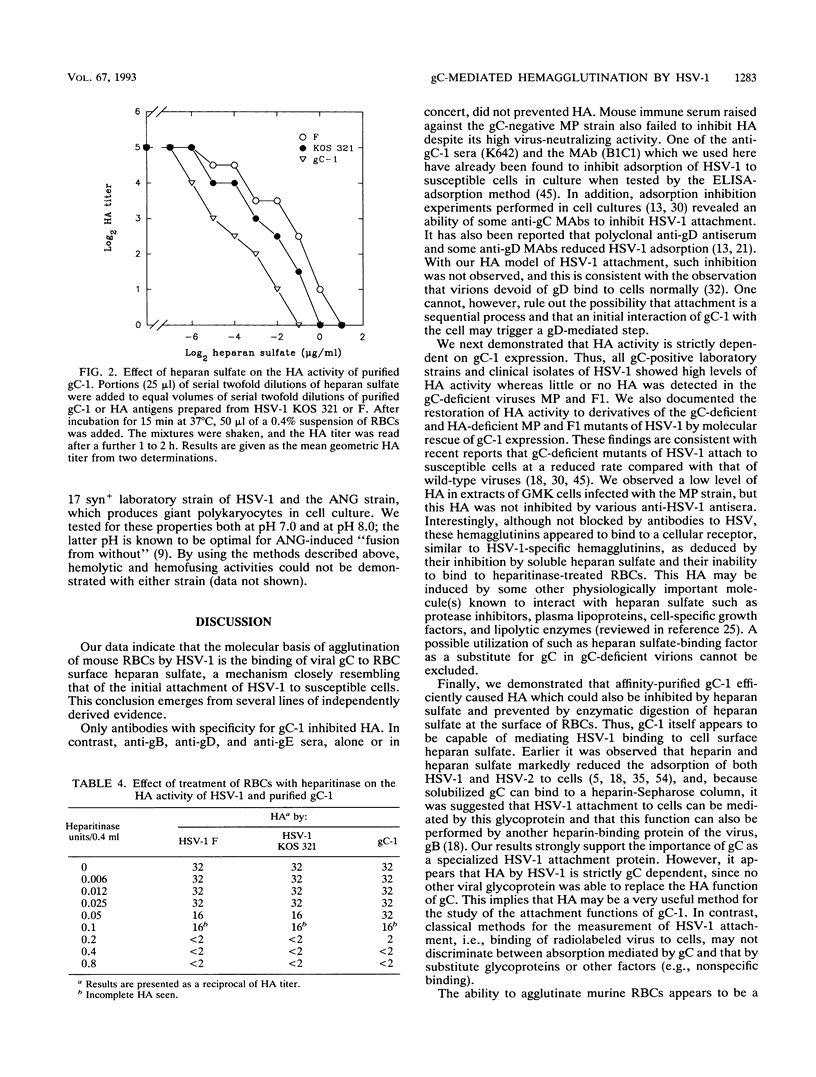
Abstract
We recently reported that herpes simplex virus type 1 (HSV-1) can cause agglutination of murine erythrocytes (E. Trybala, Z. Larski, and J. Wisniewski, Arch. Virol. 113:89-94, 1990). We now demonstrate that the mechanism of this hemagglutination is glycoprotein C-mediated binding of virus to heparan sulfate moieties at the surface of erythrocytes. Hemagglutination was found to be a common property of all gC-expressing laboratory strains and clinical isolates of HSV-1 tested. Mutants of HSV-1 deficient in glycoprotein C caused no specific hemagglutination, whereas their derivatives transfected with a functional gC-1 gene, thus reconstituting gC expression, regained full hemagglutinating activity. Hemagglutination activity was inhibited by antibodies against gC-1 but not by antibodies with specificity for glycoproteins gB, gD, or gE or by murine antiserum raised against the MP strain of HSV-1, which is gC deficient. Finally, purified gC-1 protein, like whole HSV-1 virions, showed high hemagglutinating activity which was inhibited by heparan sulfate and/or heparin and was completely prevented by pretreatment of erythrocytes with heparitinase, providing evidence that gC-1 mediates hemagglutination by binding to heparan sulfate at the cell surface. Thus, HSV-1-induced hemagglutination is gC-1 dependent and resembles the recently proposed mechanism by which HSV-1 attaches to surface heparans on susceptible cells, providing a simple model for initial events in the virus-cell interaction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström T., Sjögren-Jansson E., Jeansson S., Lycke E. Mapping neuroinvasiveness of the herpes simplex virus type 1 encephalitis-inducing strain 2762 by the use of monoclonal antibodies. Mol Cell Probes. 1992 Feb;6(1):41–49. doi: 10.1016/0890-8508(92)90070-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström T., Vahlne A., Alestig K., Jeansson S., Forsgren M., Lycke E. Primary and recurrent herpes simplex virus type 2-induced meningitis. J Infect Dis. 1990 Aug;162(2):322–330. doi: 10.1093/infdis/162.2.322. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Stirpe D., Boscaro A., Avitabile E., Foá-Tomasi L., Barker D., Roizman B. Glycoprotein C-dependent attachment of herpes simplex virus to susceptible cells leading to productive infection. Virology. 1990 Sep;178(1):213–222. doi: 10.1016/0042-6822(90)90396-9. [DOI] [PubMed] [Google Scholar]
- Draper K. G., Costa R. H., Lee G. T., Spear P. G., Wagner E. K. Molecular basis of the glycoprotein-C-negative phenotype of herpes simplex virus type 1 macroplaque strain. J Virol. 1984 Sep;51(3):578–585. doi: 10.1128/jvi.51.3.578-585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Falke D., Knoblich A., Müller S. Fusion from without induced by herpes simplex virus type 1. Intervirology. 1985;24(4):211–219. doi: 10.1159/000149645. [DOI] [PubMed] [Google Scholar]
- Forrester A., Farrell H., Wilkinson G., Kaye J., Davis-Poynter N., Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992 Jan;66(1):341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. L., Cook M. L., Sederati F., Izumi K., Stevens J. G. Identification, transfer, and characterization of cloned herpes simplex virus invasiveness regions. J Virol. 1989 Mar;63(3):1153–1161. doi: 10.1128/jvi.63.3.1153-1161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Herold B. C., WuDunn D., Soltys N., Spear P. G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991 Mar;65(3):1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka Y., Sakai Y., Toh Y., Mori R. Glycoprotein C of herpes simplex virus type 1 is essential for the virus to evade antibody-independent complement-mediated virus inactivation and lysis of virus-infected cells. J Gen Virol. 1991 Apr;72(Pt 4):915–921. doi: 10.1099/0022-1317-72-4-915. [DOI] [PubMed] [Google Scholar]
