Abstract
Introduction
Various perinatal factors, including birth weight, birth order, maternal age, gestational age, twin status, and parental smoking, have been postulated to affect breast cancer risk in daughters by altering the hormonal environment of the developing fetal mammary glands. Despite ample biologic plausibility, epidemiologic studies to date have yielded conflicting results. We investigated the associations between perinatal factors and subsequent breast cancer risk through meta-analyses.
Methods
We reviewed breast cancer studies published from January 1966 to February 2007 that included data on birth weight, birth order, maternal age, gestational age, twin status, and maternal or paternal smoking. Meta-analyses using random effect models were employed to summarize the results.
Results
We found that heavier birth weights were associated with increased breast cancer risk, with studies involving five categories of birth weight identifying odds ratios (ORs) of 1.24 (95% confidence interval [CI] 1.04 to 1.48) for 4,000 g or more and 1.15 (95% CI 1.04 to 1.26) for 3,500 g to 3,999 g, relative to a birth weight of 2,500 to 2,599 g. These studies provided no support for a J-shaped relationship of birthweight to risk. Support for an association with birthweight was also derived from studies based on three birth weight categories (OR 1.15 [95% CI 1.01 to 1.31] for ≥4,000 g relative to <3,000 g) and two birth weight categories (OR 1.09 [95% CI 1.02 to 1.18] for ≥3,000 g relative to <3,000 g). Women born to older mothers and twins were also at some increased risk, but the results were heterogeneous across studies and publication years. Birth order, prematurity, and maternal smoking were unrelated to breast cancer risk.
Conclusion
Our findings provide some support for the hypothesis that in utero exposures reflective of higher endogenous hormone levels could affect risk for development of breast cancer in adulthood.
Introduction
Intrauterine environmental exposures to endogenous or exogenous hormones, notably estrogens, may influence the subsequent development of breast cancer in offspring [1]. During pregnancy, levels of circulating estrogens and other hormones with growth-enhancing properties are at least 10 times higher than those in nonpregnant women, with increases seen with advancing gestational age [2-4]. The hypothesis that breast cancer in daughters may be influenced by the intrauterine environment is receiving increased attention [5]. Perinatal factors, including birth weight, birth order, maternal age, gestational age, twin status, and parental smoking, have been postulated as risk factors for breast cancer through altered hormonal influences on the developing fetal mammary glands [1]. Despite ample biologic plausibility, this hypothesis is difficult to evaluate directly [5], and previous epidemiologic studies have reported conflicting results [6,7].
Here we review the epidemiologic studies that have assessed the association between perinatal factors and breast cancer risk in daughters. A meta-analytical approach was applied in order to clarify further the possible role played by the intrauterine environment in the etiology of breast cancer.
Materials and methods
Identification of studies
The data retrieved for the systematic review were based on searches of all published papers, letters, abstracts, and review articles on birth weight, birth order, maternal age, gestational age, twin status, and maternal or paternal smoking and breast cancer using the MEDLINE database from January 1966 through February 2007. We used keywords combining text words, with terms for six perinatal factors combined with terms for breast cancer (Table 1). We also manually searched the reference lists of all studies that fulfilled the inclusion criteria for further relevant publications. Articles were included in our systematic review if they fulfilled the following three criteria: the exposure status of at least one of six perinatal risk factors of interest was compared with nonexposure status; the outcome focused on the daughter's breast cancer morbidity or mortality using an epidemiologic study design (case-control design, data linkage study, or cohort study design); and the article was written in English language. We excluded animal studies, investigations focusing on male breast cancer, reviews, and studies that did not provide separate relative risks for breast cancer. We also excluded studies if odds ratios (ORs) or relative risks (RRs) were not specifically provided, raw data were not available for calculation of risks, or the emphasis of analyses was on hazard ratios or standardized incidence ratios.
Table 1.
Search terms used in systematic review
| Subject | Search term |
| Breast neoplasm | Breast neoplasms, subsequent breast neoplasm, breast neoplasm and daughter |
| Birth weight | Cirth weight, birthweight, birth size |
| Birth order | Birth order, birth rank |
| Maternal age | Maternal age, mother's age, parental age |
| Gestational age | Gestational age, preterm, prematurity, abruption placenta, pre-eclampsia, eclampsia |
| Twinship | Twin, twining, multiple births, multiple pregnancy, monozygote twin, dizygote twin, |
| Parental smoking | Maternal smoking, mother's smoking, paternal smoking, father's smoking, parental smoking |
| Others | Prenatal factors, perinatal factors, intrauterine environment, intrauterine factor, In-utero exposure |
Statistical analyses
For the purposes of meta-analysis, birth weight was classified in three different ways: five categories (<2,500 g, 2,500 to 2,999 g [referent], 3,000 to 3,499 g, 3,500 to 3,999 g, and ≥4,000 g); three categories (<3,000 g [referent], 3,001 to 3,999 g, and ≥4,000 g); and two categories (<3,000 g [or ≤3,000 g; referent] and ≥3,000 g [or >3,000 g]). Birth order was examined using two different categorical schemes: 1 (referent) versus ≥2; and 1 (referent), 2 to 4, and ≥5. Maternal age was classified into three categories: <25 years old (referent), 25 to 29 years old, and ≥30 years old. Gestational age was also analyzed in two ways: ≤36 weeks versus ≥37 weeks (referent); and ≤32 weeks versus ≥33 weeks (referent). To examine twin status, three classification schemes were employed: twin versus singleton (referent); monozygotic twin (or sister twins if zygosity was not reported) versus singleton (referent); and dizygotic twin (or sister-brother twins if zygosity was not reported) versus singleton (referent). Maternal or paternal smoking was considered as follows: no smoking during pregnancy (referent) versus smoking during pregnancy. If the criteria utilized in an article were slightly different from our criteria, then we included the data and described the difference in a footnote.
ORs and 95% confidence intervals (CIs) were recalculated from published frequency tables of individual studies using the Mantel-Haenszel common OR estimate. However, the reported OR (95% CI) was used when the published studies did not provide further details as to the frequencies of the exposure variables. If the manuscript reported the results after performing a stratified analysis, then we re-calculated the crude OR by combining across strata. A random-effects model was used to obtain summary ORs and 95% CIs.
Heterogeneity was assessed by heterogeneity test using Cochran Q statistics [8]. Publication bias was assessed according to the Egger regression asymmetry test, and the Begg and Mazumdar adjusted rank correlation tests [9,10]. The Egger test is a simple linear regression of the natural log of ORs or RRs against its precision (the inverse of its standard error) [10]. The Begg and Mazumdar rank correlation test reports the rank correlation (Kendall's tau) between the standardized effect size and the standard errors of these effects. If asymmetry is caused by publication bias, then we would expect that high standard errors (small studies) would be associated with larger effect sizes (ORs or RRs) or low standard errors (large studies) would be associated with smaller effect sizes [9]. The Begg and Mazumdar test makes fewer assumptions than does the Egger test, but it is insensitive to many types of bias (lower power) that the Egger test is sensitive to [11].
When significant heterogeneity or publication bias was found, we performed subgroup analyses by study design (case-control study versus cohort study), and the source of information (data linkage versus self-report) to assess the impact on between-study variations (heterogeneity). Many of the large-scale studies, especially cohort studies, were published after 2000; thus, we classified publication years into before 2000 versus 2000 or later in order to assess publication biases associated with small study sizes. Few studies focused on either Asians or African-Americans, and so it was not possible to examine ethnicity effects. All statistical analyses were conducted using STATA (Version 8.2 [special edition]; Stata Corp., College Station, TX, USA).
Results
We identified 34 studies that assessed the association between birth weight and breast cancer risk (Table 2): 19 case-control studies (eight population based, three nested, six record linkage based, and two twin-based) and 15 cohort studies (seven population based and eight record linkage based). Many studies showed positive association between heavier birth weight and breast cancer risk [12-31], and some of the studies observed stronger effects among younger (<45 years) or premenopausal women [14,22,29,30]. In contrast, studies observing no association [32-41] or a negative one [42-45] also have been reported. Additionally, some authors reported a J-shaped relationship between birth weight and breast cancer risk [12,14,15,18,25,33,35,37,45], particularly for early-onset cancers [18].
Table 2.
Studies assessing the association of birth weight and the risk for breast cancer
| Type of study | Ref. | Year | Design | Cases | Controls (or cohort) | Country/place of study | Birthweight (g) | OR (95% CI) | Comments |
| Case-control studies | [42] | 1988 | PCC | 153 | 461 | USA | 1,162–2,948 | Referent | Matched analysis; P for trend = 0.41 |
| 2,949–3,340 | 0.65 (0.33–1.26) | ||||||||
| 3,341–4,451 | 0.76 (0.41–1.43) | ||||||||
| [12]a | 1992 | LCC | 458 | 1,197 | Sweden | <2,500 | 1.18 (0.60–2.33) | Adjusted for age and birth date | |
| 2,500–2,999 | Referent | ||||||||
| 3,000–3,499 | 1.29 (0.90–1.91) | ||||||||
| 3,500–3,999 | 1.47 (1.00–2.18) | ||||||||
| ≥ 4,000 | 1.23 (0.80–2.00) | ||||||||
| [13] | 1996 | NCC | 550 | 1,478 | USA | <2,500 | 0.56 (0.34–0.93) | Adjusted for age | |
| 2,500–2,999 | 0.68 (0.47–0.99) | ||||||||
| 3,000–3,499 | 0.71 (0.50–0.99) | ||||||||
| 3,500–3,999 | 0.85 (0.59–1.22) | ||||||||
| ≥ 4,000 | Referent | ||||||||
| [14] | 1996 | PCC | 922 | 1,194 | USA | Age 21–45 years: | Adjusted for age, menopausal status, and maternal smoking; P for trend = 0.06 among both groups. The OR (95% CI) for birth weight ≥ 4,000 g among patients with early-onset breast cancer (≤ 30 years old) was 3.3 (1.0–11.0) | ||
| <2,500 | 1.3 (0.9–2.0) | ||||||||
| 2,500–2,999 | Referent | ||||||||
| 3,000–3,499 | 1.3 (1.0–1.7) | ||||||||
| 3,500–3,999 | 1.2 (0.8–1.6) | ||||||||
| ≥ 4,000 | 1.7 (1.1–2.5) | ||||||||
| Age 50–64 years: | |||||||||
| <2,500 | 0.9 (0.5–1.7) | ||||||||
| 2,500–2,999 | Referent | ||||||||
| 3,000–3,499 | 1.1 (0.7–1.7) | ||||||||
| 3,500–3,999 | 0.8 (0.4–1.3) | ||||||||
| ≥ 4,000 | 0.6 (0.3–1.1) | ||||||||
| [32] | 1997 | NCC | 1068 | 2,027 | Sweden | <2,500 | 0.80 (0.50–1.26) | Adjusted for maternal age, socioeconomic status, parity, and pre-eclampsia or eclampsia, neonatal jaundice, severe prematurity, and twinship | |
| 2,500–2,999 | Referent | ||||||||
