Abstract
Combat exposure is associated with increased risk of psychiatric and substance use disorders in veterans. However, it is not known whether combat exposure independently increases risk for these disorders or whether this association is accounted for by genetic vulnerability common to posttraumatic stress disorder (PTSD). This paper tests competing explanations for the association of combat exposure and PTSD with nicotine dependence (ND), alcohol dependence (AD), and major depression (MD). Data was obtained from 6,099 members of the Vietnam Era Twin Registry, a national registry of male-male twin pairs who served in the military during the Vietnam Era. Twin models were fit to estimate the genetic and environmental variance common and specific to DSM-III-R lifetime diagnoses of PTSD, combat trauma, and three comorbid conditions: ND and AD and MD. Variance specific to ND, AD and MD was due to genetic factors (48%, 36% and 12%, respectively), and unique environmental factors (36%, 42% and 58%, respectively). After accounting for variance common to PTSD, no residual genetic and environmental variance overlapped between combat and ND, combat and AD, and combat and MD. Combat exposure is not independently associated with lifetime MD, AD, and MD. The association of combat exposure with these three disorders is due to genetic and unique environmental contributions in common with PTSD. These findings suggest comorbid PTSD may represent a genetically mediated vulnerability to psychopathology following trauma.
1. INTRODUCTION
Combat exposure is associated with increased risk of psychiatric and substance use disorders among men in the veteran population [1,2]. This association is especially strong among Vietnam veterans [3]. According to re-examination of the National Vietnam Veterans Readjustment Study, approximately 19% of male Vietnam theater veterans developed posttraumatic stress disorder (PTSD) and 9% had PTSD a decade after the war [4]. Risk for PTSD was greater with higher levels of combat exposure [4]. Moreover, nicotine dependence (ND), alcohol dependence (AD), and major depression (MD) are frequently comorbid with PTSD in Vietnam theater veterans [3,5–10].
PTSD may account for the association between combat exposure and ND, AD, or MD. This hypothesis was tested in a sample of monozygotic (MZ) twins discordant on the outcome of interest (e.g. ND) drawn from the larger Vietnam Era Twin (VET) Registry cohort used in the present study. The authors found evidence for a direct association between combat exposure and AD, but not for ND or MD, which were entirely explained by combat-related PTSD [11]. These findings are somewhat different than those for civilians whereby there is some evidence that trauma exposure increases risk of first onset of ND, independent of PTSD [12]. However, the association between trauma and AD and MD appears to be greatest in subjects with PTSD with the exception of AD in women in which case trauma alone appears to increase risk. [12,13]. As Breslau et al. [12,13] acknowledge, this mediation could be explained by vulnerability factors common to PTSD and other disorders. The current report models genetic and environmental influences on the associations among combat exposure, PTSD and ND, AD, and MD. Previous investigators have tested similar hypotheses in the VET Registry by controlling for genetic influences between combat related PTSD and these other disorders using a co-twin control design [11]. This previous study can conclude that combat related PTSD remains associated with comorbid psychopathology after controlling for genetic and unique environmental contributions. However, the co-twin design did not disentangle the magnitude of genetic, shared and unique environmental influences that may contribute to combat related PTSD and comorbid psychopathology. The current report extends earlier findings by explicitly modeling, rather than controlling for, these genetic and environmental influences.
Prior research studies using data from the VET Registry provides the basis for this work. There are substantial genetic influences on variation in combat exposure [14]. Prior research has also established that there are significant common genetic and non-shared environmental influences on the association of PTSD with ND [9], AD [15], and MD [16].
Our competing hypotheses make different predictions with regard to the model of genetic and environmental influences that would best explain the phenotypic association of PTSD, combat exposure and ND, AD and MD. If genetic or environmental contributions to combat exposure overlaps with these other disorders independent of PTSD, then genetic or environmental influences specific to combat should significantly influence ND, AD and MD after accounting for those influences common to PTSD. Alternatively, if the genetic or environmental factors between combat exposure and ND, AD and MD are accounted for by PTSD, genetic and environmental influences specific to combat should not significantly influence the variation between these disorders and PTSD.
2. Methods
Participants were drawn from the VET Registry, a nationally distributed cohort consisting of male-male twin pairs born between 1939 and 1957 in which both siblings served on active military duty during the Vietnam War era [17]. Zygosity was determined using a questionnaire and blood group typing methodology that achieved 95% accuracy [18]. Registry members are representative of all twins who served in the military during the Vietnam War on a variety of sociodemographic and other variables [19,20]. The data used in the present study came from 6,527 respondents to the 1987 Survey of Health and the 1992 Harvard Twin Study of Drug Abuse and Dependence [21]. The individual response rate to the latter study was 79.6%. The mean age of respondents was 44.6 years (S.D. + 2.8, range 36–55 years); 90.4% were non-Hispanic white, 4.9% were African-American, 2.7% were Hispanic, 1.3% were Native American and 0.7% were “other.” 8% of respondents had not graduated from high school, 92% were high school graduates, 22% college graduates, and 10.2% had post college education; 92.6% were employed full-time and 1.8% part-time. Three-quarters were married at the time of the study and 11% were never married. Registry members lived in all 50 states of the United States.
