Abstract
There is now extensive evidence that mammographic density is an independent risk factor for breast cancer that is associated with large relative and attributable risks for the disease. The epidemiology of mammographic density, including the influences of age, parity and menopause, is consistent with it being a marker of susceptibility to breast cancer, in a manner similar to the concept of 'breast tissue age' described by the Pike model. Mammographic density reflects variations in the tissue composition of the breast. It is associated positively with collagen and epithelial and nonepithelial cells, and negatively with fat. Mammographic density is influenced by some hormones and growth factors as well as by several hormonal interventions. It is also associated with urinary levels of a mutagen. Twin studies have shown that most of the variation in mammographic density is accounted for by genetic factors. The hypothesis that we have developed from these observations postulates that the combined effects of cell proliferation (mitogenesis) and genetic damage to proliferating cells by mutagens (mutagenesis) may underlie the increased risk for breast cancer associated with extensive mammographic density. There is clearly a need for improved understanding of the specific factors that are involved in these processes and of the role played by the several breast tissue components that contribute to density. In particular, identification of the genes that are responsible for most of the variance in percentage density (and of their biological functions) is likely to provide insights into the biology of the breast, and may identify potential targets for preventative strategies in breast cancer.
Introduction
Following Wolfe's original studies [1,2], the proportion of the breast area in the mammogram that is occupied by radiologically dense breast tissue (mammographic density) is now recognized to be a strong risk factor for breast cancer that may account for a large fraction of the disease [3,4] (see the review by Vachon and coworkers in this series [5]). In the present paper we review what is known of the aetiology of mammographic density and outline hypotheses for its association with risk for breast cancer.
We describe below the evidence that mammographic density is a marker of susceptibility to breast cancer, and we review what is known of the histology of radiologically dense breast tissue, and the influence of other risk factors for breast cancer. We describe associations of hormones, growth factors and a mutagen with mammographic density, and the evidence that mammographic density is influenced by genetic variants.
We propose that cumulative exposure to mammographic density may be an important determinant of breast cancer incidence, and that the risk for breast cancer associated with mammographic density may be explained by the combined effects of mitogens, which influence cell proliferation and the size of the cell population in the breast, and mutagens, which influence the likelihood of genetic damage to those cells. Figure 1 panels a and b, respectively, provide a schematic overview and a more detailed description of aspects of these hypotheses that are examined in the sections that follow. The available evidence is incomplete in many of these areas, however. In addition, all studies of the aetiology of mammographic density are constrained by the limitations of current methods of measuring density (see the review by Yaffe and coworkers in this series [5]).
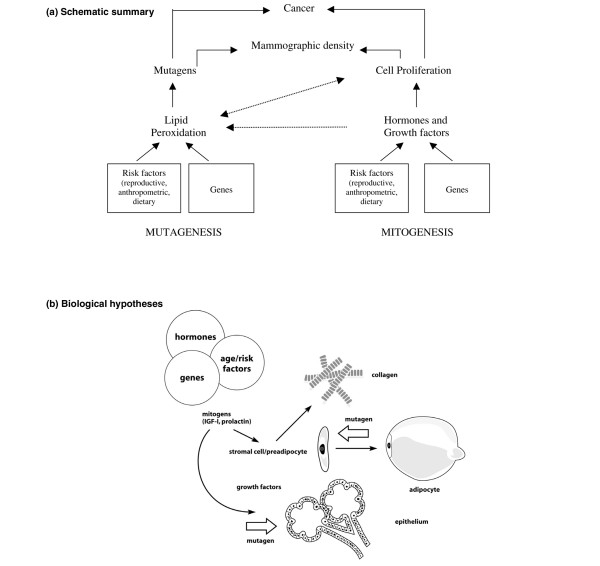
Figure 1.
Hypotheses. (a) Schematic summary. We postulate that the combined effects of cell proliferation (mitogenesis) and genetic damage to proliferating cells caused by mutagens (mutagenesis) may underlie the increased risk for breast cancer associated with extensive mammographic density. Mitogenesis and mutagenesis are related processes. Increased cell proliferation increases susceptibility to mutations but also increases lipid peroxidation, which can in turn increase cell proliferation (see text). (b) Biological hypothesis. The tissue components (epithelial cells, stromal cells, collagen and fat) that are responsible for variations in mammographic density are related to each other in several ways. Stromal fibroblasts produce collagen, and some are pre-adiopocytes that differentiate into adipocytes. Stromal and epithelial cells influence each other through paracrine growth factors, and both cell types are influenced by endocrine stimuli to cell proliferation (mitogenesis). Genetic damage to either stromal or epithelial cells caused by mutagens (mutagenesis) could initiate carcinogenesis (see text).
Ultimately, the risk for breast cancer associated with mammographic density will be elucidated by an improved understanding of the biology of the breast (see the review by Tisty and coworkers in this series [5]). However, just as epidemiological methods have identified mammographic density as an important risk factor for breast cancer, whose biology is likely to play an important role in the aetiology of the disease, epidemiological approaches may be able to suggest potential pathways and mechanisms that are responsible for risk.
Cumulative exposure to mammographic density and breast cancer incidence
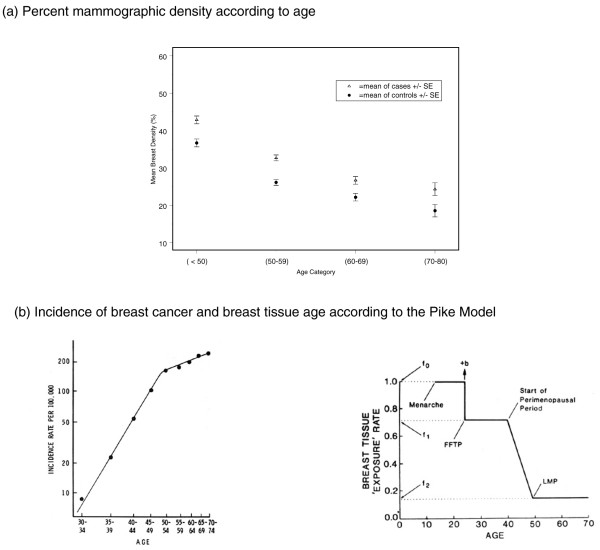
The average percentage mammographic density declines with increasing age (Figure 2a), whereas breast cancer incidence increases with age (Figure 2b [left]). This apparent paradox may be resolved, however, by reference to a model of breast cancer incidence proposed by Pike and coworkers [6]. This model is based on the concept that it is the rate of breast tissue 'ageing' or 'exposure', rather than chronological age, that is the relevant measure for describing the age-specific incidence of breast cancer (Figure 2b [right]). Breast tissue ageing is thought to be closely related to the mitotic activity of breast epithelial or stem cells and their susceptibility to genetic damage. According to the model, shown in Figure 2b (right), the rate of breast tissue ageing is most rapid at the time of menarche, slows with pregnancy, slows further during the peri-menopausal period, and is least after the menopause. After fitting numeric values for these parameters, Pike and coworkers [6] showed that cumulative exposure to breast tissue ageing, given by the area under the curve in Figure 2b (right), described the age-incidence curve for breast cancer in the USA, also shown in Figure 2b (left). Thus, cumulative exposure to breast tissue ageing and the age-specific breast cancer incidence both increase with age, but the rate of increase slows with age, particularly after menopause.
Figure 2.
Age, mammographic density and the incidence of breast cancer. (a) Baseline percentage mammographic density in women from three mammographic screening programmes according to those who developed breast cancer 1 to 8 years later (cases) or remained free from breast cancer (control individuals). Average percentage density in the baseline mammogram declined with increasing age at enrolment, both in women who eventually developed breast cancer and in those who remained free from disease. At all ages, percentage density was greater in those who developed breast cancer. Data from Boyd and coworkers [4]. (b) On the left is shown a log-log plot of the age-specific incidence of breast cancer. Adapted from Pike and coworkers [6]. To the right is shown the Pike model of breast tissue ageing. 'b' represents a one time increase in risk associated with first full-term pregnancy (FFTP). See Pike and coworkers [6]. LMP, last menstrual period.