| 3,000–3,499 | 1.00 (0.79–1.28) | ||||||||
| 3,500–3,999 | 0.99 (0.77–1.26) | ||||||||
| ≥ 4,000 | 1.04 (0.77–1.41) | ||||||||
| [33] | 1998 | PCC | 510 | 436 | USA | <2,500 | 1.2 (0.7–2.1) | Crude ORs | |
| 2,500–2,999 | Referent | ||||||||
| 3,000–3,499 | 1.0 (0.7–1.5) | ||||||||
| 3,500–3,999 | 1.0 (0.7–1.5) | ||||||||
| ≥ 4,000 | 1.3 (0.7–2.3) | ||||||||
| [15] | 2000 | LCC | 484 | 2,870 | USA | <1,500 | 1.59 (0.61–4.11) | Crude ORs | |
| 1,500–2,499 | 1.33 (0.94–1.90) | ||||||||
| 2,500–3,499 | Referent | ||||||||
| 3,500–4,499 | 1.08 (0.87–1.34) | ||||||||
| ≥ 4,500 | 3.29 (1.37–7.92) | ||||||||
| [34] | 2001 | LTCC | 87 | 87 | Sweden | <1,999 | Referent | Matched analysis by conditional logistic regression | |
| 2,000–2,499 | 1.6 (0.6–4.0) | ||||||||
| 2,599–2,999 | 2.4 (0.9–6.2) | ||||||||
| ≥ 3,000 | 1.6 (0.4–5.6) | ||||||||
| (P trend = 0.05) | |||||||||
| [43] | 2001 | LCC | 319 | 768 | USA | <2,500 | 1.4 (0.55–3.4) | Crude ORs. Higher birth weight (≥ 3,500 g) carried a marginal significantly higher risk for breast cancer (OR 1.76 [95% CI 0.90–3.35]) relative to lower birth weight (<3,500 g) | |
| 2,500–3,750 | Referent | ||||||||
| ≥ 3,750 | 0.9 (0.50–1.6) | ||||||||
| [16] | 2001 | LTCC | 90 | 90 | Sweden | ≤ 2,000 | Referent | Crude ORs. Study subjects were women with opposite-sexed pair twins | |
| 2,001–2,500 | 3.2 (0.8–12.6) | ||||||||
| 2,501–3,000 | 3.5 (1–13) | ||||||||
| 3,001–3,500 | 5.8 (1.3–25.7) | ||||||||
| ≥ 3,501 | 12.1 (1.1–138.8) | ||||||||
| [35] | 2002 | PCC | 2,088 | 2,187 | USA | <2,500 | 1.10 (0.90–1.35) | Adjusted for age and residential regions (states) | |
| 2,500–2,999 | 0.90 (0.70–1.10) | ||||||||
| 3,000–3,499 | Referent | ||||||||
| 3,500–3,999 | 1.07 (0.90–1.30) | ||||||||
| 4,000–4,499 | 0.89 (0.70–1.14) | ||||||||
| ≥ 4,500 | 1.18 (0.90–1.51) | ||||||||
| [44] | 2002 | PCC | 288 | 350 | China | <2,500 | 0.9 (0.4–2.0) | Adjusted for age income, family history of breast cancer in first-degree relative, history of fibroadenoma, age at menarche, parity, and age at first live birth. | |
| 2,500–2,999 | Referent | ||||||||
| 3,000–3,499 | 1.1 (0.8–1.6) | ||||||||
| 3,500–3,999 | 0.8 (0.4–1.4) | ||||||||
| ≥ 4,000 | 0.7 (0.4–1.4) | ||||||||
| [17] | 2002 | LCC | 373 | 1,150 | USA | <3,090 | Referent | Adjusted for parity and age at first birth. P for trend = 0.02 | |
| 3,090–3,410 | 1.1 (0.8–1.5) | ||||||||
| 3,420–3,720 | 1.2 (0.9–1.6) | ||||||||
| ≥ 3,630 | 1.4 (1.1–1.9) | ||||||||
| [18] | 2003 | LCC | 881 | 3,423 | Denmark | <2,500 | 1.66 (1.00–2.51) | Adjusted for mother's marital status, maternal age, and birth order | |
| 2,500–2,999 | 0.83 (0.60–1.10) | ||||||||
| 3,000–3,499 | Referent | ||||||||
| 3,500–3,999 | 0.98 (0.80–1.17) | ||||||||
| ≥ 4,000 | 1.25 (1.00–1.55) | ||||||||
| [19] | 2004 | NCC | 89 | 238 | Sweden | 100 g increase | 1.06 (1.00–1.12) | Adjusted for gestational age, birth year, and maternal hypertension/proteinuria | |
| [45] | 2004 | LCC | 2471 | 9801 | USA | <1,500 | 0.64 (0.40–1.11) | Adjusted for age and maternal age at first birth | |
| 1,500–1,999 | 1.05 (0.70–1.68) | ||||||||
| 2,000–2,499 | 1.02 (0.80–1.31) | ||||||||
| 2,500–3,499 | Referent | ||||||||
| 3,500–3,999 | 0.97 (0.90–1.08) | ||||||||
| 4,000–4,499 | 0.93 (0.80–1.11) | ||||||||
| ≥ 4,500 | 0.69 (0.40–1.09) | ||||||||
| [36] | 2004 | PCC | 196 | 167 | USA | All subjects: | Adjusted for age, race and sampling fractions, body mass index, household income, and maternal age. Tertiles are race specific with cutpoints derived from controls. White women: <3,062, 3,062–3,458, >3,458 g; black women: <3,146, 3,146–3,488, >3,488 g. Restricted data using birth weight measured in pounds and ounces and participant delivered in a medical facility by a physician | ||
| Lower tertile | 1.0 (0.6–1.7) | ||||||||
| Central tertile | Referent | ||||||||
| Upper tertile | 0.7 (0.4–1.2) | ||||||||
| White, restricted | |||||||||
| data: | |||||||||
| Lower tertile | 1.1 (0.5–2.4) | ||||||||
| Central tertile | Referent | ||||||||
| Upper tertile | 1.4 (0.6–2.0) | ||||||||
| [20] | 2006 | PCC | 2,386 | 2,502 | Poland | <2,500 | Referent | Adjusted for: age, education, age at menarche, menopausal status and age at menopause, age at first full-term pregnancy, number of full-term pregnancies, family history of breast cancer among first-degree relatives, mammography screening, and current body mass index. Lower birth weight (<2,500 g) carries greater risk than birth weight of 2,500–4,000 g among women under 45 years old | |
| 2,500–4,000 | 1.22 (0.92–1.62) | ||||||||
| >4,000 | 1.54 (1.08–2.19) | ||||||||
| (p-trend = 0.01) | |||||||||
| [37] | 2006 | PCC | 1,166 | 2,105 | USA | <2,495 | 1.19 (0.85–1.66) | Adjusted for age (years), education (years), race, body mass index, history of breast benign disease, family history of breast cancer, lactation (months), age at menarche (years), age at first full-term pregnancy (years), age at menopause (years), parity | |
| 2,495–3,130 | Referent | ||||||||
| 3,131–3,855 | 0.97 (0.75–1.25) | ||||||||
| >3,855 | 1.03 (0.74–1.44) | ||||||||
| Cohort studies | [21] | 1999 | LCohort | 57 | 152,590 | Sweden | <2,500 | Referent | Standardization for sex, age, and age-specific incidence rate |
| 2,500–3,999 | 1.3 (0.6–2.4) | ||||||||
| 4,000–4,499 | 1.2 (0.0–6.7) | ||||||||
| ≥ 4,500 | 1.3 (0.7–2.3) | ||||||||
| [22] | 2000 | Cohort | 37 | 2,221 | UK | All ages | Adjusted for age. P for trend = 0.03 among premenopausal women | ||
| <3,000 | Referent | ||||||||
| 3,000–3,499 | 1.05 (0.41–2.71) | ||||||||
| 3,500–3,999 | 1.76 (0.72–4.33) | ||||||||
| ≥ 4,000 | 2.02 (0.59–6.90) | ||||||||
| Premenopausal ages | |||||||||
| <3,000 | Referent | ||||||||
| 3,000–3,499 | 1.99 (0.40–9.86) | ||||||||
| 3,500–3,999 | 3.26 (0.69–15.36) | ||||||||
| ≥ 4,000 | 5.65 (0.95–33.84) | ||||||||
| [38] | 2001 | LCohort | 177 | 3,447 | Sweden | ≤ 2,000 | Referent | Crude hazard ratios | |
| 2,001–2,500 | 1.4 (0.6–3.4) | ||||||||
| 2,501–3,000 | 1.9 (0.8–4.3) | ||||||||
| 3,001–3,500 | 1.5 (0.6–3.5) | ||||||||
| ≥ 3,501 | 1.9 (0.7–5.0) | ||||||||
| [39] | 2001 | Cohort | 62 | 1260 | Sweden | ≤ 3,000 | Referent | Singleton only; adjusted for gestational age and cohort membership | |
| 3,010–3,349 | 1.16 (0.47–2.87) | ||||||||
| 3,350–3,590 | 1.65 (0.71–3.86) | ||||||||
| 3,600–3,960 | 1.58 (0.67–3.72) | ||||||||
| ≥ 4,000 | 1.57 (0.67–3.64) | ||||||||
| [23] | 2003 | LCohort | 63 | 5,352 | Sweden | <3,000 | Referent | Crude ORs;P for trend = 0.01 | |
| 3,000–3,499 | 1.46 (0.60–3.43) | ||||||||
| 3,500–3,999 | 2.09 (0.90–4.85) | ||||||||
| ≥ 4,000 | 2.78 (1.10–7.15) | ||||||||
| [24] | 2003 | LCohort | 2,334 | 106,504 | Denmark | 1,000 g increase | 9 (0.02–17)% | Adjusted for age and calendar period. Additional adjustment for parity and age at first birth did not indicate confounding | |
| [25] | 2003 | LCohort | 39 | 1483 | Sweden | 500–1,999 | 1.14 (0.70–1.85) | Standardized incidence ratio (expected/observed) | |
| 2,000–2,999 | 0.71 (0.40–1.15) | ||||||||
| ≥ 3,000 | 2.55 (1.03–5.25) | ||||||||
| [26]a | 2004 | LCohort | 2,074 | 91,601 | Denmark | Median of each quintile | Adjusted for age and calendar period. No change in estimates when additionally adjusted for parity and age at first birth | ||
| 2.5 | Referent | ||||||||
| 3.0 | 0.98 (0.85–1.13) | ||||||||
| 3.4 | 1.06 (0.93–1.20) | ||||||||
| 3.6 | 1.05 (0.87–1.27) | ||||||||
| 4.0 | 1.17 (1.02–1.33) | ||||||||
| [27] | 2004 | Cohort | 59 | 2,176 | UK | <3,000 | Referent | Adjusted for age; P for trend = 0.03 | |
| 3,000–3,499 | 1.37 (0.34–5.47) | ||||||||
| 3,500–3,999 | 2.18 (0.58–8.21) | ||||||||
| ≥ 4,000 | 5.03 (1.13–22.47) | ||||||||
| [28] | 2005 | LCohort | 311 | 16,011 | USA | <3,040 | Referent | Adjusted for year of birth | |
| 3,040–3,310 | 1.4 (1.0–2.1) | ||||||||
| 3,320–3,550 | 1.0 (0.6–1.5) | ||||||||
| 3,560–3,830 | 1.3 (0.9–1.9) | ||||||||
| ≥ 3,840 | 1.5 (1.0–2.2) | ||||||||
| [29]a | 2005 | LCohort | 367 | 5,346 | Sweden | <50 years | |||
| <3,000 | Referent | ||||||||
| 3,000–3,499 | 1.81 (0.77–4.26) | ||||||||
| 3,500–3,999 | 2.66 (1.09–6.46) | ||||||||
| ≥ 4,000 | 4.00 (1.49–10.72) | ||||||||
| ≥ 50 years | |||||||||
| <3,000 | Referent | ||||||||
| 3,000–3,499 | 0.86 (0.62–1.19) | ||||||||
| 3,500–3,999 | 1.06 (1.20–3.34) | ||||||||
| ≥ 4,000 | 0.91 (0.57–1.46) | ||||||||
| [40] | 2006 | Cohort | 97 | 5,847 | USA | <3,000 | 0.98 (0.61–1.60) | Adjusted for age | |
| 3,000–3,499 | Referent | ||||||||
| ≥ 3,500 | 1.09 (0.66–1.80) | ||||||||
| [30] | 2006 | Cohort | 3,140 | 91,601 | USA | Premenopause | Adjusted for age: P for trend = 0.019 | ||
| <2,495 | 0.69 (0.50–0.94) | ||||||||
| 2,495–3,130 | 0.79 (0.64–0.97) | ||||||||
| 3,131–3,810 | 0.76 (0.63–0.93) | ||||||||
| >3,810 | Referent | ||||||||
| Postmenopause: | Adjusted for age: P for trend = 0.99 | ||||||||
| <2,495 | 1.04 (0.88–1.23) | ||||||||
| 2,495–3,130 | 1.00 (0.87–1.14) | ||||||||
| 3,131–3,855 | 1.05 (0.93–1.20) | ||||||||
| >3,855 | Referent | ||||||||
| [31] | 2006 | Cohort | 209 | 1,024 | USA | <2,500 | 0.9 (0.5–1.6) | Hazard ratio; adjusted for age at diagnosis, diagnosis year, stage at diagnosis, and birth order, with exception of birth order, which is adjusted for maternal age | |
| 2,500–3,999 | Referent | ||||||||
| ≥ 4,000 | 1.8 (1.0–3.1) | ||||||||
| (P trend = 0.1) | |||||||||
| [41] | 2007 | Cohort | 657 | 38,566 | Sweden | <2,500 | 0.65 (0.43–0.99) | Adjusted for adult body mass index | |
| 2,500–3,000 | 1.04 (0.86–1.25) | ||||||||
| >3,000 | Referent | ||||||||
Cohort, cohort study; LCC, case-control study with linkage with population and cancer registry data; LCohort, cohort study with linkage with population and cancer registry data; LTCCS, twin case-control study by using linkage with birth and cancer registry data; NCC, nested case-control study in cohort; PCC, population-based case-control study. aThe numbers of cases and controls were not shown in the original article.
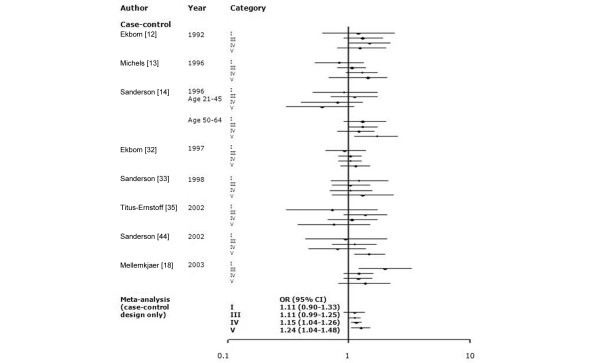
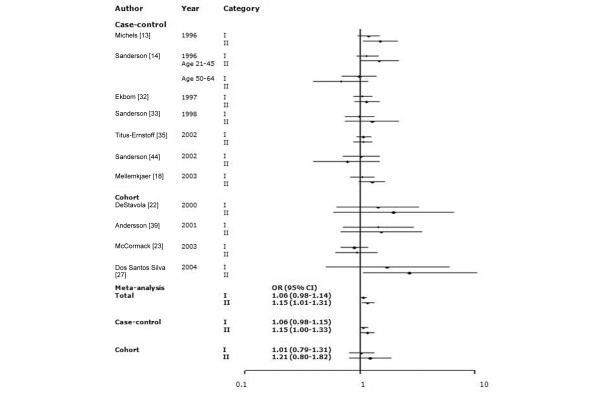
Among 34 studies of birth weight and breast cancer, we selected studies that employed the same categories of birth weight. To evaluate whether a J-shaped relationship existed, we grouped birth weight into more than three categories. The findings of meta-analysis of eight studies that utilized five categories of birth weight (<2,500, 2,500 to 2,999, 3,000 to 3,499, 3,500 to 3,999, and ≥4,000 g) and 11 studies that used three categories (<3,000, 3,000 to 3,999, and ≥4,000 g) are shown in Figures 1 and 2. To include more studies, we also categorized birthweights as <3,000 g (or ≤3,000 g) and ≥3,000 g (or >3,000 g; Figure 3). Sixteen studies among all 34 studies were included in the meta-analyses for birth weight and breast cancer: seven studies [13,14,18,32,33,35,44] were included in the all three meta-analyses; four studies [22,23,27,39] were included in the two of the three meta-analyses; and five studies were included in only one meta-analysis [12,16,28,34,38]. There was no significant heterogeneity across studies (P_Q test > 0.05 for all categories). In the five-category meta-analysis, ORs were 1.11 (95% CI 0.90 to 1.33) for birth weight <2,500 g, 1.11 (0.99 to 1.25) for 3,000 to 3,499 g, 1.15 (1.04 to 1.26) for 3,500 to 3,999 g, and 1.24 (1.04 to 1.48) for ≥4,000 g relative to the referent category of 2,500 to 2,999 g. In the three-category meta-analysis, ORs were 1.06 (95% CI 0.98 to 1.14) for 3,000 to 3,999 g and 1.15 (1.01 to 1.31) for ≥4,000 g relative to the referent category of <3,000 g. In the two-category meta-analysis, ORs were 1.09 (95% CI 1.02 to 1.18) for the category of >3,000 g (or ≥3,000 g) relative to the referent category of ≤3,000 g (or <3,000 g).
Figure 1.