- Highlander S. L., Cai W. H., Person S., Levine M., Glorioso J. C. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988 Jun;62(6):1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Marlin S. D., Levine M., Glorioso J. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J Virol. 1983 Feb;45(2):672–682. doi: 10.1128/jvi.45.2.672-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Sandri-Goldin R. M., Holland L. E., Marlin S. D., Levine M., Glorioso J. C. Physical mapping of the mutation in an antigenic variant of herpes simplex virus type 1 by use of an immunoreactive plaque assay. J Virol. 1983 May;46(2):649–652. doi: 10.1128/jvi.46.2.649-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Busch S. J., Cardin A. D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991 Apr;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Jeansson S., Elwing H., Nygren H., Olofsson S. Evaluation of solubilized herpes simplex virus membrane antigens in diffusion in gel-enzyme-linked immunosorbent assay (DIG-ELISA). J Virol Methods. 1982 Apr;4(3):167–176. doi: 10.1016/0166-0934(82)90045-3. [DOI] [PubMed] [Google Scholar]
- Jeansson S., Forsgren M., Svennerholm B. Evaluation of solubilized herpes simplex virus membrane antigen by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Nov;18(5):1160–1166. doi: 10.1128/jcm.18.5.1160-1166.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaerner H. C., Schröder C. H., Ott-Hartmann A., Kümel G., Kirchner H. Genetic variability of herpes simplex virus: development of a pathogenic variant during passaging of a nonpathogenic herpes simplex virus type 1 virus strain in mouse brain. J Virol. 1983 Apr;46(1):83–93. doi: 10.1128/jvi.46.1.83-93.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn J. E., Kramer M. D., Willenbacher W., Wieland U., Lorentzen E. U., Braun R. W. Identification of herpes simplex virus type 1 glycoproteins interacting with the cell surface. J Virol. 1990 Jun;64(6):2491–2497. doi: 10.1128/jvi.64.6.2491-2497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langeland N., Oyan A. M., Marsden H. S., Cross A., Glorioso J. C., Moore L. J., Haarr L. Localization on the herpes simplex virus type 1 genome of a region encoding proteins involved in adsorption to the cellular receptor. J Virol. 1990 Mar;64(3):1271–1277. doi: 10.1128/jvi.64.3.1271-1277.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. T., Pogue-Geile K. L., Pereira L., Spear P. G. Expression of herpes simplex virus glycoprotein C from a DNA fragment inserted into the thymidine kinase gene of this virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6612–6616. doi: 10.1073/pnas.79.21.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström M., Olofsson S., Jeansson S., Lycke E., Datema R., Månsson J. E. Host cell-induced differences in O-glycosylation of herpes simplex virus gC-1. I. Structures of nonsialylated HPA- and PNA-binding carbohydrates. Virology. 1987 Dec;161(2):385–394. doi: 10.1016/0042-6822(87)90131-0. [DOI] [PubMed] [Google Scholar]
- Lycke E., Johansson M., Svennerholm B., Lindahl U. Binding of herpes simplex virus to cellular heparan sulphate, an initial step in the adsorption process. J Gen Virol. 1991 May;72(Pt 5):1131–1137. doi: 10.1099/0022-1317-72-5-1131. [DOI] [PubMed] [Google Scholar]