Mammographic density shares many of the features of 'breast tissue age' and is influenced by similar factors. Detailed descriptions of the associations of risk factors with mammographic density can be found elsewhere [7-9]. Body size in particular is strongly and inversely associated with mammographic density, and is a risk factor for breast cancer independent of mammographic density [10]. We focus here on the associations of mammographic density with age, parity and menopause, variables in the Pike model that are also associated with variations in one or more of the histological features of the breast [11].
In addition to the effects of age referred to above, mammographic density is less extensive in women who are parous and in those with a larger number of live births (Figure 3). In these data each pregnancy was associated with an average 2% difference in percentage density [4]. Postmenopausal women have consistently been found to have less extensive mammographic density than premenopausal women, and a longitudinal study of the effects of the menopause on mammographic density [12] showed that percentage density was reduced by about 8% on average over the menopause.
Figure 3.
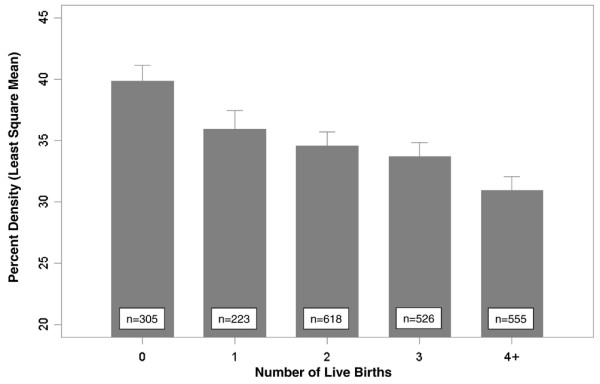
Parity and mammographic density. Least square means of percentage mammographic density according to number of live births, adjusted for age, body mass index, age at menarche, age at first birth, menopausal status, age at menopause, previous use of menopausal hormone therapy (ever/never) and breast cancer in first degree relatives (0, 1, 2+). The height of the bar is the least square mean of percentage density, and half width of the error bar represents the standard error. Data from Boyd and coworkers [4].
All risk factors for breast cancer must ultimately exert their influence by an effect on the breast. These findings suggest that, for at least some risk factors, this influence includes an effect on the number of cells and the quantity of collagen in the breast, which is reflected in differences in mammographic density and which may mediate the effect of the factor on breast cancer risk (see 'Breast histology and radiological features', below). The concept of breast tissue age in the Pike model is related to the effects of hormones on the kinetics of breast cells and the accumulation of genetic damage. As we discuss below, mammographic density may reflect cumulative exposure to stimuli to division of breast cells that predisposes them to genetic damage by mutagens.
In addition to the cross-sectional data shown in Figure 2a, longitudinal studies [13,14] have found that percentage mammographic density in women who develop breast cancer was greater than in those who remained free from disease, but neither study showed that the rate of change over time was related to breast cancer risk. Both studies were based primarily on postmenopausal women, and it remains possible that differences in rate of change in mammographic density earlier in life may be related to later risk for breast cancer.
Breast histology and radiological features
Breast histology and mammographic density
Studies of the relationship between breast tissue histology and the radiological appearance of the breast (described in detail by Boyd and coworkers [9]), using surgical biopsies or mastectomy specimens, have found greater amounts of epithelium and/or stroma to be associated with mammographic density.
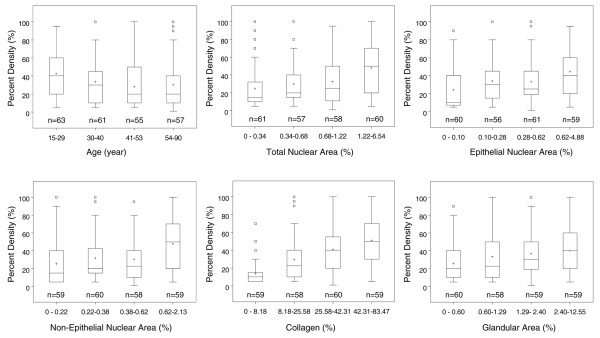
Li and coworkers used quantitative microscopy to examine histological features of randomly selected tissue blocks from breast tissue obtained at forensic autopsy [15,16] and determined the proportions of the biopsy occupied by cells (estimated by nuclear areas), glandular structures and collagen [11]. Figure 4 from that study [11] shows the inverse association of percentage density (in the image of the breast tissue slice from which the biopsy was taken) with age, and associations of percentage density with measured components of breast tissue, expressed as a percentage of the total area of the section. Greater percentage mammographic density was associated with a significantly greater total nuclear area, a greater nuclear area of both epithelial and nonepithelial cells, a greater proportion of collagen, and a greater area of glandular structures. Of the tissue components measured, collagen was present in the greatest quantity, was most strongly associated with percentage density, and explained 29% of the variance in percentage density. Nuclear area and glandular area accounted for between 4% and 7% of the variance in percentage density.
Figure 4.
Percentage mammographic density, age, and histological measures. Boxplots showing the associations of percentage density with age and histological measures. Median is shown by a horizontal line, mean by the '+' symbol, interquartile range by the columns, 1.5× the interquartile range by the whiskers, and outliers are shown separately. P values from linear regression, using continuous variables adjusted for age, were as follows: age, P = 0.04; total nuclear area, P < 0.001; epithelial nuclear area, P ≤ 0.001; nonepithelial nuclear area, P < 0.001; collagen, P < 0.001; glandular area, P < 0.001. Data from Li and coworkers [11].
Greater body weight, parity and greater number of births, and postmenopausal status were associated with quantitative differences in one or more of the tissue features measured in the autopsy samples described above (see Li and coworkers [11] for details). For example, greater body weight and postmenopausal status were inversely associated with all of the histological measures, and the percentage of collagen in the biopsy declined with parity and increasing number of live births. Each live birth was associated with an average reduction by 2% [11]. These factors were all associated with variations in mammographic density in this [11] and other studies [8,9], and with risk for breast cancer [17].
Breast histology and risk of breast cancer
Extensive mammographic density is associated with an increased risk for atypical hyperplasia and in situ breast cancer [18], which are associated with an increased risk for subsequent invasive breast cancer [19,20]. The reductions in collagen and glandular tissue, and the increase in fat that occur in the breast with increasing age have long been recognized by pathologists as 'involution', and Milanese and coworkers [21] demonstrated, using a definition that focused the degree of involution in the terminal duct lobular unit, that greater involution was associated with a reduced risk for breast cancer. The reduction in mammographic density with age is associated with smaller proportions of collagen and glandular tissue in the breast [11] and may be related to involution of the terminal duct lobular unit.
Relationships among histological structures that are responsible for density
Epithelial and stromal cells, collagen and fat – the tissue components that contribute to mammographic density – are related to each other in several ways that are illustrated in Figure 1b. Epithelial and stromal cells communicate by means of paracrine growth factors (see the review by Tisty and coworkers in this series [5]). Collagen is a product of stromal fibroblasts, and adipocytes develop from stromal pre-adipocytes [22]. Factors that affect one of these components may therefore affect the others, directly or indirectly, and each component has properties that may influence risk for and progression of breast cancer.
Breast cancer arises from epithelial cells, and the number and proliferative state of these cells may influence both the radiological density of the breast and the probability of genetic damage that may give rise to cancer. In addition, collagen and stromal matrix are products of stromal cells that may, through their mechanical properties, facilitate tumour invasion [23]. Metalloproteinases that regulate stromal matrix can also regulate the activation of growth factors and influence susceptibility to breast cancer [24,25].
Mitogenesis
Mitogens as potential mediators of effects: hormones and growth factors
As shown in Figure 1a,b, the effects of age and other factors on breast tissue composition that are described above (and further below) are likely to be mediated at least in part by one or more of the several endocrine, paracrine and autocrine mechanisms that regulate the growth and development of breast stroma and epithelium. Variations in exposure or response to one or more of these mechanisms may explain the effects that genetic and environmental factors have on differences in breast tissue composition. Most studies to date have focused on endocrine influences.