Meta-analysis of the association between birth weight (five categories) and risk for breast cancer. The tests for homogeneity and for publication bias in the studies analyzed are as follows. Category I (birth weight <2,500 g) versus reference: Q = 9.66 (8 degrees of freedom), P = 0.29; Begg test, P = 0.75; Egger test, P = 0.66. Category II (2,500 to 2,999 g) is the reference. Category III (3,000 to 3,499 g) versus reference: Q = 6.53 (8 degrees of freedom), P = 0.59; Begg test, P = 0.25; Egger test, P = 0.46. Category IV (3,500 to 3,999 g) versus reference: Q = 4.17 (8 degrees of freedom), P = 0.84; Begg test, P = 0.60; Egger test, P = 0.93. Category V (≥4,000 g) versus reference: Q = 11.18 (8 degrees of freedom), P = 0.19; Begg test, P = 0.25; Egger test, P = 0.30. 1We used adjusted odds ratios (ORs) for meta-analysis because the numbers of cases and controls were not represented in the original article. CI, confidence interval.
Figure 2.
Meta-analysis of the association between birth weight (three categories) and risk for breast cancer. The tests for homogeneity and for publication bias in the studies analyzed are as follows. Category I (birth weight 3,000 to 3,999 g) versus reference (<3,000 g): Q = 4.97 (11 degrees of freedom), P = 0.93; Begg test, P = 0.54; Egger test, P = 0.27. Category II (≥4,000 g) versus reference: Q = 13.44 (11 degrees of freedom), P = 0.27; Begg test, P = 0.54; Egger test, P = 0.53. CI, confidence interval; OR, odds ratio.
Figure 3.
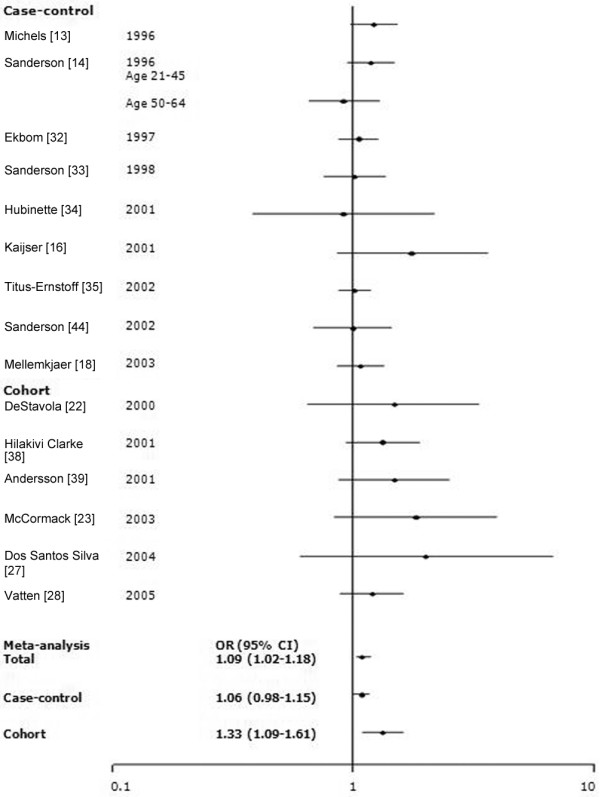
Meta-analysis of the association between birth weight (two categories) and risk for breast cancer. The tests for homogeneity and for publication bias in the studies analyzed are as folows. Reference (<3,000 g [or ≤3,000 g]) versus ≥3,000 g (or >3,000 g): Q = 11.57 (15 degrees of freedom), P = 0.93; Begg test, P = 0.15; Egger test, P = 0.50. CI, confidence interval; OR, odds ratio.
We identified 17 studies (15 case-control and two cohort) that assessed the association between birth order and breast cancer risk (Table 3). Eight of the studies reported an inverse relationship [14,20,31,35,36,42,46,47]. Some studies found significantly lower risks for second or later born children versus first-born children [31,46]. Some studies found significantly or marginally significantly reduced risk among women whose birth had been preceded by the birth of at least five siblings [20,35]. Other several studies noted an increased risk associated with higher birth order [15,37,48,49], whereas some studies failed to observe such an association [12,18,32,50]. One study did not supply the estimated risk but describe the P value by the mean difference of birth order [51].
Table 3.
Studies assessing the association of birth order and the risk of breast cancer
| Type of study | Ref. | Year | Design | Cases | Controls (or cohort) | Country/place of study | Birth order | OR (95% CI) | Comments |
| Case-control studies | [51] | 1967 | LCC-D | 229 | 229 | USA | 1 | - | The authors measured the mean value of birth weight instead of providing ORs (95% CIs). The mean difference between cases and matched controls was not significant (P > 0.2). They provided the frequency of each case and control in the tables and we calculated crude ORs |
| 2 | |||||||||
| 3 | |||||||||
| 4 | |||||||||
| 5 | |||||||||
| 6 | |||||||||
| ≥ 7 | |||||||||
| [50]a | 1980 | MCC | 4339 | 12,760 | USA, Japan, Slovenia, Athens, Taipei | 1 | Referent | The risks (point estimates) only by birth order were shown in the figure in the original article. | |
| 2 | 0.93 | ||||||||
| 3 | 1.08 | ||||||||
| 4 | 0.99 | ||||||||
| 5 | 1.05 | ||||||||
| 6 | 1.07 | ||||||||
| 7 | 1.18 | ||||||||
| ≥ 8 | 1.02 | ||||||||
| [42] | 1988 | PCC | 153 | 461 | USA | 1 | Referent | P for trend = 0.16 | |
| 2 | 0.92 (0.55–1.54) | ||||||||
| 3 | 0.98 (0.58–1.72) | ||||||||
| 4 | 0.69 (0.36–1.32) | ||||||||
| ≥ 5 | 1.03 (0.60–1.79) | ||||||||
| [46] | 1991 | MCC | 927 | 2,616 | USA/Wales/Japan | All ages | Adjusted for age, study center, parity, age at first birth, age at menarche, height, body mass index, maternal age at birth, and menopausal status | ||
| 1 | Referent | ||||||||
| 2 | 0.91 (0.73–1.02) | ||||||||
| 3 | 1.11 (0.87–1.27) | ||||||||
| ≥ 4 | 1.09 (0.81–1.18) | ||||||||
| Premenopausal | |||||||||
| 1 | Referent | ||||||||
| ≥ 2 | 0.76 (0.60–0.96) | ||||||||
| [12] | 1992 | LCC | 458 | 1,197 | Sweden | 1 | Referent | Adjusted for age and birth date | |
| ≥ 2 | 1.00 (0.76–1.32) | ||||||||
| [47] | 1994 | PCC | 2,414 | 9,138 | USA | 1 | Referent | Adjusted for age at first birth and number of children | |
| 2 | 0.90 (0.78–1.03) | ||||||||
| 3 | 0.98 (0.84–1.14) | ||||||||
| 4 | 0.86 (0.73–1.02) | ||||||||
| 5 | 0.93 (0.78–1.11) | ||||||||
| 6 | 1.02 (0.84–1.23) | ||||||||
| 7 | 0.91 (0.73–1.14) | ||||||||
| ≥ 8 | 0.88 (0.75–1.04) | ||||||||
| [14] | 1996 | PCC | 1,129 | 1,393 | USA | 1 | Referent | Adjusted for age, menopausal status, and maternal smoking; P for trend = 0.06 among both groups | |
| 2 | 1.0 (0.7–1.4) | ||||||||
| ≥ 3 | 0.8 (0.6–1.1) | ||||||||
| [32] | 1997 | NCC | 1,068 | 2,727 | Sweden | 1 | Referent | Adjusted for maternal age, socioeconomic status, parity, and preeclampsia or eclampsia, neonatal jaundice, severe prematurity, and twinship | |
| 2 | 1.01 (0.83–1.22) | ||||||||
| ≥ 3 | 1.01 (0.81–1.26) | ||||||||
| [15] | 2000 | LCC | 481 | 2,863 | USA | 1 | 1.07 (0.84–1.35) | Crude ORs | |
| 2–3 | Referent | ||||||||
| 4–5 | 1.06 (0.81–1.38) | ||||||||
| ≥ 6 | 1.50 (1.06–2.13) | ||||||||
| [35] | 2002 | PCC | 1,555 | 1,539 | USA | 1 | Referent | Adjusted for age and residential regions (states) | |
| 2 | 1.07 (0.88–1.30) | ||||||||
| 3 | 1.07 (0.85–1.35) | ||||||||
| 4 | 1.01 (0.77–1.31) | ||||||||
| 5 | 0.66 (0.48–0.92) | ||||||||
| ≥ 6 | 0.81 (0.62–1.08) | ||||||||
| [18] | 2003 | LCC | 881 | 3,423 | Denmark | 1 | Referent | Adjusted for mother's marital status, maternal age, and birth order | |
| ≥ 2 | 1.01 (0.83–1.12) | ||||||||
| [36] | 2004 | PCC | 854 | 785 | USA | All subjects | Adjusted for age, race and sampling fractions, body mass index, hosehold income, maternal age | ||
| 1 | Referent | ||||||||
| 2–4 | 0.9 (0.7–1.1) | ||||||||
| ≥ 5 | 1.0 (0.8–1.3) | ||||||||
| Born ≥ 1948 | |||||||||
| 1 | Referent | ||||||||
| 2–4 | 0.9 (0.6–1.4) | ||||||||
| ≥ 5 | 0.6 (0.3–1.3) | ||||||||
| [48]a | 2005 | MCC | 24 | 34 | Nigeria | ≤ 3 | Referent | Crude ORs | |
| ≥ 4 | 1.50 (0.25–8.98) | ||||||||
| [20] | 2005 | PCC | 1642 | 1,713 | Poland | 1 | Referent | Adjusted for age, education, age at menarche, menopausal status and age at menopause, age at first full-term pregnancy, number of full-term pregnancies, family history of breast cancer among first-degree relatives, mammography screening, and current body mass index | |
| 2 | 1.07 (0.91–1.24) | ||||||||
| 3–5 | 0.99 (0.85–1.15) | ||||||||
| ≥ 6 | 0.81 (0.61–1.06) | ||||||||
| P for trend = 0.81 | |||||||||
| [37] | 2006 | PCC | 1,166 | 2,105 | USA | 1 | Referent | Adjusted for age (years), education (years), race, body mass index, history of breast benign disease, family history of breast cancer, lactation (months), age at menarche (years), age at first full-term pregnancy (years), age at menopause (years), parity | |
| ≥ 2 | 1.27 (0.88–1.85) | ||||||||
| Cohort studies | [31] | 2006 | Cohort | 209 | 1,024 | USA | 1 | Referent | Hazard ratio for breast cancer mortality: adjusted for age at diagnosis, diagnosis year, stage at diagnosis, and birth order, with exception of birth order, which is adjusted for maternal age |
| 2 | 0.2 (0.2–0.3) | ||||||||
| ≥ 3 | 0.2 (0.2–0.3) | ||||||||
| P for trend < 0.01 | |||||||||
| [49]a | 2001 | Cohort | - | - | Sweden | Continuous scale | 1.05 (1.01–1.10) |
aWe did not include these studies in the meta-analysis because they employed different categories or a continuous scale, or they did not provide the numbers of cases and controls in the original article. Cohort, cohort study; LCC, case-control study with linkage with population and cancer registry data; LCC-D, case-control study with linkage with population and cancer death certification data; MCC, multicenter case-control study; NCC, nested case-control study in cohort; PCC, population-based case-control study.
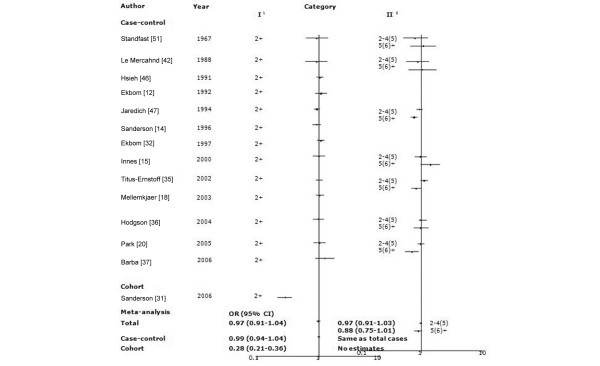
For the meta-analysis, we included 14 studies (13 case-control studies and one cohort) that used two birth order categories: 1 (referent) and ≥2. There was significant heterogeneity across all studies (P_Q test < 0.01), although there was no significant heterogeneity across the case-control studies (P_Q test = 0.90). As shown in Figure 4, there was no difference in risk according to birth order across all studies (OR 0.97 [95% CI 0.91 to 1.04]) or within the case control studies (OR 0.99 [95% CI 0.94 to 1.04]). We calculated the crude odds ratio from the cohort study [31], and the result was very different from the summary OR (calculated crude OR 0.28 [95% CI 0.21 to 0.36]). The results of all case-control studies were near null, whereas the cohort study found a significant risk reduction in birth orders of 2 or greater. We also examined the seven studies that classified individuals according to three birth order levels (1 [referent], 2 to 4, ≥5; Figure. 4). There was significant heterogeneity across studies (all of which were case-control studies) for the highest birth order category (P_Q test = 0.03) Women with a birth order of ≥5 were at nonsignificantly reduced risk compared with first-born women (OR 0.88 [95% CI 0.75–1.01]). There was no difference in risk for women of birth orders 2 to 4 (OR 0.97 [95% CI 0.91–1.03]).
Figure 4.
Meta-analysis of the association between birth order and risk for breast cancer. The tests for homogeneity and for publication bias in the studies analyzed are as follows. Category I (birth order 2+) versus reference (birth order 1): Q = 87.79 (13 degrees of freedom), P < 0.01; Begg test, P = 0.44; Egger test, P = 0.46. Category II (birth order 5+ and 2 to 4) versus reference: Q = 4.56 (6 degrees of freedom), P = 0.60; Begg test, P = 0.37; Egger test, P = 0.44. Category II (birth order ≥6, 2 to 5) versus reference: Q = 14.42 (6 degrees of freedom), P = 0.60; Begg test, P = 0.37; Egger test, P = 0.44. 1Category I of birth order was 2+ vs 1. 2Category II of birth order was composed of two conditions: 5+ and 2 to 4; and ≥6 and 2 to 5 vs 1. 3We used adjusted odds ratios (ORs) for meta-analysis because the numbers of cases and controls were not represented in the original article. CI, confidence interval.
We identified 28 studies (22 case-control and six cohort) that assessed the association between maternal age and breast cancer risk (Table 4). Seven studies observed modestly increased risks for daughters born to older mothers [15,31,32,36,42,46,52]. A pattern of slight decrease after modest increase in risk was found in five other studies [50,53-56]. Fourteen studies, however, no association was observed [12,14,18,20,35,37,38,47,49,57-60]. Two studies did not estimate the risks [51,61].