Because this study focuses on the relationship between combat experiences and psychiatric disorders, 1,215 twins who experienced non-combat trauma before entering the military and/or only after discharge from military service were excluded from the study. Thus the present study sample consists of 5,312 twins consisting of 1,224 MZ pairs, 886 DZ twin pairs and 1,092 singletons (singletons were not included in biometrical models).
2.1. Measures
Lifetime diagnoses of PTSD, ND, AD and MD were obtained using the Mental Health Diagnostic Interview Schedule Version III - Revised (DIS-III-R) [22]. Data obtained from the DIS-III-R were used to derive clinical diagnoses based on Diagnostic and Statistical Manual Third Edition Revised (DSM-III-R). Details of the interview procedure, types of traumatic events reported, and PTSD diagnostic data have been previously reported [23]. Trained, experienced staff from the Institute for Survey Research, Temple University, interviewed twins after verbal informed consent was obtained, a method approved by the Institutional Review Boards at participating institutions.
Combat exposure was measured using a Combat Exposure Index [24] which asked twins about exposure to 18 specific combat activities, such as flying in an attack helicopter, being wounded, and receiving incoming fire. A global index of combat exposure was constructed by summing all positive responses from an individual. The global index is used to classify combat exposure into five ordinal categories: 0 (did not serve in Southeast Asia (SEA), 1 (served in SEA but did not experience combat), 2 (1–2 combat experiences, ‘low combat’), 3 (3–6 combat experiences, ‘medium combat’), 4 (7 or more combat experiences, ‘high combat’). The ordinal combat categories demonstrated good internal consistency (α = 0.86) and test-retest reliability (κ = 0.84). The validity of the index are supported by a strong association between the combat exposure index and being awarded a military combat medal [24].
All data collection involving members of the VET Registry was approved by human subject committees at participating institutions.
2.2. Statistical analysis
Analyses began by first computing descriptive statistics for the association of PTSD with ND, AD and MD. To test if PTSD contributes to the association between combat and ND, AD and MD we fit logistic regression models. In each model the dependent variable was ND, AD or MD. We used the surveylogistic procedure in SAS version 9.1 to fit regression models. The surveylogistic procedure was computed with the option to adjust for the observations clustering in twin data and correctly compute the standard errors.
Twin modeling was performed by first computing tetrachoric correlations for MZ and DZ pairs to measure the association among PTSD, combat, and ND, AD and MD. The tetrachoric correlation assumes a continuous, normally distributed latent liability underlies the observed dichotomous distribution of diagnosis. It estimates the correlation between the underlying liability distribution rather than the observed dichotomous variables.
We then computed three trivariate Cholesky decomposition models of the association between: 1) PTSD, combat and ND; 2) PTSD, combat and AD; and 3) PTSD, combat and MD. We ordered the three phenotypes in the trivariate modeling to test if residual variance remained between combat and AD, combat and ND and combat and MD after accounting for variance shared with PTSD.
In the Cholesky model, variation in phenotypes is partitioned into variance due to additive genetic influences (A), shared or common environmental influences (C), and nonshared or unique environmental influences (E). Shared environmental influences are defined as environmental influences that make twins similar. Non-shared or individual-specific environmental influences are defined as environmental influences that make twins different. Measurement error is assumed to be random, i.e., uncorrelated across twins; it is, therefore, also included in the non-shared environmental variance. Cholesky models were fit to the tetrachoric correlation matrix using Mx [25.26].
When conducting model-fitting in Mx using nested models, the difference in fit between models can be tested by the change in chi-square value with the degrees of freedom equal to the difference in degrees of freedom between the two models. If the chi-square difference is not significant, then the more parsimonious model is selected, because this indicates model fit does not deteriorate with the additional constraints. When comparing two models that are not nested, the Akaike Information Criterion (AIC) [27] is used to determine the best fitting model. The smaller AIC indicates the better fit.
Figure 1 illustrates the full model for the association between PTSD, combat exposure, and the outcome (either AD, MD, or ND). Path loadings are estimated for each source of influence, and these are used to determine the contribution of A, C, and E to the variance of PTSD, combat and the outcome, and the covariance between them. These influences may be common to all three phenotypes, as is represented by the A1, C1 and E1 factors, common to only combat and the outcome as is represented by the A2, C2 and E2 factors or specific to the outcome as is represented by the A3, C3 and E3 factors. Hypotheses are tested by setting specific paths to zero. For example, we can test whether combat exposure is significantly associated with ND independently of PTSD by setting the a32’, c32’, and/or e32’ to zero. If the chi-square is not significant, then combat exposure is not significantly associated with the outcomes independently of PTSD.