Blood levels of hormones and growth factors
The results of cross-sectional studies that have examined blood levels of steroid sex hormones and growth factors in relation to mammographic density are summarized in Tables 1 and 2. The studies vary in design, size, method of sampling patients, methods of measuring density, and methods of analysis. All have assessed the association between the blood and mammographic measures after adjustment for other factors that are known to influence density.
Table 1.
Studies of steroid sex hormones, SHBG, and mammographic density
| Direction of association [ref.] | |||||
| Hormone | Number of studiesa | Menopausal status | Positive | None | Inverse |
| Estrone | 7 | Premenopausal | Noh et al. [26] | ||
| Postmenopausal | Bremnes et al. [32], Greendale et al. [33] | Tamimi et al. [27]2, Aiello et al. [28]b, Warren et al. [29], Verheus et al. [30]2 | Aiello et al. [28]c | ||
| Estradiol | 8 | Premenopausal | Noh et al. [26]1, Boyd et al. [31] | ||
| Postmenopausal | Greendale et al. [33] | Tamimi et al. [27]2, Aiello et al. [28]b, Warren et al. [29], Verheus et al. [30]2, Boyd et al. [31], Bremnes et al. [32] | Aiello et al. [28]c | ||
| Free estradiol | 8 | Premenopausal | Noh et al. [26], Boyd et al. [31] | ||
| Postmenopausal | Greendale et al. [33] | Tamimi et al. [27]2, Aiello et al. [28]b, Warren et al. [29], Verheus et al. [30]2, Bremnes et al. [32] | Aiello et al. [28]c, Boyd et al. [31] | ||
| Progesterone | 5 | Premenopausal | Noh et al. [26]1, Boyd et al. [31]1 | ||
| Postmenopausal | Tamimi et al. [27], Warren et al. [29], Boyd et al. [31], Greendale et al. [33] | ||||
| SHBG | 8 | Premenopausal | Noh et al. [26]1, Boyd et al. [31]1 | ||
| Postmenopausal | Boyd et al. [31], Bremnes et al. [32] | Tamimi et al. [27]1, Aiello et al. [28]b,c, Warren et al. [29], Verheus et al. [30]1, Greendale et al. [33]1 | |||
| Testosterone | 6 | Premenopausal | NA | ||
| Postmenopausal | Tamimi et al. [27], Aiello et al. [28]b, Warren et al. [29], Verheus et al. [30]2, Bremnes et al. [32], Greendale et al. [33] | Aiello et al. [28]c | |||
| Androstenedione | 5 | Premenopausal | NA | ||
| Postmenopausal | Tamimi et al. [27], Aiello et al. [28]b, Warren et al. [29], Verheus et al. [30], Bremnes et al. [32] | Aiello et al. [37]c | |||
Associations shown are for percentage density. Associations were classified as positive, none, or inverse according to the direction of effect and the statistical significance of the values after adjustment for other factors, using a criterion of P < 0.05. Several associations were statistically significant before adjustment for other factors and these are indicated as follows: 1positive association before adjustment and 2inverse association before adjustment. aSome studies included both premenopausal and postmenopausal women. bResults for never users of hormones. cResults for users of hormones. SHBG, sex hormone binding globulin; NA, not assessed.
Table 2.
Studies of pituitary hormones, related growth factors, and mammographic density
| Direction of association [ref.] | |||||
| Hormone | Number of studiesa | Menopausal status | Positive | None | Inverse |
| Growth hormone | 1 | Premenopausal | Boyd et al. [31]1 | ||
| Postmenopausal | Boyd et al. [31]1 | ||||
| Prolactin | 4 | Premenopausal | Boyd et al. [31] (dense area only) | ||
| Postmenopausal | Boyd et al. [31], Greendale et al. [34] | Tamimi et al. [27]1, Bremnes et al. [39] | |||
| IGF-I | 7 | Premenopausal | Boyd et al. [31], Byrne et al. [35], Diorio et al. [36] | Maskarinec et al. [37], dos Santo Silva et al. [38] | |
| Postmenopausal | Bremnes et al. [39] | Aiello et al. [28]b,c, Boyd et al. [31], Byrne et al. [35], Diorio et al. [36]1, dos Santo Silva et al. [38] | |||
| IGFBP-3 | 7 | Premenopausal | Boyd et al. [31], Byrne et al. [35], Maskarinec et al. [37], dos Santos Silva et al. [38] | Diorio et al. [36] | |
| Postmenopausal | Aiello et al. [28]b,c, Boyd et al. [31], Byrne et al. [35], dos Santos Silva et al. [38]2, Bremnes et al. [39] | ||||
| IGF-I/IGFBP-3 ratio | 7 | Premenopausal | Boyd et al. [31], Byrne et al. [35], Maskarinec et al. [37] | Diorio et al. [36]1, dos Santos Silva et al. [38] | |
| Postmenopausal | Bremnes et al. [39] | Aiello et al. [28]b, Boyd et al. [31], Byrne et al. [35], Diorio et al. [36]1, dos Santos Silva et al. [38] | Aiello et al. [28]c | ||
Associations shown are for percentage density, unless otherwise indicated. Associations were classified as positive, none, or inverse according to the direction of effect and the statistical significance of the values after adjustment for other factors, using a criterion of P < 0.05. Several associations were statistically significant before adjustment for other factors and these are indicated as follows: 1positive association before adjustment and 2inverse association before adjustment. aSome studies included both premenopausal and postmenopausal women. bResults for never users of hormones. cResults for users of hormones. IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein.
Most studies of blood oestrogen levels and percentage mammographic density have found either no association or an inverse association with estrone levels (five out of seven studies) [26-30] or total or free estradiol (seven out of eight studies) [26-32] in premenopausal or postmenopausal women. An exception is the study carried out in the Postmenopausal Estrogen/Progestin Intervention Trial [33], which identified a positive association between percentage density and estrone, estradiol and free estradiol levels in postmenopausal women. Bremnes and coworkers [32] found a positive association of mammographic density with estrone levels (which was statistically significant only in women with insulin-like growth factor [IGF]-I levels below the median) but not with estradiol or free estradiol levels. Progesterone levels have not been shown to be associated with mammographic density in premenopausal or postmenopausal women. Sex hormone binding globulin has been found to have a significant positive association with mammographic density in two studies after adjustment for other variables [31,32], and in four other studies before adjustment [26,27,30,33]. Testosterone and androstenedione have not been shown to be associated with mammographic density in postmenopausal women and have not yet been studied in premenopausal women.
Blood levels of growth hormone have been found to be positively associated with mammographic density in premenopausal women, but this association became non-significant after adjustment for body size [31]. Because growth hormone is one of the factors that influences body size, this may be over-adjustment. Prolactin levels were found to be positively associated with the area of dense tissue in premenopausal women in one study [31], with percentage mammographic density in postmenopausal women in two studies [31,34], and in a further study statistical significance was lost after adjustment for other variables [27]. Mammographic density was found to be positively associated with serum IGF-I levels in premenopausal women in three [31,35,36] out of five studies [37,38], and one study found an association in postmenopausal women [39]. Results with IGF-binding protein (IGFBP)-3 and the ratio of IGF-I to IGFBP-3 have been inconsistent. In a longitudinal study, women with higher levels of serum IGF-I during the premenopausal period experienced a smaller increase nondense area and a slightly smaller decrease in dense area during menopause [40].
Growth factors in breast tissue
To date, few studies have examined growth factors or stromal matrix proteins in breast tissue in relation to mammographic density. One study [41] was conducted in formalin-fixed paraffin blocks of breast tissue (n = 92) surrounding benign lesions, half from breasts with little or no radiological density and half from breasts with extensive density, and included groups matched for age at the time of biopsy. Similar to the results of the study conducted by Li and coworkers [11] described above, breast tissue from women with extensive densities had a greater nuclear area and a larger stained area of collagen. In addition, stained areas of immunohistochemistry for tissue inhibitor of matrix metalloproteinase-3 and IGF-I were greater in women with extensive density than in those with little breast density [41]. Stromal proteoglycans that are expressed in association with breast cancer have also been found to be associated with mammographic density [42].