Table 4.
Studies assessing the association of maternal age with risk for breast cancer
| Ref. | Year | Design | Cases | Controls (or cohort) | Country/place of study | Maternal age (years) | OR (95% CI) | Comments | |
| Case-control studies | [51] | 1967 | LCC-D | 229 | 229 | USA | ≤ 19 | Mean maternal age among cases was higher than that among controls (P < 0.005). The frequency of each case and control were shown in the tables provided and we calculated crude ORs | |
| 20–24 | |||||||||
| 25–29 | |||||||||
| 30–34 | |||||||||
| 35–39 | |||||||||
| ≥ 40 | |||||||||
| [61]a | 1974 | PCC | 308 | 308 | USA | Matched analysis; the mean maternal age was 27.3 years among cases and 26.3 years among controls (P < 0.01) | |||
| [50]b | 1980 | MCC | 4339 | 12760 | USA, Japan, Slovenia, Athens, Taipei | ≤ 19 | Referent | Authors showed point estimates of ORs without 95% CIs. The frequencies for each case and control were given in the tables provided and we calculated crude ORs | |
| 20–24 | 1.05 | ||||||||
| 25–29 | 1.22 | ||||||||
| 30–34 | 1.19 | ||||||||
| 35–39 | 1.31 | ||||||||
| ≥ 40 | 1.18 | ||||||||
| [53]a | 1984 | MCC | 1,176 | 1,176 | England | ≤ 20 | Referent | Adjusted for age, social class, family history of breast cancer, age at first-term birth, past history of benign breast disease, age at menarche, menopausal status, cigarette smoking, and oral contraceptive use | |
| 21–25 | 1.41 (0.92–2.18) | ||||||||
| 26–30 | 1.19 (0.78–1.81) | ||||||||
| 31–35 | 1.29 (0.83–1.98) | ||||||||
| ≥ 36 | 1.19 (0.68–1.67) | ||||||||
| [42]a | 1988 | PCC | 153 | 461 | USA | All women | Matched analysis | ||
| 15–22 | 1.18 (0.71–1.97) | ||||||||
| 23–26 | Referent | ||||||||
| 27–30 | 1.22 (0.71–2.10) | ||||||||
| 31–46 | 1.66 (0.99–2.78) | ||||||||
| P for trend = 0.67 | |||||||||
| Younger women | |||||||||
| 15–23 | 1.39 (0.65–2.95) | ||||||||
| 24–28 | Referent | ||||||||
| 29–46 | 2.21 (1.02–4.80) | ||||||||
| P for trend = 0.08 | |||||||||
| [52]a | 1989 | PCC | 801 | 1,573 | USA | Continuous | 1.24 (1.09–1.41) | Crude OR | |
| [54] | 1990 | PCC | 2,291 | 3,144 | USA | ≤ 19 | Referent | Adjusted for age and parity, age at first pregnancy, total duration of breast feeding, race, age at menarche, menopausal status, body mass index, family history of breast cancer, and breast biopsy | |
| 20–24 | 0.95 (0.77–1.16) | ||||||||
| 25–29 | 1.13 (0.92–1.38) | ||||||||
| 30–34 | 1.16 (0.93–1.45) | ||||||||
| 35–39 | 1.46 (1.10–1.93) | ||||||||
| ≥ 40 | 1.20 (0.79–1.83) | ||||||||
| [55] | 1991 | PCC | 1761 | 1,116,553 person-years | USA | ≤ 19 | Referent | Crude ORs | |
| 20–24 | 1.02 (0.82–1.46) | ||||||||
| 25–29 | 1.12 (1.04–1.38) | ||||||||
| 30–34 | 1.16 (0.93–1.44) | ||||||||
| 35–39 | 1.17 (0.92–1.48) | ||||||||
| ≥ 40 | 1.08 (0.80–1.46) | ||||||||
| [46]a | 1991 | MCC | 927 | 2616 | USA, Wales, Japan | Each 5-yrs | 1.06 (1.01–1.10) | Adjusted for age, study center, parity, age at first birth, age at menarche, height, BMI, maternal age at birth, and menopausal status | |
| [12]b | 1992 | LCC | 458 | 1,197 | Sweden | Each 5-year band | 1.01 (0.92–1.12) | Adjusted for age and birth date. The authors estimated breast cancer risk according to each 5-year band of maternal age. The frequency of each case and control were given in the tables provided and we calculated crude ORs | |
| [47] | 1994 | PCC | 2,412 | 9,138 | USA | ≤ 19 | Referent | Adjusted for age at first birth and number of children | |
| 20–24 | 1.05 (0.85–1.30) | ||||||||
| 25–29 | 1.10 (0.89–1.37) | ||||||||
| 30–34 | 1.10 (0.88–1.37) | ||||||||
| 35–39 | 1.09 (0.87–1.37) | ||||||||
| ≥ 40 | 0.99 (0.76–1.28) | ||||||||
| [14] | 1996 | PCC | 1,934 | 2,161 | USA | ≤ 24 | Referent | Adjusted for age, menopausal status, and maternal smoking | |
| 25–29 | 1.0 (0.8–1.2) | ||||||||
| 30–34 | 0.9 (0.6–1.1) | ||||||||
| ≥ 35 | 1.0 (0.7–1.5) | ||||||||
| [57] | 1997 | PCC | 1,253 | 1,121 | USA | ≤ 19 | Referent | Adjusted for age, menopausal status, age at menarche, parity, age at first birth, body mass index, past history of benign breast disease, and recent alcohol intake | |
| 20–24 | 0.84 (0.62–1.14) | ||||||||
| 25–29 | 1.02 (0.76–1.37) | ||||||||
| 30–34 | 0.93 (0.68–1.28) | ||||||||
| 35–39 | 1.16 (0.82–1.65) | ||||||||
| ≥ 40 | 0.92 (0.62–1.37) | ||||||||
| [58] | 1997 | PCC | 2,106 | 1,926 | USA | ≤ 19 | Referent | Adjusted for age, study site, family history of breast cancer, breast biopsy, a combination variable including number of full-term births and age at first full-term pregnancy, age at menarche, menopausal status, body mass index, average lifetime alcohol consumption, and the number of mammograms | |
| 20–24 | 0.96 (0.7–1.2) | ||||||||
| 25–29 | 0.96 (0.7–1.2) | ||||||||
| 30–34 | 0.91 (0.7–1.2) | ||||||||
| ≥ 35 | 0.93 (0.7–1.3) | ||||||||
| [32]a | 1997 | NCC | 1,067 | 2,725 | Sweden | Each 5-year band | 1.06 (0.99–1.14) | Adjusted for maternal age, socioeconomic status, parity, and pre-eclampsia or eclampsia, neonatal jaundice, severe prematurity, and twinship | |
| [15] | 2000 | LCC | 481 | 2863 | USA | ≤ 19 | 1.19 (0.83–1.72) | Crude ORs | |
| 20–24 | Referent | ||||||||
| 25–29 | 1.26 (0.97–1.64) | ||||||||
| 30–34 | 1.38 (1.04–1.84) | ||||||||
| ≥ 35 | 1.70 (1.23–2.35) | ||||||||
| [35] | 2002 | PCC | 1,555 | 1,539 | USA | ≤ 19 | 1.02 (0.75–1.39) | Adjusted for age and state | |
| 20–24 | 0.98 (0.81–1.18) | ||||||||
| 25–29 | Referent | ||||||||
| 30–34 | 1.15 (0.93–1.42) | ||||||||
| 35–39 | 1.22 (0.94–1.58) | ||||||||
| ≥ 40 | 1.27 (0.90–1.69) | ||||||||
| [18] | 2003 | LCC | 881 | 3,423 | Denmark | ≤ 24 | Referent | Adjusted for mother's marital status, maternal age, and birth order | |
| 25–29 | 1.08 (0.88–1.32) | ||||||||
| ≥ 30 | 1.11 (0.90–1.36) | ||||||||
| [36]a | 2004 | PCC | 854 | 785 | USA | ≤ 18 | 1.8 (0.9–3.4) | ||
| 19–22 | Referent | Adjusted for age, race and sampling fractions; tertiles are race specific with cutpoints derived from controls | |||||||
| 23–27 | 3.0 (1.8–5.0) | ||||||||
| ≥ 28 | 2.5 (1.6–4.0) | ||||||||
| [56] | 2005 | MCC | 1,060 | 1,060 | Korea | ≤ 24 | Referent | Adjusted for age, family history of breast cancer in first-or second-degree relatives, menopausal status, and lifetime estrogen exposure duration | |
| 25–29 | 1.2 (0.93–1.47) | ||||||||
| 30–34 | 1.4 (1.12–1.83) | ||||||||
| ≥ 35 | 1.1 (0.83–1.37) | ||||||||
| [20] | 2006 | PCC | 1,642 | 1,713 | Poland | ≤ 19 | Referent | Adjusted for: age, education, age at menarche, menopausal status and age at menopause, age at first full-term pregnancy, number of full-term pregnancies, family history of breast cancer among first-degree relatives, mammography screening, and current body mass index | |
| 20–24 | 1.02 (0.75–1.39) | ||||||||
| 25–29 | 1.07 (0.79–1.46) | ||||||||
| 35–39 | 1.16 (0.84–1.60) | ||||||||
| ≥ 35 | 0.91 (0.66–1.27) | ||||||||
| P for trend = 0.76 | |||||||||
| [37]a | 2006 | PCC | 1,166 | 2,105 | USA | ≤ 24 | Referent | Adjusted for: age, education, race, body mass index, history of breast benign disease, family history of breast cancer, lactation, age at menarche, age at first full-term pregnancy, age at menopause, and parity | |
| 25–35 | 0.87 (0.67–1.13) | ||||||||
| >35 | 0.87 (0.59–1.27) | ||||||||
| Cohort studies | [59] | 1995 | Cohort | 149 | 75,237 | USA | ≤ 24 | Referent | Adjusted for age, education, menopausal status, parity, body mass index, height, smoking, and alcohol drinking |
| 25–29 | 1.3 (0.8–2.0) | ||||||||
| 30–34 | 1.4 (0.9–2.1) | ||||||||
| ≥ 35 | 1.2 (0.7–2.0) | ||||||||
| [60] | 1995 | Cohort | 1,967 | 384,769 | Sweden | ≤ 19 | Referent | Breast cancer mortality; adjusted for age | |
| 20–24 | 0.99 (0.82–1.21) | ||||||||
| 25–29 | 1.00 (0.82–1.22) | ||||||||
| 30–34 | 0.97 (0.79–1.18) | ||||||||
| 35–39 | 1.04 (0.84–1.29) | ||||||||
| 40–44 | 0.93 (0.71–1.21) | ||||||||
| ≥ 45 | 1.39 (0.91–2.13) | ||||||||
| [49]a | 2001 | Cohort | Sweden | Continuous scale | 1.07 (0.91–1.27) | Adjusted for spouse age, year of diagnosis, and birth order | |||
| [38]a | 2001 | Cohort | 177 | 3,447 | Filand | Continuous scale | - | No association | |
| [31] | 2006 | Cohort | 249 | 1,024 | USA | ≤ 24 | Referent | Hazard ratio; adjusted for age at diagnosis, diagnosis year, stage at diagnosis, and birth order, with exception of birth order, which is adjusted for maternal age | |
| 25–29 | 1.2 (0.9–1.7) | ||||||||
| 30–34 | 1.4 (0.9–1.9) | ||||||||
| ≥ 35 | 1.7 (1.1–2.8) | ||||||||
| P for trend = 0.03 |
aWe did not include these studies in the meta-analysis because they employed different categories or a continuous scale, or they did not provide the numbers of cases and controls in the original articlebWe included this study in the meta-analysis because we calculated the crude OR using the number of subjects represented the original article. Cohort, cohort study; LCC, case-control study with linkage with population and cancer registry data; LCC-D, case-control study with linkage with population and cancer death certification data; MCC, multicenter case-control study; NCC, nested case-control study in cohort; PCC, population-based case-control study.
In our meta-analyses, we included the 18 studies that reported categorical data and examined three age categories (≤24 [referent], 25 to 29, and ≥30 years; Figure 5). There was, however, significant study heterogeneity (P_Q test < 0.01 for 25 to 29 years and for ≥30 years). Heterogeneity was also present across case-control studies and studies published after 2000 (P_Q test < 0.01). The ORs (95% CI) were 1.18 (1.05 to 1.11) for 25 to 29 years and 1.23 (1.07 to 1.15) for ≥30 years across all studies.
Figure 5.
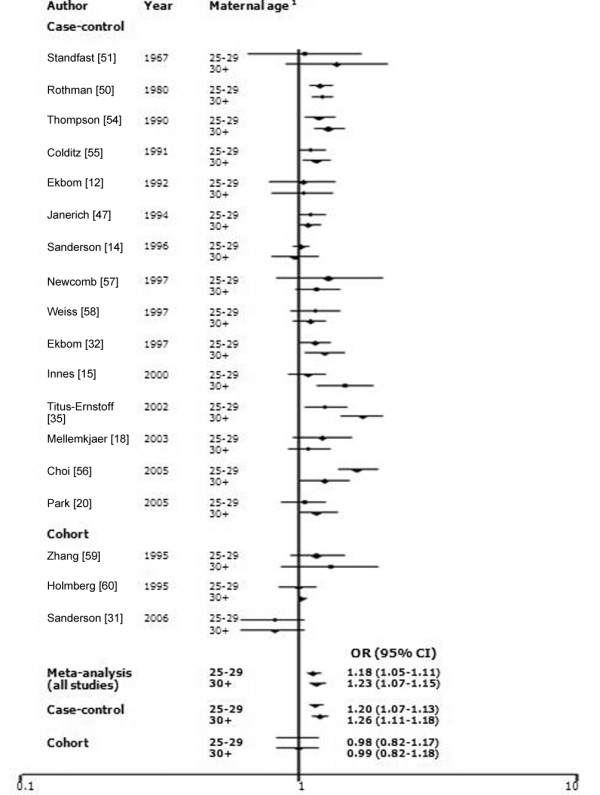
Meta-analysis for the association between maternal age and the risk of breast cancer. The tests for homogeneity and for publication bias in the studies analyzed are as follows. Maternal age 25 to 29 years: Q = 39.40 (17 degrees of freedom), P < 0.01; Begg test, P = 0.85; Egger test, P = 0.38, Maternal age 30+ years: Q = 67.34 (17 degrees of freedom), P < 0.01; Begg test, P = 0.88; Egger test, P = 0.07. 1The reference for maternal age is ≤24 years old. CI, confidence interval; OR, odds ratio.