- Morein B., Simons K. Subunit vaccines against enveloped viruses: virosomes, micelles and other protein complexes. Vaccine. 1985 Jun;3(2):83–93. doi: 10.1016/0264-410x(85)90055-6. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk K., Ludwig G. Properties of plaque variants of herpes virus hominis strains of genital origin. Arch Gesamte Virusforsch. 1972;37(4):308–315. doi: 10.1007/BF01241453. [DOI] [PubMed] [Google Scholar]
- Noda M., Miura K., Yamanaka K., Inaba Y. Hemagglutination with avian infectious laryngotracheitis virus. Arch Virol. 1990;114(1-2):137–142. doi: 10.1007/BF01311017. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Sjöblom I., Glorioso J. C., Jeansson S., Datema R. Selective induction of discrete epitopes of herpes simplex virus type 1-specified glycoprotein C by interference with terminal steps in glycosylation. J Gen Virol. 1991 Aug;72(Pt 8):1959–1965. doi: 10.1099/0022-1317-72-8-1959. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Sjöblom I., Lundström M., Jeansson S., Lycke E. Glycoprotein C of herpes simplex virus type 1: characterization of O-linked oligosaccharides. J Gen Virol. 1983 Dec;64(Pt 12):2735–2747. doi: 10.1099/0022-1317-64-12-2735. [DOI] [PubMed] [Google Scholar]
- Pauls F. P., Dowdle W. R. A serologic study of herpesvirus hominis strains by microneutralization tests. J Immunol. 1967 May;98(5):941–947. [PubMed] [Google Scholar]
- Shieh M. T., WuDunn D., Montgomery R. I., Esko J. D., Spear P. G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992 Mar;116(5):1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard L. M., Fuller A. O., Spear P. G. Herpes simplex virus glycoproteins associated with different morphological entities projecting from the virion envelope. J Gen Virol. 1987 Mar;68(Pt 3):715–725. doi: 10.1099/0022-1317-68-3-715. [DOI] [PubMed] [Google Scholar]
- Svennerholm B., Jeansson S., Vahlne A., Lycke E. Involvement of glycoprotein C (gC) in adsorption of herpes simplex virus type 1 (HSV-1) to the cell. Arch Virol. 1991;120(3-4):273–279. doi: 10.1007/BF01310482. [DOI] [PubMed] [Google Scholar]
- Svennerholm B., Vahlne A., Jeansson S., Lundén R., Olofsson S., Svantesson G., Lycke E. Separation of herpes simplex virus virions and nucleocapsids on Percoll gradients. J Virol Methods. 1980;1(6):303–309. doi: 10.1016/0166-0934(80)90047-6. [DOI] [PubMed] [Google Scholar]
- Szilágyi J. F., Cunningham C. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J Gen Virol. 1991 Mar;72(Pt 3):661–668. doi: 10.1099/0022-1317-72-3-661. [DOI] [PubMed] [Google Scholar]
- Tetsu N., Inaba Y., Yukawa M., Yoshiki K., Hirahara T., Furuya Y., Ito S., Yonemochi A., Ishikawa H. Hemagglutination with pseudorabies virus. Brief report. Arch Virol. 1989;106(3-4):321–326. doi: 10.1007/BF01313960. [DOI] [PubMed] [Google Scholar]
- Trybała E., Larski Z., Wiśniewski J. Hemagglutination by herpes simplex virus type 1. Arch Virol. 1990;113(1-2):89–94. doi: 10.1007/BF01318356. [DOI] [PubMed] [Google Scholar]
- Trépanier P., Séguin C., Bastien Y., Boulay G., Lussier G., Trudel M. Hemagglutinating activity associated with bovine herpesvirus type 1. Vet Microbiol. 1985 Dec;10(6):517–523. doi: 10.1016/0378-1135(85)90060-4. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Blomberg J., Olofsson S., Lycke E. Subtyping of herpes simplex virus. Acta Pathol Microbiol Scand B. 1975 Oct;83(5):506–512. doi: 10.1111/j.1699-0463.1975.tb00131.x. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]