Breast tissue response to hormones
Combined estrogen-progesterone menopausal hormone therapy, but not estrogen therapy alone, is associated with a small increase in risk for breast cancer [43], and increases mammographic density [44-46]. Percentage density is reduced by tamoxifen [47], and by a gonadotrophin-releasing hormone agonist [48] that reduces exposure to estrogen and progesterone in premenopausal women. The average reductions in percentage density associated with these hormonal interventions are modest, in general less than 10%.
Hormones and growth factors: risk factors and risk for breast cancer
Estradiol and testosterone blood levels have been shown to be related to risk for breast cancer in premenopausal and postmenopausal women [49,50] but, as discussed above, have not consistently been shown to be associated with mammographic density, suggesting that they may influence risk through pathways that are unrelated to density. In support of this idea, Tamimi and coworkers [51] recently reported that circulating sex steroid levels and mammographic density are independently associated with breast cancer risk in postmenopausal women. However, it remains possible that other forms of estrogen not measured in these studies, including estrogen metabolites, may influence mammographic density [52] and the associated risk for breast cancer [53]. Estrogens can induce lipid peroxidation (see below) [54], and catechol estrogens (metabolites of estrone and estradiol) can react with DNA to form adducts [55] that may initiate cancer.
Blood levels of IGF-I and prolactin have also been found to be associated with risk for breast cancer, IGF-I predominantly in premenopausal women, and prolactin in both premenopausal and postmenopausal women [56,57]. IGF-I is a known mitogen for breast epithelium that is produced in the breast stroma, as well as by the liver in response to growth hormone [58], and administration of growth hormone to ageing primates has been shown to induce epithelial proliferation [59]. Prolactin increases cell proliferation and decreases apoptosis in the breast, and higher blood levels have been found to be associated with an increased risk for breast cancer in both premenopausal and postmenopausal women [60]. Prolactin plays an important role in the development and progression of mammary tumours in rodents [61]. Mammographic density, IGF-I and prolactin levels are all influenced by age, parity and number of births in a similar manner [62,63].
Mutagenesis
Mutagens as potential mediators of effects: oxidative stress
Oxidative stress occurs when an excess of reactive oxygen species (ROS) is produced in relation to antioxidant defences and can cause oxidative damage to DNA, protein and lipid molecules. DNA damage can lead to mutagenesis and increased risk for cancer [64]. Inflammation is also associated with increased ROS and may be an additional pathway that relates oxidative stress to cancer risk [65].
A variety of biomarkers for measurement of oxidative stress in vivo have been proposed, including markers of oxidative damage to DNA, protein and lipids [66], but a recent validation study in rats indicated that blood or urinary isopros-tanes and urinary malondialdehyde (MDA) are the best indicators of in vivo oxidative stress [67]. These compounds are products of lipid peroxidation produced from the free radical mediated oxidation of arachidonic acid. Isoprostane is a prostaglandin-like compound [68] and MDA is a known mutagen [69,70].
Urinary malondialdehyde and mammographic density
A positive association between mammographic density and 24-hour urinary MDA excretion was observed in three independent studies [71-73]. In premenopausal and postmenopausal women, representing a wide range of mammographic density [72,73], urinary MDA excretion was 23% to 30% higher in the highest quintile of mammographic density as compared with the lowest, after adjustment for age and body mass index or waist circumference (Table 3). Measures of body size, which are negatively associated with mammographic density and positively associated with oxidative stress, are important potential confounders of the relationship between urinary MDA and percentage mammographic density. This relationship becomes stronger [71], or is only evident [72,73], after adjustment for body size. Serum levels of MDA and MDA DNA adducts were not associated with mammographic density [73].
Table 3.
Urinary excretion of MDA by quintile of percentage mammographic density
| Quintiles of percentage density | ||||||
| 1 | 2 | 3 | 4 | 5 | P value for trend | |
| Premenopausal (n = 160) | 2.76a | 2.29 | 2.86 | 2.75 | 3.62 | 0.02 |
| Postmenopausal (n = 175) | 3.19 | 3.23 | 3.67 | 3.46 | 3.88 | 0.13 |
| All women (n = 335) | 3.02 | 2.76 | 3.10 | 3.38 | 3.68 | 0.01 |
Values for malondialdehyde (MDA) are expressed as mmol/day. For 'All women', values are also adjusted for menopausal status. aLeast square mean of MDA adjusted for age and waist circumference (negative inverse). Data are from Hong and coworkers [73].
It is unknown whether systemic levels of lipid peroxidation markers, such as urinary MDA, reflect breast tissue levels. However, women with breast cancer who received radiation to the breast (which induces oxidative stress and inflammation) exhibited significantly increased urinary excretion of isoprostanes as compared with those women with breast cancer who did not receive such treatment [74].
Oxidative stress: risk factors and risk of breast cancer
The protective effects of higher fruit and vegetable intake and serum antioxidant levels on breast cancer risk seen in some studies, and studies showing that genetic polymorphisms in some antioxidant enzymes are associated with breast cancer risk provide indirect evidence for a role of oxidative stress in the development of breast cancer [75,76]. Direct evidence of an association of oxidative stress with breast cancer risk arises from case control studies of patients with and without breast cancer. Plasma MDA was elevated in breast cancer patients relative to levels in healthy control individuals [77-79]. Levels of MDA DNA adducts and 8-hydroxy-2-deoxyguannosine (markers of DNA damage) were significantly higher in normal breast tissue of cancer patients than in the breast tissue of control individuals without cancer [80-82]. Recently, a large case control study identified a significant trend toward increasing breast cancer risk with increasing urinary excretion of isoprostanes [74]. A limitation of the studies cited above is that the markers of oxidative stress were measured in biological samples collected after breast cancer diagnosis, and therefore the higher levels of oxidative stress in cases could be due to the presence of cancer or its treatment.
Several lines of evidence suggest that there is an association between oxidative stress and some factors that are known or suspected to influence risk for breast cancer. Chinese women living in China have lower levels of urinary MDA excretion [83] and lower breast cancer risk than do Chinese women living the USA, and Chinese American women have lower urinary isoprostane excretion than Caucasian American women [84]. The lower risk and oxidative stress observed in Asian women may be related to their lower body weight and dietary fat intake compared with Caucasian women. Lower body weight is associated with lower breast cancer risk [85] and lower levels of isoprostane [86,87]. Lower dietary fat intake may be associated with reduced breast cancer risk [88] and with reduced oxidative stress [84,89]. Chronic moderate levels of activity increase antioxidant activity [90,91] and are associated with reduced breast cancer risk [92]. However, the role that these factors play in risk for breast cancer associated with mammographic density is not yet clear. For example, Asian women tend to have greater percentage density than do Caucasian women (probably as a result of smaller breast size) [93], the effect of body weight on breast cancer is probably independent of mammographic density [10], and physical activity does not appear to be associated with mammographic density [94,95].
In terms of reproductive risk factors known to be associated with mammographic density, markers of oxidative stress are higher in postmenopausal than in premenopausal women [73,96] and may be reduced by menopausal hormone therapy [97] and tamoxifen [98]. However, estrogen and its metabolites have both anti-oxidant and pro-oxidant effects [99], and urinary isoprostane excretion was not associated with blood estrogen levels [100]. Higher alcohol intake is associated with higher breast cancer risk [101], plasma isoprostane levels [102], and mammographic density [72,103].
Relationship of mitogenesis and mutagenesis
Increased cell proliferation can cause an increase in production of ROS and lipid peroxidation, and the products of lipid peroxidation themselves can promote cell proliferation via cell signalling [104] (Figure 1a). Interestingly, MDA and isoprostanes (products of lipid peroxidation) have been reported to be mediators of the increased cell proliferation and collagen production seen in hepatic fibrosis [105]. Fibrosis, a response to tissue injury and inflammation (which increase oxidative stress), involves the proliferation and activation of fibroblasts and results in accumulation of extracellular matrix and collagen [106]. It is unknown whether the process of fibrosis is related to mammographic density and increased risk for breast cancer. However, chronic inflammation and/or the wound healing response may be involved in the initiation or promotion of cancer [24,107], and the presence of breast cancer is associated with reactive stroma, a process that resembles fibrosis [108] that is thought to promote tumour progression and invasion. Thus, the association of increased MDA with higher mammographic density may be either a cause or an effect of increased cell proliferation and collagen production, and the risk for breast cancer may be increased by these processes as well as by mutagenesis. As shown in the Figure 1b both stromal and epithelial cells are potential sites of mutagenesis, either of which might initiate processes that ultimately give rise to breast cancer.