We identified 15 studies (10 case-control and five cohort) that assessed the association between prematurity and breast cancer risk (Table 5). Most studies did not observe a significant relationship [13-16,20,25,29,31,33,40,42,45]. Two studies found that extreme prematurity was associated with an increased risk (OR 3.96 [95% CI 1.46 to 10.81] for ≤32 weeks relative to ≥33 weeks [32], and SIR (standardized incidence ratio) 6.7 [95% CI 1.4 to 19.5] for <31 weeks [62]). In contrast, another study [34] found that longer gestation was associated with a significantly increased risk (OR 8.4 [95% CI 1.3 to 54.4] for ≥40 weeks relative to ≤32 weeks).
Table 5.
Table 5 Studies assessing the association of premature birth and the risk of breast cancer
| Type of study | Author | Year | Design | Cases | Controls (or cohort) | Country/place of study | Gestational age (weeks) | OR (95% CI) | Comments |
| Case-control studies | [42]a | 1988 | PCC | 153 | 461 | USA | 25–32 | 1.16 (0.50–1.54) | Matched analysis |
| 33–40 | Referent | ||||||||
| [13]b | 1996 | NCC | 571 | 1,525 | USA | Categorical | Adjusted for age | ||
| 40 | Referent | ||||||||
| 38–39 | 0.76 (0.44–1.32) | ||||||||
| 36–37 | 0.96 (0.59–1.56) | ||||||||
| Binomial | |||||||||
| ≥ 37 | Referent | ||||||||
| ≤ 36 | 0.82 (0.37–1.82) | ||||||||
| [14] | 1996 | PCC | 1123 | 1371 | USA | Nonpreterm | Referent | Adjusted for age, menopausal status, and maternal smoking | |
| Preterm | 1.1 (0.5–2.1) | ||||||||
| [32]a | 1997 | NCC | 1,010 | 2,625 | Sweden | ≥ 33 | Referent | Adjusted for maternal age, matermal socioeconomic status, maternal parity, maternal pre-eclampsia or eclampsia, neonatal jaundice, severe prematurity, twin, and birth weight | |
| ≤ 32 | 3.96 (1.46–10.81) | ||||||||
| [33]b | 1998 | PCC | 502 | 433 | USA | ≥ 43 | 1.5 (0.8–2.6) | Crude ORs | |
| 37–42 | Referent | ||||||||
| ≤ 36 | 0.9 (0.5–1.8) | ||||||||
| [15]a,b | 2000 | LCC | 480 | 2,854 | USA | ≥ 37 | Referent | Crude ORs | |
| 33–36 | 1.34 (0.85–2.13) | ||||||||
| ≤ 32 | 0.55 (0.19–1.57) | ||||||||
| [34]a,b | 2001 | LCC | 87 | 87 | Sweden | ≥ 40 | 8.4 (1.3–54.4) | Matched analysis by conditional logistic regression | |
| 37–40 | 3.4 (0.7–17.0) | ||||||||
| 33–36 | 3.5 (0.7–17.5) | ||||||||
| ≤ 32 | Referent | ||||||||
| [25]a | 2003 | LCohort | 127 | (1,483) | Sweden | ≥ 33 | 1.08 (0.64–1.70) | Standardized incidence ratio (expected/observed) | |
| ≤ 32 | 0.92 (0.57–1.41) | ||||||||
| [45]a,b | 2004 | LCC | 2,471 | 9,801 | USA | ≥ 37 | Referent | Adjusted for age and maternal age at first birth | |
| 32–36 | 0.91 (0.72–1.13) | ||||||||
| ≤ 31 | 1.43 (0.90–2.28) | ||||||||
| [20]b | 2005 | PCC | 1,424 | 1,457 | Poland | ≥ 37 | Referent | Adjusted for age, education, age at menarche, menopausal status and age at menopause, age at first full-term pregnancy, number of full-term pregnancies, family history of breast cancer among first-degree relatives, mammography screening, and current body mass index | |
| ≤ 36 | 1.01 (0.75–1.32) | ||||||||
| Cohort studies | [62]c | 2000 | LCohort | 12 | 273 | Sweden | 35 | 0.2 (0.01–1.3) | Standardized incidence ratio |
| 33–34 | 0.7 (0.1–2.0) | ||||||||
| 31–32 | 2.3 (0.7–5.3) | ||||||||
| <31 | 6.7 (1.4–19.5) | ||||||||
| [16]c | 2001 | LTCCS | 2,265 | 9,060 | Sweden | 33–36 | Referent | Crude ORs | |
| 37–38 | 1.8 (0.83–4.0) | ||||||||
| 40–44 | 2.0 (0.88–4.6) | ||||||||
| [29]c | 2005 | LCohort | 367 | 5,346 | Sweden | 1 week increase | <50 years | ||
| 0.94 (0.83–1.07) | |||||||||
| [40]c | 2006 | Cohort | 97 | 5,847 | USA | <39 | 0.77 (0.42–1.4) | Adjusted for age | |
| 39 | 1.38 (0.78–2.4) | ||||||||
| 40 | Referent | ||||||||
| 41+ | 1.33 (0.67–2.6) | ||||||||
| [31]b | 2006 | Cohort | 249 | 1024 | USA | ≥ 43 | 0.7 (0.2–2.7) | Adjusted for: age at diagnosis, diagnosis year, stage at diagnosis, and birth order, with exception of birth order, which is adjusted for maternal age | |
| 37–42 | Referent | ||||||||
| <37 | 1.4 (0.7–2.9) | ||||||||
| P for trend = 0.3 |
Cohort, cohort study; LCC, case-control study with linkage with population and cancer registry data; LTCCS, twin case-control study by using linkage with birth and cancer registry data; NCC, nested case-control study in cohort; PCC, population-based case-control study. aWe included this study in the meta-analysis with categories of ≥33 versus ≤32 months (reference). bWe included this study in the meta-analysis with categories of ≥37 versus ≤36 months (reference). cWe did not include these studies in the meta-analysis because they employed different categories or a continuous scale, or they did not provide the numbers of cases and controls in the report.
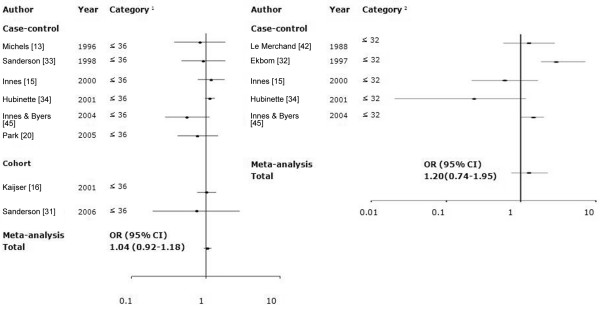
There was no significant heterogeneity across studies (P-Q test = 0.55), whereas we found no association between prematurity (≤36 weeks) and risk (OR 1.04 [95% CI 0.92 to 1.18]; Figure 6). However, a strong publication bias was observed (P-Egger test = 0.03 and P-Begg test = 0.11; Figure 7). A significant publication bias occurred because three studies with smaller standard errors of log RR [15,16,34] reported RRs near 1.0, whereas five studies with larger standard errors [13,20,31,33,45] reported substantially reduced RRs. When the analysis was performed for extreme prematurity (≤32 weeks), heterogeneity was also evident across the studies (P-Q test = 0.04), and the association was not significant (OR 1.20 [95% CI 0.74 to 1.95]).
Figure 6.
Meta-analysis of studies assessing the association of prematurity and risk for breast cancer. The tests for homogeneity and for publication bias in the studies analyzed are as follows. Category 36+: Q = 5.91 (7 degrees of freedom), P = 0.55; Begg test, P = 0.11; Egger test, P = 0.03. Category 32+: Q = 10.10 (4 degrees of freedom), P = 0.04; Begg test, P = 0.09; Egger test, P = 0.40. 1Category of prematurity (week): ≤36 versus ≥37 (reference). 2Category of prematurity (week): ≤32 versus ≥33 (reference). CI, confidence interval; OR, odds ratio.
Figure 7.
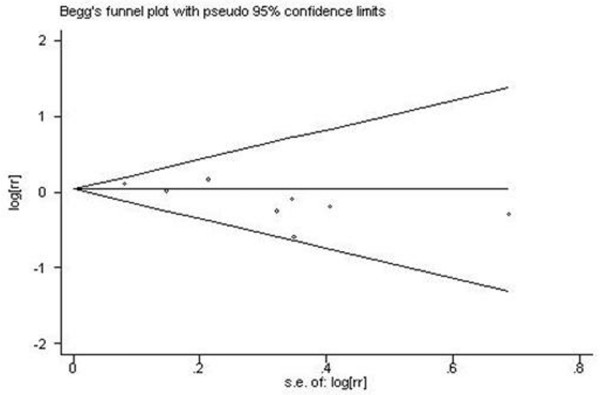
Begg's funnel plot for publication bias in meta-analysis of premature birth and breast cancer risk. Premature birth (gestational age ≤36 weeks) was compared with gestational age ≥37 weeks. Egger test, P = 0.03; Begg test, P = 0.11. rr, relative risk; s.e., standard error.
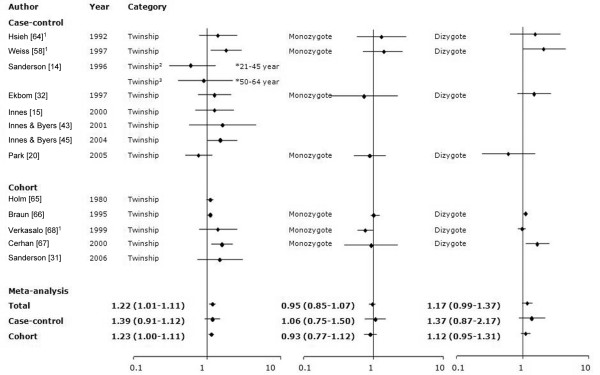
We examined 13 studies (eight case-control and five cohort) that assessed the association between twin status and risk (Table 6). Most studies identified a slightly increased risk among twins [15,31,32,43,45,58,63-66], with five studies demonstrating significant associations [31,58,64-66]. In contrast, some studies observed a slightly reduced risk [14,20,67], with one of the risks being marginally significant [67]. Seven studies [20,32,58,63,65,66] had information on zygosity. Of these studies, two [58,63] used the twins' sex as a proxy for zygosity. For monozygotic twins, a reduction in risk was significant in one study [68]. Most studies failed to observe an association [20,32,58,63,65,66]. Three studies reported a significantly increased risk associated with being a dizygotic twin [58,65,66], whereas other studies reported no association [20,32,63,67] (Figure 8).
Table 6.
Studies assessing the association of twinship with risk for breast cancer
| Type of study | Ref. | Year | Design | Cases | Controls (or cohort) | Country/place of study | Category | OR (95% CI) | Comments |
| Case-control studies | [63]a | 1992 | MCC | 870 | 2,741 | UK, USA | Singleton | Referent | Adjusted for age, study center, parity, age at first birth, age at menarche, height, body mass index, maternal age at birth, birth order, and menopausal status |
| Twinship | 1.40 (0.77–2.55) | ||||||||
| Singleton | Referent | ||||||||
| Monozygote twin | 1.30 (0.58–2.92) | ||||||||
| Dizygote twin | 1.54 (0.64–3.71) | ||||||||
| [14] | 1996 | PCC | 1,134 | 1,380 | USA | Age 21–45 | Adjusted for age, menopausal status, and maternal smoking | ||
| Singleton | Referent | ||||||||
| Twinship | 0.6 (0.3–1.3) | ||||||||
| Age 50–64 | |||||||||
| Singleton | Referent | ||||||||
| Twinship | 0.9 (0.4–2.2) | ||||||||
| [58]a | 1997 | PCC | 2,150 | 1,961 | USA | Singleton | Referent | Adjusted for age, study site, family history of breast cancer, breast biopsy, a combination variable including number of full-term births and age at first full-term pregnancy, age at menarche, menopausal status, body mass index, average lifetime alcohol consumption, and the number of mammograms | |
| Twinship | 1.6 (1.0–2.7) | ||||||||
| Singleton | Referent | ||||||||
| Monozygote twin | 1.39 (0.7–2.6) | ||||||||
| Dizygote twin | 2.06 (1.0–4.5) | ||||||||
| [32] | 1997 | NCC | 1,068 | 2,727 | Sweden | Singleton | Referent | Adjusted for maternal age, matermal socioeconomic status, maternal parity, maternal pre-eclampsia or eclampsia, neonatal jaundice, severe prematurity, twin, and birth weight | |
| Twinship | 1.3 (0.8–2.1) | ||||||||
| Singleton | Referent | ||||||||
| Monozygote twin | 0.7 (0.2–2.2) | ||||||||
| Dizygote twin | 1.5 (0.8–2.7) | ||||||||
| [15] | 2000 | LCC | 481 | 2,863 | USA | Singleton | Referent | Crude ORs | |
| Twinship | 1.04 (0.51–2.11) | ||||||||
| [43] | 2001 | LCC | 319 | 768 | USA | Singleton | Referent | Crude ORs | |
| Twinship | 1.6 (0.2–10.1) | ||||||||
| [45] | 2004 | LCC | 2,522 | 10,052 | USA | Singleton | Referent | Adjusted for age and maternal age at first birth | |
| Twinship | 1.77 (1.05–2.97) | ||||||||
| [20] | 2005 | PCC | 2,338 | 2,476 | Poland | Singleton | Referent | Adjusted for age, education, age at menarche, menopausal status and age at menopause, age at first full-term pregnancy, number of full-term pregnancy, family history of breast cancer among first-degree relatives, mammography screening, and current body mass index | |
| Twinship | 0.76 (0.49–1.16) | ||||||||
| Singleton | Referent | ||||||||
| Monozygote twin | 0.90 (0.53–1.52) | ||||||||
| Dizygote twin | 0.58 (0.23–1.47) | ||||||||
| Cohort studies | [64] | 1980 | LTCohort | 270 | (16,922) | Denmark | Twinship | 1.1 (1.0–1.2) | Observed/expected ratio (95% CI) |
| [65] | 1995 | LTCohort | 740 | (25,541) | Sweden | Twinship | 1.1 (1.0–1.1) | Observed/expected ratio (95% CI) | |
| Monozygote twin | 1.0 (0.9–1.2) | ||||||||
| Dizygote twin | 1.1 (1.0–1.2) | ||||||||
| [67] | 1999 | LTCohort | 245 | (13,176) | Finland | Twinship | 0.91 (0.81–1.00) | Observed/expected ratio (95% CI) | |
| Monozygote twin | 0.76 (0.59–0.97) | ||||||||
| Dizygote twin | 0.98 (0.84–1.10) | ||||||||
| [66] | 2000 | Cohort | 1,230 | (29,197) | USA | Singleton | Referent | Adjusted for age, education, family history of breast cancer, age at menarche, age at first birth, height, current body mass index, body mass index at age 18, waist:hip ratio, alcohol drinking, and hormone replacement therapy | |
| Twinship | 1.72 (1.22–2.42) | ||||||||
| Singleton | Referent | ||||||||
| Monozygote twin | 1.04 (0.43–2.5) | ||||||||
| Dizygote twin | 1.77 (1.16–2.7) | ||||||||
| [31] | 2006 | Cohort | 249 | 1,024 | USA | Singleton | Referent | Adjusted for age at diagnosis, diagnosis year, stage at diagnosis, and birth order, with exception of birth order, which is adjusted for maternal age | |
| Twinship | 2.5 (1.0–6.2) | ||||||||
aAuthors used the female twins as the proxy of the monozygote twin and the female twin with male twin as the proxy of the dizygote twin. Cohort, cohort study; LCC, case-control study with linkage with population and cancer registry data; LTCohort, twin cohort study by using linkage with birth and cancer registry data; MCC, multicenter case-control study; NCC, nested case-control study in cohort; PCC, population-based case-control study.
Figure 8.
Meta-analysis for the association between twinship and risk for breast cancer. The tests for homogeneity and for publication bias in the studies analyzed are as follows. Twinship: Q = 18.79 (13 degrees of freedom), P = 0.13; Begg test, P = 0.78; Egger test, P = 0.24. Monozygote twin: Q = 5.79 (6 degrees of freedom), P = 0.45; Begg test, P = 0.55; Egger test, P = 0.85. Dizygote twin: Q = 12.53 (6 degrees of freedom), P = 0.06; Begg test, P = 1.0; Egger test, P = 0.3. 1The authors used the female twins as the proxy for the monozygote twin and the female twin with male twin as the proxy for the dizygote twin. 2Women aged 21 to 45 years. 3Women aged 50 to 64 years. CI, confidence interval; OR, odds ratio.
The Q test for heterogeneity was not significant (P-Q test = 0.13), and the meta-analysis of 13 studies examining twin status (without regard to zygosity) found an OR of 1.22 (95% CI 1.01 to 1.11). There was no evidence of any publication bias (P-Egger test or P-Begg test >0.1). There were little evidence of heterogeneity (P-Q test > 0.1 for monozygotic or dizygotic twins), and breast cancer risk was not significantly increased among either monozygotic (OR 0.95 [95% CI 0.85 to 1.07]) or dizygotic (OR 1.17 [95% CI 0.99 to 1.37]) twins, albeit based on limited statistical power. In subgroup analysis by study design, cohort studies identified significantly increased risk (OR 1.23 [95% CI 1.00 to 1.11]) for breast cancer in twins versus singletons, with no study heterogeneity (P-Q test = 0.07). Case-control studies showed no association with twin status (OR 1.39 [95% CI 0.91 to 1.12]). There was no evidence of any publication bias (P-Egger test or P-Begg test > 0.05) among the case-control or cohort studies. In subgroup analysis by study design and zygosity, there were no heterogeneity in studies (P-Q test > 0.1). In subgroup analysis by study year, significant heterogeneity by publication year was identified (P = 0.01), and the OR (95% CI) for studies published before 2000 was 1.06 (0.97 to 1.47), whereas the OR (95% CI) for studies published in 2000 or later was 1.27 (1.03 to 1.58).
We identified nine studies that assessed the association between maternal or paternal smoking and risk (Table 7). Two cohort studies reported nonsignificantly reduced risks associated with maternal smoking (OR 0.49 [95% CI 0.29 to 1.03] [68]; OR 0.8 [95% CI 0.5 to 1.1] [31]), whereas a case-control study [43] identified a significant positive association (age-adjusted OR 2.7 [95% CI 1.1 to 6.3]), although its crude OR was not statistically significant (OR 1.1 [95% CI 0.7 to 1.7]). The majority of studies, however, identified no associations with maternal [14,20,33,35,58,69] or paternal [20,35,69] smoking during pregnancy.
Table 7.
Studies assessing the association of maternal or paternal smoking and the risk of breast cancer
| Type of study | Ref. | Year | Design | Cases | Controls (or cohort) | Country/place of study | Smoking status | OR (95% CI) | Comments |
| Case-control studies | [69] | 1996 | PCC | 53 | 470 | USA | Maternal smoking | Crude ORs | |
| No | Referent | ||||||||
| Yes | 0.9 (0.4–2.1) | ||||||||
| Paternal smoking | |||||||||
| No | Referent | ||||||||
| Yes | 1.3 (0.9–1.7) | ||||||||
| [14] | 1996 | PCC | 1,086 | 1,321 | USA | Maternal smoking | Adjusted for age, menopausal status, and maternal smoking; OR (95% CI) for maternal smoking among early-onset breast cancer patients (≤ 30 years old) was 1.9 (1.0–3.4) | ||
| Age 21–45 years | |||||||||
| No | Referent | ||||||||
| Yes | 1.1 (0.9–1.3) | ||||||||
| Age 50–64 years | |||||||||
| No | Referent | ||||||||
| Yes | 1.3 (0.9–2.1) | ||||||||
| [58] | 1997 | PCC | 522 | 484 | USA | Maternal smoking | Adjusted for age, study site, family history of breast cancer, breast biopsy, a combination variable including number of full-term births and age at first full-term pregnancy, age at menarche, menopausal status, body mass index, average lifetime alcohol consumption, and the number of mammograms | ||
| No | Referent | ||||||||
| Yes | 1.1 (0.8–1.4) | ||||||||
| [33] | 1998 | PCC | 507 | 433 | USA | Maternal smoking | Crude ORs | ||
| No | Referent | ||||||||
| Yes | 1.1 (0.9–1.5) | ||||||||
| [43] | 2001 | LCC | 319 | 768 | USA | Maternal smoking | Adjusted for attained age | ||
| No | Referent | ||||||||
| Yes | 2.7 (1.1–6.3) | ||||||||
| [35]a | 2002 | PCC | 1,535 | 1,534 | USA | Smoking | Adjusted for age and residential regions (states) | ||
| No | Referent | ||||||||
| Paternal smoking | 1.00 (0.88–1.13) | ||||||||
| Maternal/parental smoking | 1.10 (0.84–1.42) | ||||||||
| [20] | 2005 | PCC | 2380 | 2,497 | Poland | Maternal smoking | Unadjusted; recalculated | ||
| No | Referent | ||||||||
| Yes (any exposure) | 1.19 (0.97–1.47) | ||||||||
| Paternal smoking | |||||||||
| No | Referent | ||||||||
| Yes (any exposure) | 0.90 (0.77–1.05) | ||||||||
| Cohort studies | [31] | 2006 | Cohort | 249 | 1,024 | USA | Maternal smoking | Referent | Adjusted for age at diagnosis, diagnosis year, stage at diagnosis, and birth order, with exception of birth order, which is adjusted for maternal age Crude relative rates |
| No | 0.8 (0.5–1.1) | ||||||||
| Yes (any exposure) | |||||||||
| [68] | 2005 | Cohort | 42 | (3,989) | USA | Maternal smoking | |||
| No | Referent | ||||||||
| Yes (any exposure) | 0.49 (0.29–1.03) | ||||||||
| ≤ 15 cigarettes a day | 0.33 (0.12–0.94) | ||||||||
| >15 | 0.68 (0.26–1.73) |
aTitus-Ernstoff and coworkers [35] classified three categories: nonparental smoking, either paternal or maternal smoking only or both parents smoking during pregnancy. Thus, in this study, the maternal or both parents smoking versus nonparental smoking can be regarded as maternal smoking versus no maternal smoking. Cohort, cohort study; LCC, case-control study by linkage with population data and cancer registry data; PCC, population-based case-control study.
There was no heterogeneity or publication bias (P-Q test > 0.05, P-Egger test and P-Begg test > 0.1 among all studies, case-control or cohort). The meta-analysis for maternal smoking (Figure 9) found no significant association with risk (OR 0.98 [95% CI 0.86 to 1.13]), although cohort studies [40,68] noted a significant negative association with maternal smoking (OR 0.59 [95% CI 0.41 to 0.85]).
Figure 9.
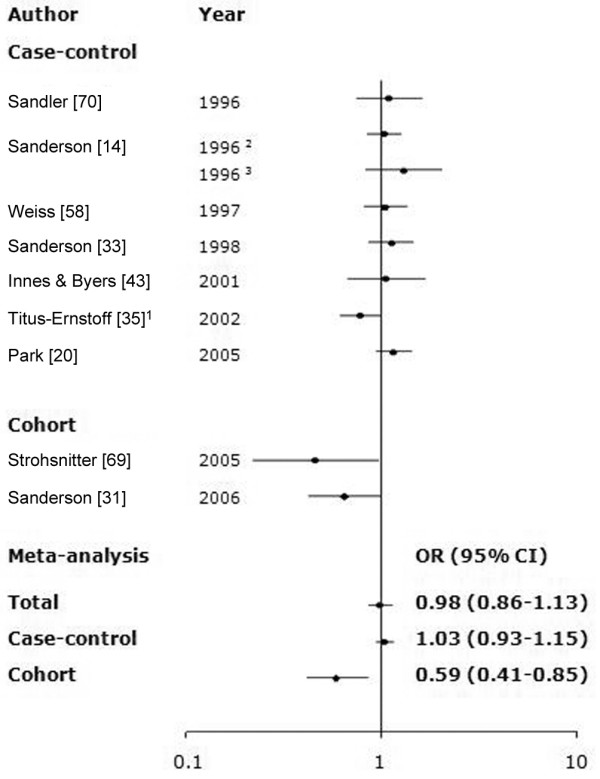
Meta-analysis for the association of maternal smoking during pregnancy with risk for subsequent breast cancer. The tests for homogeneity and for publication bias in the studies analyzed are as follows: Q = 16.90 (9 degrees of freedom), P = 0.06; Begg test, P = 0.59; Egger test, P = 0.31. 1Titus-Ernstoff and coworkers [35] classified three categories: nonparental smoking, paternal or maternal smoking only or both parents smoking during pregnancy. The odds ratios (ORs) of father smoking on breast cancer risk was almost unity (OR 1.0, 95% confidence intrval [CI] 0.9 to 1.1). Thus, in this study, the mother smoking and both parents smoking versus nonparental smoking can be considered to the maternal smoking versus no maternal smoking. 2Women aged 21 to 45 years. 3Women aged 50 to 64 years.
Discussion
The main finding of our meta-analysis was that heavier birth weight was associated with increased breast cancer risk (18% increased risk for the heaviest weight). Twin status was associated with 1.2-fold higher risk for breast cancer relative to a singleton birth. Although we found some evidence of increased risk associated with older maternal age (OR 1.16 for maternal age ≥30 years), there were heterogeneous findings across study designs.
Most studies identified an increased risk for breast cancer with heavier birth weight, with the association being particularly strong for premenopausal or early-onset breast cancers [14,22,29,30]. Our result was similar to the findings of a recent meta-analysis of 26 studies, which revealed that high birth weight was associated with a RR of 1.23 and restricted to premenopausal women (OR 1.25 [95% CI 1.14 to 1.38) [70]. This analysis grouped birth weight into two categories (classified into high and low birth weight in each study, regardless of specific weight in terms of grams), preventing evaluation of dose-response relationships. We did in fact observe evidence of a dose-response relationship of risk with birth weight, although this was based on a relatively small number of studies involving three or four categories.
Although some studies identified a J-shaped relationship between birth weight and breast cancer risk [12,14,15,18,25,33,35,37,45], others failed to note an increased risk associated with very low birth weights. A recent study involving 3,066 breast cancer patients and 106,504 comparison individuals in a Danish cohort also found no elevated risk among those with very low birth weights [71]. Similarly, our meta-analysis provided little evidence of increased risk for very low birth weights.
Although the mechanisms underlying the association between high birth weight and breast cancer risk remain unclear, it has been suggested that heavier birth weights may result from increased in utero exposuresto factors such as insulin-like growth factor-I or estrogens [72-76]. These substances may act as mitogens by increasing the likelihood of genetic mutations [75,77]. However, several studies have failed to find any correlation between umbilical cord estrogen levels and birth weight [78,79]. One study, however, reported a significant positive relationship with estriol [80]. Further studies should be undertaken to assist in the resolution of these conflicting data.
Our analysis found no association of breast cancer risk with birth orders between 2 and 4, but we did note a somewhat reduced risk associated with higher birth orders (at least 5), although the results were heterogeneous across studies. Biologically, pregnancy estrogen levels appear to be higher during first pregnancies and decline in successive pregnancies [81]. Furthermore, cord blood levels of estradiol, estrone, and progesterone are lower for later born than first born children [82]. These findings suggest that the reduced risk associated with higher birth orders may relate to lower estrogen levels. However, evidence supporting birth order as a risk factor for breast cancer is limited, with further investigations needed to evaluate dose-response relationships more fully.
In our meta-analysis, we found some evidence that having been born to an older mother was associated with higher breast cancer risk, although the results were heterogeneous across studies. Our data failed to support the previous studies that suggested a J-shaped relationship between maternal age and breast cancer risk. It was previously suggested that older maternal age may have an adverse effect on the primordial mammary gland of their daughters because of altered hormonal profiles [37] or may linked to the epigenetic change of mtDNA which can lead to breast carcinogenesis by oocyte inheritance [83]. However, the two studies that examined pregnancy estrogen levels according to maternal age found that both total estrogen and estradiol levels were lowest in youngest mothers (<20 years of age), highest in those aged 20 to 24 years, and intermediate in mothers over 25 years of age [78,81]. Thus, it remains unclear from both our meta-analysis as well as from biologic data whether maternal age is a proxy for estrogen or estradiol exposure to fetus. Although it has been suggested that older paternal age may cause germ cell mutations, previous epidemiologic studies have failed to support an association [35,20,69,82,84,85]. Because the purpose of this study was to evaluate whether the intrauterine hormone environment affects subsequent breast cancer risk, our meta-analysis did not include paternal age.