Heritability of mammographic density
Parity, menopause and other risk factors explain only 20% to 30% of the variance in mammographic density [8,109]. Early studies of mother-daughter sets [110,111] and small twin studies [111,112] suggested that genetic factors might explain a proportion of the variation (the heritability) of breast tissue patterns within a given population. A segregation analysis of nuclear family data conducted Pankow and coworkers [113] yielded findings consistent with a single mode of inheritance of one or more major genes, but it could not distinguish between dominant, recessive, or co-dominant models.
Twin studies conducted in Australia and North America identified correlations between twin pairs in percentage mammographic density that were, respectively, 0.61 and 0.67 for monozygotic twin pairs, and 0.25 and 0.27 for dizygotic twin pairs [114]. After adjustment for the other risk factors associated with differences in mammographic density, the proportion of the residual variation accounted for by additive genetic factors (heritability) was 63% (95% confidence interval 59% to 67%) in the studies combined, and was similar in each of the two studies. These two twin studies thus replicate each other in providing compelling evidence that the wide variation in percentage mammographic density among women is strongly influenced by genetic factors.
The search for genes associated with mammographic density is in its infancy and few have been found to date. Several large-scale genome-wide linkage and association studies are in progress and can be expected to report their findings within the next few years. The preliminary results from one genome-wide sib-pair linkage study [115] provide evidence for linkage at a region on chromosome 6. Vachon and coworkers [116] recently reported results of a genome-wide linkage scan that showed that a putative locus on chromosome 5p may account for a large proportion of the variance in mammographic density. Among association studies conducted to date, variations in genes concerned with estrogen metabolism [117-120], the estrogen [121] and androgen [122] receptors, IGFBP-3 [123], IGF [124] and growth hormone [125] have been shown to be associated with mammographic density. To date, few of these findings have been replicated, and some that have been replicated [117,118] have also been contradicted [119,120].
Among potential genetic influences suggested by our hypothesis shown in Figure 1a,b are effects on the production and metabolism of breast mitogens [126], effects on the change in mitogens that occurs with ageing [127], the response of stromal and epithelial breast tissue to stimulation by mitogens [128], and tissue modelling in the breast [129]. The production and metabolism of mutagens may also be under genetic control [130], as is the repair of DNA damage caused by mutagens [131]. Some factors already found to be associated with mammographic density are also involved in processes that generate mutagens or modify their effects. These include catechol-O-methyltransferase, which is involved in the metabolism of catechol estrogens with pro-oxidant and anti-oxidant activities [117], and cytochrome P450 1A2 [73], which has been found to be associated with serum and urinary MDA levels. These associations require confirmation, however.
Summary
There is now extensive evidence that mammographic density is a risk factor for breast cancer, independent of other risk factors, and is associated with large relative and attributable risks for the disease. The hypotheses that we have developed from the observations described above are summarized here and illustrated in Figure 1a,b.
Cumulative exposure to mammographic density and breast cancer risk
Mammographic density reflects variations in the tissue composition of the breast, and is associated positively with collagen and epithelial and nonepithelial cells, and negatively with fat. Increasing age, parity, and menopause are all associated with reductions in the epithelial and stromal tissues in the breast, and with an increase in fat. These histological changes are reflected in the radiological appearance of the breast, and are consistent with mammographic density being a marker of susceptibility to breast cancer, in a manner similar to the concept of 'breast tissue age' described in the Pike model [6,132]. Like breast tissue age, variations in mammographic density may reflect the mitotic activity of breast cells and differences in susceptibility to genetic damage, and cumulative exposure to density may have an important influence on breast cancer incidence.
Mitogens, mutagens and mammographic density
Mammographic density is influenced by some hormones and growth factors, as well as by several hormonal interventions, and is associated with urinary levels of a mutagen. We postulate that the combined effects of cell proliferation (mitogenesis) and genetic damage to proliferating cells by mutagens (mutagenesis) may underlie the increased risk for breast cancer associated with extensive mammographic density. As described above under 'Relationship of mitogenesis and mutagenesis', mitogenesis and mutagenesis are not independent processes. Increased cell proliferation can increase lipid peroxidation, and the products of lipid peroxidation can increase cell proliferation.
Blood levels of IGF-I and prolactin are among the endocrine stimuli to cell proliferation that have been found to be positively associated with both mammographic density and breast cancer risk, respectively, in premenopausal and postmenopausal women [56,57].
Autocrine and paracrine stimuli to the proliferation of epithelial and stromal cells, which regulate the growth, development and involution of the breast [25,133], have to date received little attention in relation to mammographic density, but one study [41] has identified an association of density with IGF-I in breast tissue. Animal models have shown that growth factors from fibroblasts can stimulate or inhibit epithelial proliferation, and that the genetic modification of fibroblasts can induce cancer [24,25,133].
The proliferation of cells that results from stimulation by endocrine, autocrine and paracrine growth factors increases risk for mutation [134]. To date the association of only one mutagen has been examined in relation to mammographic density, but greater levels of urinary excretion of MDA (a mutagenic product of lipid peroxidation) was found in three independent studies to be associated with more extensive mammographic density.
Potential areas for genetic influence include variation in the regulation of the hormones and growth factors that act on the breast, the response and modelling of breast tissue to these stimuli, and the processes that are involved in oxidative stress and the generation of mutagens.
Conclusion
Although there is evidence that both mitogenic and mutagenic processes are involved in determining the risk for breast cancer associated with mammographic density, there is clearly a need for an improved understanding of the specific factors involved and of the role played by the several breast tissue components that contribute to density. In particular, the identification of the genes that are responsible for most of the variance in percentage density (and of their biological functions) is likely to provide insights into the biology of the breast and may identify potential targets for preventative strategies for breast cancer.
Abbreviations
IGF = insulin-like growth factor; IGFBP = insulin-like growth factor binding protein; ROS = reactive oxygen species; MDA = malondialdehyde.
Competing interests
The authors declare that they have no competing interests.
Note
This article is part of a review series on Mammographic density, edited by Norman Boyd.
Other articles in the series can be found online at http://breast-cancer-research.com/articles/review-series.asp?series=bcr_Density
Acknowledgments
Acknowledgements
Much in this paper has arisen from collaborations with others, in particular the following: Martin Yaffe (Sunnybrook Health Sciences Centre), Salomon Minkin (Ontario Cancer Institute), Johanna Rommens and Andrew Paterson (Hospital for Sick Children), all of Toronto, Canada; and John Hopper (University of Melbourne, Australia).
Contributor Information
Lisa J Martin, Email: lmartin@uhnres.utoronto.ca.
Norman F Boyd, Email: boyd@uhnres.utoronto.ca.