We observed no association between prematurity and breast cancer risk. Biologically, women having abruptio placentae or an extremely premature birth (<32 week) have been shown to have elevated levels of human chorionic gonadotropin and α-fetoprotein, which could inhibit the differentiation of stem cells in human breast tissue cells [15]. Gestational age is related to birth weight, of course, because birth weights in infants born prematurely are lower than those in infants born at term [13].
Twin pregnancies are associated with an approximate doubling of estrogen levels compared with singleton pregnancies [86,87]. Dizygotic twin pregnancies have elevated levels of estrogens and gonadotropins [88-90]. It has therefore been postulated that twins, especially dizygotic twins, could be at an elevated risk for breast cancer. In general, our results did not support differences in risk between monozygotic and dizygotic twins, and there was evidence that risk estimates published after 2000 were qualitatively different from those of earlier studies.
Studies of parental smoking, especially maternal smoking, and daughter's breast cancer risk have yielded inconsistent results. Biologically, maternal smoking, rather than paternal smoking, has a greater impact on the fetus. In the meta-analytic results, both factors failed to exhibit a significant association with risk. Some studies have reported that maternal smoking in pregnancy reduces serum estrogen levels [91,92]. A recent experimental study reported that both estradiol-17β levels and progesterone:estradiol-17β ratios were reduced in pregnant mice exposed to cigarette smoke [93]. However, the relevance of these findings to humans is unclear.
These meta-analyses are based on results from studies involving heterogeneous designs and methodology. We did note between-study heterogeneity for the associations of birth order, maternal age, and twin status. To resolve the heterogeneous findings, we considered the influence of study design and the date of study publication on the results by subgroup analyses. However, heterogeneity in studies could only be explained partially.
Effects of maternal age, birth order, prematurity (cut-off value 32 weeks), and maternal smoking were found to be heterogeneous across study designs, but birth weight and twinning were comparable. Self-reported measures of perinatal factors may be vulnerable to misclassification biases, with differential or nondifferential effects [94,95]. Because studies based on data linkage to medical records have a lower chance of misclassification bias, we conducted subgroup meta-analyses stratified by source of information (data linkage versus self-report) and found no substantial differences in the results. Although the completeness of records is a critical factor in evaluating biases in studies based on data linkage, most papers did not provide details about the completeness of records. We also conducted subgroup meta-analyses stratified by publication year. Only twin status exhibited significant heterogeneity according to publication year (<2000 versus ≥2000).
Our findings may be somewhat inflated because of our dependence on crude rather than adjusted ORs or RRs. A possible misclassification bias for zygosity might have resulted in studies that used sex as a proxy for zygosity [96]. Because this bias would probably attenuate associations, additional investigations are needed to determine the extent of any true association of risk with twin status.
Conclusion
It has been hypothesized that certain perinatal factors, including birth weight and order, twin pregnancies, prematurity, maternal age, and smoking, may reflect higher estrogenic environments in utero, thereby increasing the subsequent risk of breast cancer. Findings of an increase in breast cancer risk among daughters exposed to diethylstilbestrol in utero supports this hypothesis [97,98]. Although the current meta-analysis found evidence that higher birth weights are associated with increased breast cancer risk, older maternal age and twin status were less convincingly related, and birth order and prematurity appeared unrelated. Greater birth weights have been attributed to higher maternal estrogens levels, which could affect fetal development [72-74] through epigenetic modifications of breast stem cells [1,99,100]. Although our findings regarding birth weight support the hypothesis that higher estrogen exposures in utero may be involved in the subsequent development of breast cancer, further biologic data are needed to elucidate the relationship fully.
Abbreviations
CI = confidence interval; OR = odds ratio; RR = relative risk.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SKP collected and selected the all of breast cancer studies, analyzed the data in the study, drafted the manuscript, critically revised the manuscript for important intellectual content, and takes responsibility for the study concept and design, the integrity of the data, and the accuracy of the data analysis. DK participated in design of the study, drafting of the manuscript and interpretation of results, and critically revised the manuscript for important intellectual content. KAM was responsible for the study concept and design, interpreted the findings, and revised the manuscript for important intellectual content. MGC participated in the interpretation of the data and revision of the manuscript. YK was involved in data analysis and revision for important intellectual content. KYY contributed to interpreting the findings and critically revised the manuscript for important intellectual content. LAB led conception and design of the study, the analysis and interpretation of the findings, and revision to the manuscript, and obtained part funding for this research. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health (National Cancer Institute).
Contributor Information
Sue Kyung Park, Email: suepark@snu.ac.kr.
Daehee Kang, Email: dhkang@snu.ac.kr.
Katherine A McGlynn, Email: mcglynnk@mail.nih.gov.
Montserrat Garcia-Closas, Email: garciacm@exchange.nih.gov.
Yeonju Kim, Email: kyju@snu.ac.kr.
Keun Young Yoo, Email: kyyoo@plaza.snu.ac.kr.
Louise A Brinton, Email: brintonl@exchange.nih.gov.
References
- Trichopoulos D. Hypothesis: does breast cancer originate in utero? Lancet. 1990;335:939–40. doi: 10.1016/0140-6736(90)91000-Z. [DOI] [PubMed] [Google Scholar]
- Tulchinsky D, Hobel CH, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone and 17-hydroxyprogesterone in human pregnancy: I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–1100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- Buster JE, Sakakini J, Jr, Killam AP, Scragg WH. Serum unconjugated estriol levels in the third trimester and their relationship to gestational age. Am J Obstet Gynecol. 1976;125:672–676. doi: 10.1016/0002-9378(76)90792-4. [DOI] [PubMed] [Google Scholar]
- Lindberg BS, Johansson EDB, Nilsson BA. Plasma levels of nonconjugated estrone, estradiol, 17β, and estriol during uncomplicated pregnancy. Acta Obstet Gynecol Scand Suppl. 1974;32:21–36. doi: 10.3109/00016347409156390. [DOI] [PubMed] [Google Scholar]
- Trichopoulos D. Intrauterine environment, mammary gland mass and breast cancer risk. Breast Cancer Res. 2003;5:42–44. doi: 10.1186/bcr555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potischman N, Troisi R. In-utero and early life exposures in relation to risk of breast cancer. Cancer Causes Control. 1999;10:561–573. doi: 10.1023/A:1008955110868. [DOI] [PubMed] [Google Scholar]
- Grotmol T, Weiderpass E, Tretli S. Conditions in utero and cancer risk. Eur J Epidemiol. 2006;21:561–570. doi: 10.1007/s10654-006-9036-7. [DOI] [PubMed] [Google Scholar]
- Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17:841–856. doi: 10.1002/(SICI)1097-0258(19980430)17:8<841::AID-SIM781>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20:641–654. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- Ekbom A, Trochopoulos D, Adami HO, Hsieh CC, Land SJ. Evidence of prenatal influences on breast cancer risk. Lancet. 1992;340:1015–1018. doi: 10.1016/0140-6736(92)93019-J. [DOI] [PubMed] [Google Scholar]
- Michels KB, Trichopoulos D, Robins JM, Rosner BA, Manson JE, Hunter DJ, Colditz GA, Hankinson SE, Speizer FE, Willett WC. Birth weight as a risk factor for breast cancer. Lancet. 1996;348:1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Williams MA, Malone KE, Stanford JL, Emanuel I, White E, Daling JR. Perinatal factors and risk of breast cancer. Epidemiology. 1996;7:34–37. doi: 10.1097/00001648-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Innes K, Byers T, Schymura M. Birth characteristics and subsequent risk for breast cancer in very young women. Am J Epidemiol. 2000;152:1121–1128. doi: 10.1093/aje/152.12.1121. [DOI] [PubMed] [Google Scholar]
- Kaijser M, Lichtenstein P, Granath F, Erlandsson G, Cnattingius S, Ekbom A. In utero exposures and breast cancer: a study of opposite-sexed twins. J Natl Cancer Inst. 2001;93:60–62. doi: 10.1093/jnci/93.1.60. [DOI] [PubMed] [Google Scholar]
- Vatten LJ, Maehle BO, Lund Nilsen TI, Tretli S, Hsieh CC, Trichopoulos D, Stuver SO. Birth weight as a predictor of breast cancer: a case-control study in Norway. Br J Cancer. 2002;86:89–91. doi: 10.1038/sj.bjc.6600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellemkjaer L, Olsen ML, Sorensen HT, Thulstrup AM, Olsen J, Olsen JH. Birth weight and risk of early-onset breast cancer (Denmark) Cancer Causes Control. 2003;14:61–64. doi: 10.1023/A:1022570305704. [DOI] [PubMed] [Google Scholar]
- Lahmann PH, Gullberg B, Olsson H, Boeing H, Berglund G, Lissner L. Birth weight is associated with postmenopausal breast cancer risk in Swedish women. Br J Cancer. 2004;91:1666–1668. doi: 10.1038/sj.bjc.6602203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Garcia-Closas M, Lissowska J, Sherman ME, McGlynn KA, Peponska B, Bardin-Mikoajczak A, Zatonski W, Szeszenia-Dabrowska N, Brinton LA. Intrauterine environment and breast cancer risk in a population-based case-control study in Poland. Int J Cancer. 2006;119:2136–2141. doi: 10.1002/ijc.21974. [DOI] [PubMed] [Google Scholar]
- Mogren I, Damber L, Tavelin B, Hogberg U. Characteristics of pregnancy and birth and malignancy in the offspring (Sweden) Cancer Causes Control. 1999;10:85–94. doi: 10.1023/A:1008813701634. [DOI] [PubMed] [Google Scholar]
- De Stavola BL, Hardy R, Kuh D, Silva IS, Wadsworth M, Swerdlow AJ. Birthweight, childhood growth and risk of breast cancer in a British cohort. Br J Cancer. 2000;83:964–968. doi: 10.1054/bjoc.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I, De Stavola BL, Mohsen R, Leon DA, Lithell HO. Fetal growth and subsequent risk of breast cancer: results from long term follow up of Swedish cohort. BMJ. 2003;326:248. doi: 10.1136/bmj.326.7383.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren M, Sorensen T, Wohlfahrt J, Haflidadottir A, Holst C, Melbe M. Birth weight and risk of breast cancer in a cohort of 106,504 women. Int J Cancer. 2003;107:997–1000. doi: 10.1002/ijc.11481. [DOI] [PubMed] [Google Scholar]
- Kaijser M, Akre O, Cnattinguis S, Ekbom A. Preterm birth, birth weight, and subsequent risk of female breast cancer. Br J Cancer. 2003;89:1664–1666. doi: 10.1038/sj.bjc.6601357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- dos Santos Silva I, De Stavola BL, Hardy RJ, Kuh DJ, McCormack VA, Wadsworth ME. Is the association of birth weight with premenopausal breast cancer risk mediated through childhood growth? Br J Cancer. 2004;91:519–524. doi: 10.1038/sj.bjc.6601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatten LJ, Nilsen TI, Tretli S, Trichopoulos D, Romundstad PR. Size at birth and risk of breast cancer: prospective population-based study. Int J Cancer. 2005;114:461–464. doi: 10.1002/ijc.20726. [DOI] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I, Koupil I, Leon DA, Lithell HO. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115:611–7. doi: 10.1002/ijc.20915. [DOI] [PubMed] [Google Scholar]
- Michels KB, Xue F, Terry KL, Willett WC. Longitudinal study of birthweight and the incidence of breast cancer in adulthood. Carcinogenesis. 2006;27:2464–2468. doi: 10.1093/carcin/bgl105. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Daling JR, Doody DR, Malone KE. Perinatal factors and mortality from breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1984–1987. doi: 10.1158/1055-9965.EPI-06-0350. [DOI] [PubMed] [Google Scholar]
- Ekbom A, Hsieh CC, Lipworth L, Adami HQ, Trichopoulos D. Intrauterine environment and breast cancer risk in women: a population-based study. J Natl Cancer Inst. 1997;89:71–76. doi: 10.1093/jnci/89.1.71. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Williams MA, White E, Daling JR, Holt VL, Malone KE, Self SG, Moore DE. Validity and reliability of subject and mother reporting of perinatal factors. Am J Epidemiol. 1998;147:136–140. doi: 10.1093/oxfordjournals.aje.a009425. [DOI] [PubMed] [Google Scholar]
- Hubinette A, Lichtenstein P, Ekbom A, Cnattingius S. Birth characteristics and breast cancer risk: a study among like-sexed twins. Int J Cancer. 2001;91:248–251. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1025>3.3.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Egan KM, Newcomb PA, Ding J, Trentham-Dietz A, Greenberg ER, Baron JA, Trichopoulos D, Willett WC. Early life factors in relation to breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:207–210. [PubMed] [Google Scholar]