References
- Wolfe JN. Breast patterns as an index of risk for developing breast cancer. A J Roentgenol. 1976;126:1130–1137. doi: 10.2214/ajr.126.6.1130. [DOI] [PubMed] [Google Scholar]
- Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::AID-CNCR2820370542>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- Breast Cancer Research Thematic Review Series on Mammographic Density http://breast-cancer-research.com/articles/review-series.asp?series=BCR_Density
- Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. 'Hormonal' risk factors, 'breast tissue age' and the age-incidence of breast cancer. Nature. 1983;303:767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- Grove JS, Goodman MJ, Gilbert F, Mi MP. Factors associated with mammographic pattern. Br J Radiol. 1985;58:21–25. doi: 10.1259/0007-1285-58-685-21. [DOI] [PubMed] [Google Scholar]
- Vachon CM, Kuni CC, Anderson K. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11:653–662. doi: 10.1023/A:1008926607428. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Lockwood GA, Byng J, Tritchler DL, Yaffe M. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–1144. [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Sun L, Guo H, Chiarelli A, Hislop G, Yaffe M, Minkin S. Body size, mammographic density and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2086–2092. doi: 10.1158/1055-9965.EPI-06-0345. [DOI] [PubMed] [Google Scholar]
- Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- Boyd N, Martin L, Stone J, Little L, Minkin S, Yaffe M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11:1048–1053. [PubMed] [Google Scholar]
- Vachon CM, Pankratz VS, Scott CG, Maloney SD, Ghosh K, Brandt KR, Milanese T, Carston MJ, Sellers TA. Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:921–928. doi: 10.1158/1055-9965.EPI-06-1047. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:732–739. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- Hart BL, Steinbock RT, Mettler FA, Jr, Pathak DR, Bartow SA. Age and race related changes in mammographic parenchymal patterns. Cancer. 1989;63:2537–2539. doi: 10.1002/1097-0142(19890615)63:12<2537::AID-CNCR2820631230>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bartow SA, Mettler FA, Jr, Black WC., III Correlations between radiographic patterns and morphology of the female breast. Rad Patterns Morph. 1997;13:263–275. [Google Scholar]
- Kelsey JL, Gammon MD, John ES. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Jensen H, Cooke G, Lee Han HW. Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst. 1992;84:1170–1179. doi: 10.1093/jnci/84.15.1170. [DOI] [PubMed] [Google Scholar]
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- Zangani D, Darcy KM, Masso-Welch PA, Bellamy ES, Desole MS, Ip MM. Multiple differentiation pathways of rat mammary stromal cells in vitro: acquisition of a fibroblast, adipocyte or endothelial phenotype is dependent on hormonal and extracellular matrix stimulation. Differentiation. 1999;64:91–101. doi: 10.1046/j.1432-0436.1999.6420091.x. [DOI] [PubMed] [Google Scholar]
- Provenzano PO, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JJ, Maskarinec G, Pagano I, Cheung LW, Stanczyk FZ. Mammographic densities and circulating hormones: A cross-sectional study in premenopausal women. Breast. 2006;15:20–28. doi: 10.1016/j.breast.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:2641–2647. doi: 10.1158/1055-9965.EPI-05-0558. [DOI] [PubMed] [Google Scholar]
- Aiello EJ, Tworoger SS, Yasui Y, Stanczyk FZ, Potter J, Ulrich CM, Irwin M, McTiernan A. Associations among circulating sex hormones, insulin-like growth factor, lipids, and mammographic density in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1411–1417. doi: 10.1158/1055-9965.EPI-04-0920. [DOI] [PubMed] [Google Scholar]
- Warren R, Skinner J, Sala E, Denton E, Dowsett M, Folkerd E, Healey CS, Dunning A, Doody D, Ponder B, et al. Associations among mammographic density, circulating sex hormones, and polymorphisms in sex hormone metabolism genes in postmenopausal women. Cancer Epidemiol Biomarkers & Prev. 2006;15:1502–1508. doi: 10.1158/1055-9965.EPI-05-0828. [DOI] [PubMed] [Google Scholar]
- Verheus M, Peeters PHM, van Noord PAH, van der Schouw YT, Grobbee DE, van Gils CH. No relationship between circulating levels of sex steroids and mammographic breast density: the Prospect-EPIC cohort. Breast Cancer Res. 2007;9:R53. doi: 10.1186/bcr1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, Hammond G, Minkin S. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87:876–882. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremnes Y, Ursin G, Bjurstam N, Rinaldi S, Kaaks R, Gram IT. Endogenous sex hormones, prolactin and mammographic density in postmenopausal Norwegian women. Int J Cancer. 2007;121:2506–2511. doi: 10.1002/ijc.22971. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Palla SL, Ursin G, Laughlin GA, Crandall C, Pike MC, Reboussin BA. The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Am J Epidemiol. 2005;162:826–834. doi: 10.1093/aje/kwi286. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Huang MH, Ursin G, Ingles S, Stanczyk F, Crandall C, Laughlin GA, Barrett-Connor E, Karlamangla A. Serum prolactin levels are positively associated with mammographic density in postmenopausal women. Breast Cancer Res Treat. 2007;105:337–346. doi: 10.1007/s10549-006-9454-y. [DOI] [PubMed] [Google Scholar]
- Byrne C, Colditz GA, Pollak M, Willet WC, Speizer FE, Hankinson SE. Plasma insulin-like growth factor-I, insulin-like growth factor binding protein-3 and mammographic density. Cancer Res. 2000;60:3744–3748. [PubMed] [Google Scholar]
- Diorio C, Pollak M, Byrne C, Mâsse B, Hébert-Croteau N, Yaffe M, Coté G, Bérubé S, Morin C, Brisson J. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14:1065–1073. doi: 10.1158/1055-9965.EPI-04-0706. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Williams AE, Kaaks R. A cross-sectional investigation of breast density and insulin-like growth factor I. Int J Cancer. 2003;107:991–996. doi: 10.1002/ijc.11505. [DOI] [PubMed] [Google Scholar]
- dos Santos Silva I, Johnson N, De Stavola B, Torres-Mejía G, Fletcher O, Allen DS, Allen NE, Key TJ, Fentiman IS, Holly JM, et al. The insulin-like growth factor system and mammographic features in premenopausal and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:449–455. doi: 10.1158/1055-9965.EPI-05-0555. [DOI] [PubMed] [Google Scholar]
- Bremnes Y, Ursin G, Bjurstam N, Rinaldi S, Kaaks R, Gram IT. Insulin-like growth factor and mammographic density in postmenopausal Norwegian women. Cancer Epidemiol Biomarkers Prev. 2007;16:57–62. doi: 10.1158/1055-9965.EPI-06-0788. [DOI] [PubMed] [Google Scholar]
- Verheus M, Peeters PHM, Kaaks R, van Noord PAH, Grobbee DE, van Gils CH. Premenopausal insuln-like growth factor-1 serum levels and changes in breast density over menopause. Cancer Epidemiol Biomarkers Prev. 2007;16:451–457. doi: 10.1158/1055-9965.EPI-06-0642. [DOI] [PubMed] [Google Scholar]
- Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF. Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev. 2001;10:243–248. [PubMed] [Google Scholar]
- Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–R135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. The Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- Lundstrom E, Wilczek B, von Palffy Z, Soderqvist G, von Schoultz B. Mammographic breast density during hormone replacement therapy: differences according to treatment. Am J Obstet Gynecol. 1999;181:348–352. doi: 10.1016/S0002-9378(99)70560-0. [DOI] [PubMed] [Google Scholar]
- Rutter CM, Mandelson MT, Laya MB, Seger DJ, Taplin S. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285:171–176. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Warwick J, Pinney E, Warren RML, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- Spicer DV, Ursin G, Parisky YR, Pearce JG, Shoupe D, Pike A, Pike MC. Changes in mammographic densities induced by a hormonal contraceptive designed to reduce breast cancer risk. J Natl Cancer Inst. 1994;86:431–436. doi: 10.1093/jnci/86.6.431. [DOI] [PubMed] [Google Scholar]