- Hodgson ME, Newman B, Millikan RC. Birth weight, parental age, birth order and breast cancer risk in African-American and white women: a population-based case-control study. Breast Cancer Res. 2004;6:R656–R667. doi: 10.1186/bcr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba M, McCann SE, Nie J, Vito D, Stranges S, Fuhrman B, Trevisan M, Muti P, Freudenheim JL. Perinatal exposures and breast cancer risk in the Western New York Exposures and Breast Cancer (WEB) Study. Cancer Causes Control. 2006;17:395–401. doi: 10.1007/s10552-005-0481-5. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Forsen T, Eriksson JG, Luoto R, Tuomilehto J, Osmond C, Barker DJ. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85:1680–1684. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson SW, Bengtsson C, Hallberg L, Lapidus L, Niklasson A, Wallgren A, Hulthen L. Cancer risk in Swedish women: the relation to size at birth. Br J Cancer. 2001;84:1193–1198. doi: 10.1054/bjoc.2000.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Hatch EE, Titus-Ernstoff L, Palmer JR, Hyer M, Strohsnitter WC, Robboy SJ, Kaufman R, Herbst A, Adam E, Hoover RN. Birth weight and breast cancer risk. Br J Cancer. 2006;94:1734–1737. doi: 10.1038/sj.bjc.6603122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lof M, Sandin S, Hilakivi-Clarke L, Weiderpass E. Birth weight in relation to endometrial and breast cancer risks in Swedish women. Br J Cancer. 2007;96:134–136. doi: 10.1038/sj.bjc.6603504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand L, Kolonel LN, Myers BC, Mi MP. Birth characteristics of premenopausal women with breast cancer. Br J Cancer. 1988;57:437–439. doi: 10.1038/bjc.1988.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes KE, Byers TE. Smoking during pregnancy and breast cancer risk in very young women (United States) Cancer Causes Control. 2001;12:179–185. doi: 10.1023/A:1008961512841. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Shu XO, Jin F, Dai Q, Ruan Z, Gao YT, Zheng W. Weight at birth and adolescence and premenopausal breast cancer risk in a low-risk population. Br J Cancer. 2002;86:84–88. doi: 10.1038/sj.bjc.6600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes KE, Byers TE. First pregnancy characteristics and subsequent breast cancer risk among young women. Int J Cancer. 2004;112:306–311. doi: 10.1002/ijc.20402. [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Tzonou A, Trichopoulos D. Birth order and breast cancer risk. Cancer Causes Control. 1991;2:95–98. doi: 10.1007/BF00053127. [DOI] [PubMed] [Google Scholar]
- Janerich DT, Thompson WD, Mineau GP. Maternal pattern of reproduction and risk of breast cancer in daughters: results from the Utah Population Database. J Natl Cancer Inst. 1994;86:1634–1639. doi: 10.1093/jnci/86.21.1634. [DOI] [PubMed] [Google Scholar]
- Okobia MN, Bunker CH, Lee LL, Osime U, Uche EE. Case-control study of risk factors for breast cancer in Nigerian women: a pilot study. East Afr Med J. 2005;82:14–19. doi: 10.4314/eamj.v82i1.9288. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Mutanen P. Birth order, family size, and the risk of cancer in young and middle-aged adults. Br J Cancer. 2001;84:1466–1471. doi: 10.1054/bjoc.2001.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, MacMahon B, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Trichopoulos D, Yuasa S. Maternal age and birth rank of women with breast cancer. J Natl Cancer Inst. 1980;65:719–722. doi: 10.1093/jnci/65.4.719. [DOI] [PubMed] [Google Scholar]
- Standfast SJ. Birth characteristics of women dying from breast cancer. J Natl Cancer Inst. 1967;39:33–42. [PubMed] [Google Scholar]
- Janerich DT, Hayden CL, Thompson WD, Selenskas SL, Mettlin C. Epidemiologic evidence of perinatal influence in the etiology of adult cancers. J Clin Epidemiol. 1989;42:151–157. doi: 10.1016/0895-4356(89)90088-7. [DOI] [PubMed] [Google Scholar]
- Baron JA, Vessey M, McPherson K, Yeates D. Maternal age and breast cancer risk. J Natl Cancer Inst. 1984;72:1307–1309. [PubMed] [Google Scholar]
- Thompson WD, Janerich DT. Maternal age at birth and risk of breast cancer in daughters. Epidemiology. 1990;1:101–106. doi: 10.1097/00001648-199003000-00004. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Stampfer MJ, Hennekens CH, Rosner B, Speizer FE. Parental age at birth and risk of breast cancer in daughters: a prospective study among US women. Cancer Causes Control. 1991;2:31–36. doi: 10.1007/BF00052358. [DOI] [PubMed] [Google Scholar]
- Choi JY, Lee KM, Park SK, Noh DY, Ahn SH, Yoo KY, Kang D. Association of paternal age at birth and the risk of breast cancer in offspring: a case control study. BMC Cancer. 2005;5:143. doi: 10.1186/1471-2407-5-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb PA, Trentham-Dietz A, Storer BE. Parental age in relation to risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:151–154. [PubMed] [Google Scholar]
- Weiss HA, Potischman NA, Brinton LA, Brogan D, Coates RJ, Gammon MD, Malone KE, Schoenberg JB. Prenatal and perinatal risk factors for breast cancer in young women. Epidemiology. 1997;8:181–187. doi: 10.1097/00001648-199703000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cupples LA, Rosenberg L, Colton T, Kreger BE. Parental ages at birth in relation to a daughter's risk of breast cancer among female participants in the Framingham Study (United States) Cancer Causes Control. 1995;6:23–29. doi: 10.1007/BF00051677. [DOI] [PubMed] [Google Scholar]
- Holmberg L, Ekbom A, Calle E, Mokdad A, Byers T. Parental age and breast cancer mortality. Epidemiology. 1995;6:425–427. doi: 10.1097/00001648-199507000-00018. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Powell D, Rosario I, Keys C, Hanisch R, Young M, Casagrande J, Gerkins V, Pike MC. An epidemiologic study of breast cancer. J Natl Cancer Inst. 1974;53:609–614. doi: 10.1093/jnci/53.3.609. [DOI] [PubMed] [Google Scholar]
- Ekbom A, Erlandsson G, Hsieh C, Trichopoulos D, Adami HO, Cnattingius S. Risk of breast cancer in prematurely born women. J Natl Cancer Inst. 2000;92:840–841. doi: 10.1093/jnci/92.10.840. [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Lan SJ, Ekbom A, Petridou E, Adami HO, Trichopoulos D. Twin membership and breast cancer risk. Am J Epidemiol. 1992;136:1321–1326. doi: 10.1093/oxfordjournals.aje.a116444. [DOI] [PubMed] [Google Scholar]
- Holm NV, Hauge M, Harvald B. Etiologic factors of breast cancer elucidated by a study of unselected twins. J Natl Cancer Inst. 1980;65:285–298. [PubMed] [Google Scholar]
- Braun MM, Ahlbom A, Floderus B, Brinton LA, Hoover RN. Effect of twinship on incidence of cancer of the testis, breast, and other sites (Sweden) Cancer Causes Control. 1995;6:519–524. doi: 10.1007/BF00054160. [DOI] [PubMed] [Google Scholar]
- Cerhan JR, Kushi LH, Olson JE, Rich SS, Zheng W, Folsom AR, Sellers TA. Twinship and risk of postmenopausal breast cancer. J Natl Cancer Inst. 2000;92:261–265. doi: 10.1093/jnci/92.3.261. [DOI] [PubMed] [Google Scholar]
- Verkasalo PK, Kaprio J, Pukkala E, Koskenvuo M. Breast cancer risk in monozygotic and dizygotic female twins: a 20-year population-based cohort study in Finland from 1976 to 1995. Cancer Epidemiol Biomarkers Prev. 1999;8:271–274. [PubMed] [Google Scholar]
- Strohsnitter WC, Noller KL, Titus-Ernstoff L, Troisi R, Hatch EE, Poole C, Glynn RJ, Hsieh CC. Breast cancer incidence in women prenatally exposed to maternal cigarette smoke. Epidemiology. 2005;16:342–345. doi: 10.1097/01.ede.0000158741.07645.9b. [DOI] [PubMed] [Google Scholar]
- Sandler DP, Everson RB, Wilcox AJ, Browder JP. Cancer risk in adulthood from early life exposure to parents' smoking. Am J Public Health. 1985;75:487–492. doi: 10.2105/ajph.75.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119:2007–2025. doi: 10.1002/ijc.22004. [DOI] [PubMed] [Google Scholar]
- Ahlgren M, Wohlfahrt J, Olsen LW, Sørensen TI, Melbye M. Birth weight and risk of cancer. Cancer. 2007;110:412–419. doi: 10.1002/cncr.22773. [DOI] [PubMed] [Google Scholar]
- Kaijser M, Granath F, Jacobsen G, Cnattingius S, Ekbom A. Maternal pregnancy estriol levels in relation to anamnestic and fetal anthropometric data. Epidemiology. 2000;11:315–319. doi: 10.1097/00001648-200005000-00015. [DOI] [PubMed] [Google Scholar]
- McFadyen IR, Worth HG, Wright DJ, Notta SS. High oestrogen excretion in pregnancy. Br J Obstet Gynaecol. 1982;89:994–999. doi: 10.1111/j.1471-0528.1982.tb04653.x. [DOI] [PubMed] [Google Scholar]
- Mucci LA, Lagiou P, Tamimi RM, Hsieh CC, Adami HO, Trichopoulos D. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States) Cancer Causes Control. 2003;14:311–318. doi: 10.1023/A:1023966813330. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES. In-utero exposures and breast cancer risk: joint effect of estrogens and insulin-like growth factor? Cancer Causes Control. 2002;13:505–508. doi: 10.1023/A:1016348425833. [DOI] [PubMed] [Google Scholar]
- Wang HS, Chard T. The role of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in the control of human fetal growth. J Endocrinol. 1992;132:11–19. doi: 10.1677/joe.0.1320011. [DOI] [PubMed] [Google Scholar]
- Savarese TM, Strohsnitter WC, Low HP, Liu Q, Baik I, Okulicz W, Chelmow DP, Lagiou P, Quesenberry P, Noller KL, Hsieh CC. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Res. 2007;9:R29. doi: 10.1186/bcr1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Roberts J, Siiteri P, Daftary A, Sims C, Hoover RN. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States) Cancer Causes Control. 2003;14:347–355. doi: 10.1023/A:1023934518975. [DOI] [PubMed] [Google Scholar]
- Vatten LJ, Romundstad PR, Ødegård RA, Nilsen ST, Trichopoulos D, Austgulen R. a-Fetoprotein in umbilical cord in relation to severe preeclampsia, birth weight, and future breast cancer risk. Br J Cancer. 2002;86:728–731. doi: 10.1038/sj.bjc.6600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata C, Iwasa S, Shiraki M, Shimizu H. Estrogen and alpha-fetoprotein levels in maternal and umbilical cord blood samples in relation to birth weight. Cancer Epidemiol Biomarkers Prev. 2006;15:1469–1472. doi: 10.1158/1055-9965.EPI-06-0158. [DOI] [PubMed] [Google Scholar]
- Panagiotopoulou K, Katsouyanni K, Petridou E, Garas Y, Tzonou A, Trichopoulos D. Maternal age, parity, and pregnancy estrogens. Cancer Causes Control. 1990;1:119–124. doi: 10.1007/BF00053162. [DOI] [PubMed] [Google Scholar]
- Maccoby EE, Doering CH, Nagy Jacklin C, Kraemer H. Concentrations of sex hormones in umbilical-cord blood: their relation to sex and birth order of infants. Child Dev. 1979;50:632–642. doi: 10.2307/1128928. [DOI] [PubMed] [Google Scholar]
- van Noord PAH. An alternative, non-intrauterine hypothesis, based on maternal mitochondrial oocyte inheritance, to explain inconsistent findings of birth weight on (breast) cancer risk. Br J Cancer. 2003;88:1817–1818. doi: 10.1038/sj.bjc.6600978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Colditz GA, Willett WC, Rosner BA, Michels KB. Parental age at delivery and incidence of breast cancer: a prospective cohort. study. Breast Cancer Res Treat. 2007;104:331–340. doi: 10.1007/s10549-006-9424-4. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Kyyronen P. Parental age and risk of sporadic and familial cancer in offspring: implications for germ cell mutagenesis. Epidemiology. 1999;10:747–751. [PubMed] [Google Scholar]
- Duff GB, Brown JB. Urinary oestriol excretion in twin pregnancies. J Obstet Gynaecol Br Commonw. 1974;81:695–700. doi: 10.1111/j.1471-0528.1974.tb00543.x. [DOI] [PubMed] [Google Scholar]
- TambyRaja RL, Ratnam SS. Plasma steroid changes in twin pregnancies. Prog Clin Biol Res. 1981;69A:189–195. [PubMed] [Google Scholar]
- Wald N, Cuckle H, Wu T, George L. Maternal serum unconjugated oestriol and human chorionic gonadotrophin levels in twin pregnancies: implications for screening for Down's syndrome. Br J Obstet Gynaecol. 1991;98:905–908. doi: 10.1111/j.1471-0528.1991.tb13513.x. [DOI] [PubMed] [Google Scholar]
- Martin NG, El Beaini JL, Olsen ME, Bhatnagar AS, Macourt D. Gonadotropin levels in mothers who have had two sets of DZ twins. Acta Genet Med Gemellol (Roma) 1984;33:131–139. doi: 10.1017/s0001566000007613. [DOI] [PubMed] [Google Scholar]
- Campbell DM. Aetiology of twinning. In: MacGillivray I, Campbell D, Thompson B, editor. Twinning and twins. New York, NY: John Wiley & Sons; 1988. pp. 27–36. [Google Scholar]
- Petridou E, Panagiotopoulou K, Katsouyanni K, Spanos E, Trichopoulos D. Tobacco smoking, pregnancy estrogens, and birth weight. Epidemiology. 1990;1:247–250. doi: 10.1097/00001648-199005000-00011. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Pike MC, Lobo RA, Depue RH, Ross RK, Henderson B. Cigarette smoking during pregnancy results in marked decrease in maternal HCG and oestradiol levels. Br J Obstet Gynaecol. 1989;96:92–96. doi: 10.1111/j.1471-0528.1989.tb01582.x. [DOI] [PubMed] [Google Scholar]
- Ng SP, Steinetz BG, Lasano SG, Zelikoff JT. Hormonal changes accompanying cigarette smoke-induced preterm births in a mouse model. Exp Biol Med (Maywood) 2006;231:1403–1409. doi: 10.1177/153537020623100814. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Whyatt RM, Cooper TB, Flak E, Perera FP. Exposure misclassification error in studies on prenatal effects of tobacco smoking in pregnancy and the birth weight of children. J Expo Anal Environ Epidemiol. 1998;8:347–357. [PubMed] [Google Scholar]
- Sanderson M, Williams MA, White E, Daling JR, Holt VL, Malone KE, Self SG, Moore DE. Validity and reliability of subject and mother reporting of perinatal factors. Am J Epidemiol. 1998;147:136–140. doi: 10.1093/oxfordjournals.aje.a009425. [DOI] [PubMed] [Google Scholar]
- Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979;28:225–236. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- Lagiou P. Intrauterine exposures, pregnancy estrogens and breast cancer risk: where do we currently stand? Breast Cancer Res. 2006;8:112. doi: 10.1186/bcr1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JR, Wise LA, Hatch EE, Troisi R, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Herbst AL, Noller KL, Hyer M, Hoover RN. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1509–1514. doi: 10.1158/1055-9965.EPI-06-0109. [DOI] [PubMed] [Google Scholar]
- Trichopoulos D, Lagiou P, Adami HO. Towards an integrated model for breast cancer etiology: the crucial role of the number of mammary tissue-specific stem cells. Breast Cancer Res. 2005;7:13–17. doi: 10.1186/bcr966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17:340–348. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]