- Endogenous Hormones and Breast Cancer Collaborative Group. Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99:1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- Riza E, De Stavola B, Bradlow HL, Sepkovic DW, Linos D, Linos A. Urinary estrogen metabolites and mammographic parenchymal patterns in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10:627–634. [PubMed] [Google Scholar]
- Wellejus A, Olsen A, Tjonneland A, Thomsen BL, Overvad K, Loft S. Urinary hydroxyestrogens and breast cancer risk among postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2005;14:2137–2142. doi: 10.1158/1055-9965.EPI-04-0934. [DOI] [PubMed] [Google Scholar]
- Liehr JG. Hormone-associated cancer: mechanistic similarities between human breast cancer and estrogen-induced kidney carcinogenesis in hamsters. Environ Health Perspect. 1997;105(suppl 3):565–569. doi: 10.2307/3433371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents – DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Michaud DS, Manson JE, Colditz GA, Longcope C, Rosner B, Speizer FE. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91:629–634. doi: 10.1093/jnci/91.7.629. [DOI] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nature Reviews. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- Ng ST, Zhou J, Adesanya OO, Wang J, LeRoith D, Bondy CA. Growth hormone treatment induces mammary gland hyperplasia in aging primates. Nat Med. 1997;3:1141–1144. doi: 10.1038/nm1097-1141. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Eliassen AH, Sluss P, Hankinson SE. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol. 2007;25:1482–1488. doi: 10.1200/JCO.2006.07.6356. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Pollak MN, Hankinson SE. Lifestyle correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:862–867. [PubMed] [Google Scholar]
- Eliassen AH, Tworoger SS, Hankinson SE. Reproductive factors and family history of breast cancer in relation to plasma prolactin levels in premenopausal and postmenopausal women. Int J Cancer. 2007;120:1536–1541. doi: 10.1002/ijc.22482. [DOI] [PubMed] [Google Scholar]
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Talser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/B:MCBI.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- Pathak SK, Sharma RA, Steward WP, Mellon JK, Griffiths TR, Gescher AJ. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: targets for chemopreventive strategies. Eur J Cancer. 2005;41:61–70. doi: 10.1016/j.ejca.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning. Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10:S10–S23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- Mukai FH, Goldstein BD. Mutagenicity of malondialdehyde, a decomposition product of peroxidized polyunsaturated fatty acids. Science. 1976;191:868–869. doi: 10.1126/science.766187. [DOI] [PubMed] [Google Scholar]
- Basu AK, Marnett LJ. Unequivocal demonstration that malondialdehyde is a mutagen. Carcinogenesis. 1983;4:331–333. doi: 10.1093/carcin/4.3.331. [DOI] [PubMed] [Google Scholar]
- Boyd NF, McGuire V. Evidence of lipid peroxidation in premenopausal women with mammographic dysplasia. Cancer Lett. 1990;50:31–37. doi: 10.1016/0304-3835(90)90175-W. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Connelly P, Byng J, Yaffe M, Draper H, Little L, Jones D, Martin LJ, Lockwood G, Tritchler D. Plasma lipids, lipoproteins, and mammographic densities. Cancer Epidemiol Biomarkers Prev. 1995;4:727–733. [PubMed] [Google Scholar]
- Hong CC, Tang BK, Rao V, Agarwal S, Martin L, Tritchler D, Yaffe M, Boyd NF. Cytochrome P450 1A2 (CYP1A2) activity, mammographic density, and oxidative stress: a cross-sectional study. Breast Cancer Res. 2004;6:R338–R351. doi: 10.1186/bcr797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner P, Jr, Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL, Eng SM, Gaudet MM, Neugut AI, Santella RM. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:639–644. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- Ambrosone CB. Oxidants and antioxidants in breast cancer. Antioxid Redox Signal. 2000;2:903–917. doi: 10.1089/ars.2000.2.4-903. [DOI] [PubMed] [Google Scholar]
- Toniolo P, Van Kappel AL, Akhmedkhanov A, Ferrari P, Kato I, Shore RE, Riboli E. Serum carotenoids and breast cancer. Am J Epidemiol. 2001;153:1142–1147. doi: 10.1093/aje/153.12.1142. [DOI] [PubMed] [Google Scholar]
- Gonenc A, Ozkan Y, Torun M, Simsek B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J Clin Pharm Ther. 2001;26:141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Ray G, Batra S, Shukla NK, Deo S, Raina V, Ashok S, Husain SA. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 2000;59:163–170. doi: 10.1023/A:1006357330486. [DOI] [PubMed] [Google Scholar]
- Huang YL, Sheu JY, Lin TH. Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin Biochem. 1999;32:131–136. doi: 10.1016/S0009-9120(98)00096-4. [DOI] [PubMed] [Google Scholar]
- Wang M, Dhingra K, Hittelman WN, Liehr JG, de Andrade M, Li D. Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol Biomarkers Prev. 1996;5:705–710. [PubMed] [Google Scholar]
- Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer. 1996;32A:1209–1214. doi: 10.1016/0959-8049(96)00031-7. [DOI] [PubMed] [Google Scholar]
- Li D, Zhang W, Zhu J, Chang P, Sahin A, Singletary E, Bondy M, Hazra T, Mitra S, Lau SS, et al. Oxidative DNA damage and 8-hydroxy-2-deoxyguanosine DNA glycosylase/apurinic lyase in human breast cancer. Mol Carcinog. 2001;31:214–223. doi: 10.1002/mc.1056. [DOI] [PubMed] [Google Scholar]
- Yeung KS, McKeown-Eyssen GE, Li GF, Glazer E, Hay K, Child P, Gurgin V, Zhu SL, Baptista J, Aloe M, et al. Comparison of diet and biochemical characteristics of stool and urine between Chinese populations with low and high colorectal cancer rates. J Natl Cancer Inst. 1991;83:46–50. doi: 10.1093/jnci/83.1.46. [DOI] [PubMed] [Google Scholar]
- Tomey KM, Sowers MR, Li X, McConnell DS, Crawford S, Gold EB, Lasley B, Randolph JF., Jr Dietary fat subgroups, zinc, and vegetable components are related to urine F2a-Isoprostane concentration, a measure of oxidative stress, in midlife women. J Nutr. 2007;137:2419. doi: 10.1093/jn/137.11.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ, Framingham Study Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- Djuric Z, Heilbrun LK, Reading BA, Boomer A, Valeriote FA, Martino S. Effects of a low-fat diet on levels of oxidative damage to DNA in human peripheral nucleated blood cells. J Natl Cancer Inst. 1991;83:766–769. doi: 10.1093/jnci/83.11.766. [DOI] [PubMed] [Google Scholar]
- Karolkiewicz J, Szczesniak L, Deskur-Smielecka E, Nowak A, Stemplewski R, Szeklicki R. Oxidative stress and antioxidant defense system in healthy, elderly men: relationship to physical activity. Aging Male. 2003;6:100–105. doi: 10.1080/713604768. [DOI] [PubMed] [Google Scholar]
- Covas MI, Elosua R, Fito M, Alcantara M, Coca L, Marrugat J. Relationship between physical activity and oxidative stress biomarkers in women. Med Sci Sports Exerc. 2002;34:814–819. doi: 10.1097/00005768-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev. 2001;10:287–301. [PubMed] [Google Scholar]
- Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, Pike MC, Ursin G. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol. 2004;159:140–147. doi: 10.1093/aje/kwh028. [DOI] [PubMed] [Google Scholar]
- Suijkerbuijk KP, Van Duijnhoven FJ, Van Gils CH, Van Noord PA, Peeters PH, Friedenreich CM, Monninkhof EM. Physical activity in relation to mammographic density in the Dutch prospect-European prospective investigation into cancer and nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:456–460. doi: 10.1158/1055-9965.EPI-05-0569. [DOI] [PubMed] [Google Scholar]
- Reeves KW, Gierach GL, Modugno F. Recreational physical activity and mammographic breast density characteristics. Cancer Epidemiol Biomarkers Prev. 2007;16:934–942. doi: 10.1158/1055-9965.EPI-06-0732. [DOI] [PubMed] [Google Scholar]
- Trevisan M, Browne R, Ram M, Muti P, Freudenheim J, Carosello AM, Armstrong D. Correlates of oxidative stress in the general population. Am J Epidemiol. 2001;154:348–356. doi: 10.1093/aje/154.4.348. [DOI] [PubMed] [Google Scholar]
- Wakatsuki A, Ikenoue N, Sagara Y. Effects of estrogen on susceptibility to oxidation of low-density and high-density lipoprotein in postmenopausal women. Maturitas. 1998;28:229–234. doi: 10.1016/S0378-5122(97)00072-8. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Vijayalakshmi T, Phil M, Sachdanandam P. Effect of tamoxifen on lipid peroxide and antioxidative system in postmenopausal women with breast cancer. Cancer. 1994;74:78–82. doi: 10.1002/1097-0142(19940701)74:1<78::AID-CNCR2820740114>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Gago-Dominguez M, Castelao JE, Pike MC, Sevanian A, Haile RW. Role of lipid peroxidation in the epidemiology and prevention of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2829–2839. doi: 10.1158/1055-9965.EPI-05-0015. [DOI] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22:438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt PA, Folsom AR, Goldbohm RA, Graham S, Holmberg L, Howe GR, Marshall JR, et al. Alcohol and breast cancer in women: A pooled analysis of cohort studies. JAMA. 1998;279:535–540. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- Hartman TJ, Baer DJ, Graham LB, Stone WL, Gunter EW, Parker CE, Albert PS, Dorgan JF, Clevidence BA, Campbell WS, et al. Moderate alcohol consumption and levels of antioxidant vitamins and isoprostanes in postmenopausal women. Eur J Clin Nutr. 2005;59:161–168. doi: 10.1038/sj.ejcn.1602051. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Takata Y, Pagano I, Lurie G, Wilkens LR, Kolonel LN. Alcohol consumption and mammographic density in a multiethnic population. Int J Cancer. 2006;118:2579–2583. doi: 10.1002/ijc.21705. [DOI] [PubMed] [Google Scholar]
- Davies KJA. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life. 1999;48:41–47. doi: 10.1080/713803463. [DOI] [PubMed] [Google Scholar]
- Comporti M, Arezzini B, Signorini C, Sgherri C, Monaco B, Gardi C. F2-isoprostanes stimulate collagen synthesis in activated hepatic stellate cells: a link with liver fibrosis? Lab Invest. 2005;85:1381–1391. doi: 10.1038/labinvest.3700332. [DOI] [PubMed] [Google Scholar]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- Marks F, Furstenberger G, Muller-Decker K. Tumor promotion as a target of cancer prevention. Recent Results Cancer Res. 2007;174:37–47. doi: 10.1007/978-3-540-37696-5_3. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Lockwood GA, Byng JW, Yaffe MJ, Tritchler DL. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br J Cancer. 1998;78:1233–1238. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JN, Albert S, Belle S, Salane M. Familial influences on breast parenchymal patterns. Cancer. 1980;46:2433–2437. doi: 10.1002/1097-0142(19801201)46:11<2433::AID-CNCR2820461123>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Haars G, van Noord PAH, van Gils CH, Peeters PHM, Grobbee DE. Heritable aspects of dysplastic breast glandular tissue (DY) Breast Cancer Res Treat. 2004;87:149–156. doi: 10.1023/B:BREA.0000041621.48575.9b. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Alanko A, Kivisaari L. Mammographic patterns in twin pairs discordant for breast cancer. Br J Radiol. 1987;60:459–462. doi: 10.1259/0007-1285-60-713-459. [DOI] [PubMed] [Google Scholar]
- Pankow JS, Vachon CM, Kuni CC, King RA, Arnett DK, Grabrick DM, Rich SS, Anderson VE, Sellers TA. Genetic analysis of mammographic breast density in adult women: evidence of a gene effect. J Natl Cancer Inst. 1997;89:549–556. doi: 10.1093/jnci/89.8.549. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Dite GS, Stone J, Gunasekara A, English DR, McCredie MR, Giles GG, Tritchler D, Chiarelli A, Yaffe MJ, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347:886–894. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- Vachon CM, King RA, Atwood LD, Kuni CC, Sellers TA. Preliminary sibpair linkage analysis of percent mammographic density. J Natl Cancer Inst. 1999;91:1778–1779. doi: 10.1093/jnci/91.20.1778. [DOI] [PubMed] [Google Scholar]
- Vachon CM, Sellers TA, Carlson EE, Cunningham JM, Hilker CA, Smalley RL, Schaid DJ, Kelemen LE, Couch FJ, Pankratz VS. Strong evidence of a genetic determinant for mammographic density, a major risk factor for breast cancer. Cancer Res. 2007;67:8412–8418. doi: 10.1158/0008-5472.CAN-07-1076. [DOI] [PubMed] [Google Scholar]
- Hong C-C, Thompson HJ, Jiang C, Hammond GL, Tritchler D, Yaffe M, Boyd NF. Val158Met Polymorphism in catechol-O-methyltransferase (COMT) gene associated with risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:838–847. [PubMed] [Google Scholar]
- Maskarinec G, Luire G, Williams AE, Marchand L. An investigation of mammographic density and gene variants in healthy women. Int J Cancer. 2004;112:683–688. doi: 10.1002/ijc.20448. [DOI] [PubMed] [Google Scholar]
- Haiman CA, Bernstein L, Berg D, Ingles SA, Salane M, Ursin G. Genetic determinants of mammographic density. Breast Cancer Res. 2002;4:R5. doi: 10.1186/bcr434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman CA, Hankinson SE, De Vivo I, Guillemette C, Ishibe N, Hunter DJ, Byrne C. Polymorphisms in steroid hormone pathway genes and mammographic density. Breast Cancer Res Treat. 2003;77:27–36. doi: 10.1023/A:1021112121782. [DOI] [PubMed] [Google Scholar]
- van Duijnhoven FJ, Bezemer ID, Peeters PH, Roest M, Uitterlinden AG, Grobbee DE, van Gils CH. Polymorphisms in the estrogen receptor alpha gene and mammographic density. Cancer Epidemiol Biomarkers Prev. 2005;14:2655–2660. doi: 10.1158/1055-9965.EPI-05-0398. [DOI] [PubMed] [Google Scholar]
- Lillie EO, Bernstein L, Ingles SA, Gauderman WJ, Rivas GE, Gagalang V, Krontiris T, Ursin G. Polymorphism in the androgen receptor and mammographic density in women taking and not taking estrogen and progestin therapy. Cancer Res. 2004;64:1237–1241. doi: 10.1158/0008-5472.CAN-03-2887. [DOI] [PubMed] [Google Scholar]
- Lai JH, Vesprini D, Zhang W, Yaffe MJ, Pollak M, Narod SA. A polymorphic locus in the promoter region of the IGFBP3 gene is related to mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2004;13:573–582. [PubMed] [Google Scholar]
- Tamimi RM, Cox DG, Kraft P, Pollak MN, Haiman CA, Cheng I, Freedman ML, Hankinson SE, Hunter DJ, Colditz GA. Common genetic variation in IGF1, IGFBP-1, and IGFBP-3 in relation to mammographic density: a cross-sectional study. Breast Cancer Res. 2007;9:R18. doi: 10.1186/bcr1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhall C, Hegele R, Cao H, Tritchler D, Yaffe M, Boyd NF. Mammographic density and the pituitary growth hormone and growth hormone releasing hormone receptor genes. Cancer Epidemiol Biomarkers Prev. 2005;14:2648–2654. doi: 10.1158/1055-9965.EPI-04-0374. [DOI] [PubMed] [Google Scholar]
- Dunning AM, Healey CS, Pharoah PDP, Teare MD, Ponder BAJ, Easton DF. A systematic review of genetic polymorphism and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8:843–854. [PubMed] [Google Scholar]
- Lamberts SWJ, van den Beld AW, van der Lely A-J. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- van Duijnhoven FJ, Peeters PH, Warren RM, Bingham SA, Uitterlinden AG, van Noord PA, Monninkhof EM, Grobbee DE, van Gils CH. Influence of estrogen receptor alpha and progesterone receptor polymorphisms on the effects of hormone therapy on mammographic density. Cancer Epidemiol Biomarkers Prev. 2006;15:462–467. doi: 10.1158/1055-9965.EPI-05-0754. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P 450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/S0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Lockwood GA, Martin LJ, Byng JW, Yaffe MJ, Tritchler DL. Mammographic density as a marker of susceptibility to breast cancer: a hypothesis. In: Miller AB, Bartsch H, Boffetta P, Dragsted L, Vainio H, editor. Biomarkers in Cancer Chemoprevention. Lyon, France: International Agency for Research on Cancer; 2001. pp. 163–169. [Google Scholar]
- Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11:97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE. Increased cell division as a cause of human cancer. Cancer Res. 1990;50:7415–7421. [PubMed] [Google Scholar]