Figure 1.
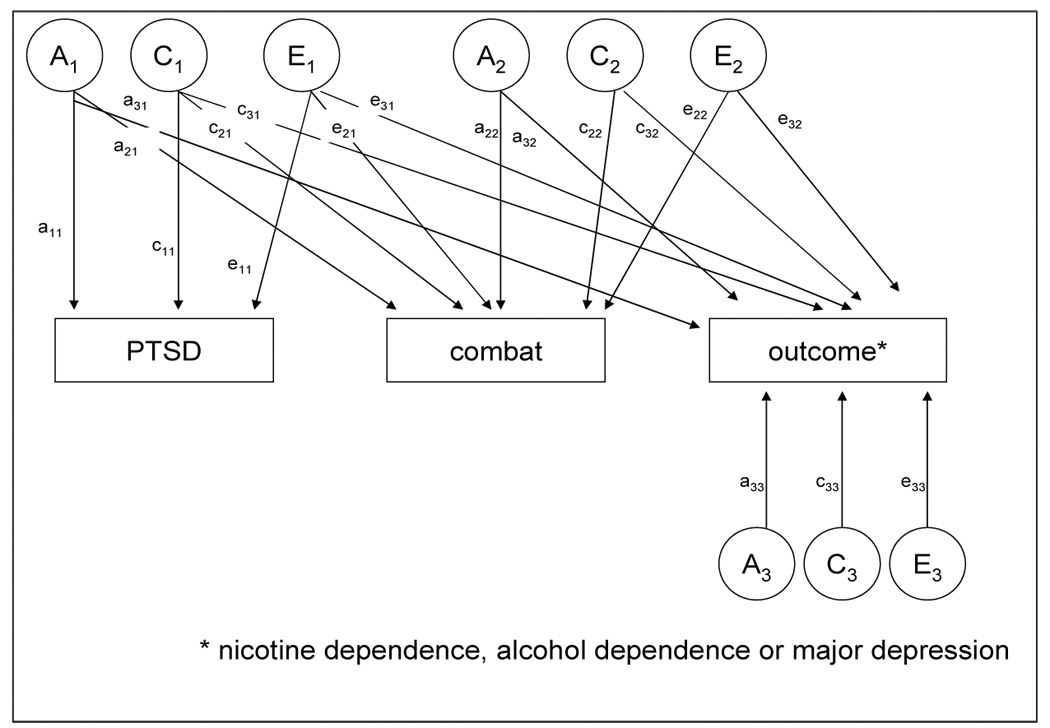
Full Cholesky model of additive genetic (A), family environmental (C) and unique environmental (E) contributions to posttraumatic stress disorder (PTSD), combat exposure and outcome phenotypes: nicotine dependence, alcohol dependence and major depression.
3. Results
Among twins in the study, 295 (5.6%) met DSM-III-R criteria for PTSD, 2,376 (44.7%) for ND, 1,728 (32.6%) for AD and 425 (8.0%) for MD. Of those who served in Southeast Asia, 644 (12.1%) had low combat (1 ≤ combat index ≤ 2), 692 (13%) subjects had medium combat (3 ≤ combat index ≤ 6), and 478 (9.0%) subjects had high combat (combat index ≥ 7). The remainder served elsewhere or had no combat experience in Southeast Asia.
Among subjects without PTSD, the prevalence of ND among low, medium and high combat was 41%, 48% and 43%; the prevalence of AD among low, medium and high combat was 30%, 36% and 32%; the prevalence of MD among low, medium and high combat was 6%, 6% and 7%. Among subjects with PTSD, the prevalence of ND among low, medium and high combat was 65%, 67% and 77%; the prevalence of AD among low, medium and high combat was 79%, 67% and 67%; the prevalence of MD among low, medium and high combat was 43%, 42% and 52%. Thus the prevalence of ND, AD and MD were higher among subjects with combat and PTSD as compared to those with combat only.
PTSD was strongly associated with ND, AD and MD in regression models that included combat level and PTSD. PTSD was significantly associated with ND (OR=3.3; 95% CI: 2.5–4.3), with AD (OR=3.6; 95% CI:2.8–4.6) and with MD (OR=7.2; 95% CI: 5.5–9.4) after adjusting for combat level.
The results for the three trivariate Cholesky biometrical models are shown in Tables 1a–1c. Based on our previous studies with the VETR cohort [14,15,28–31], shared environmental pathways (Figure 1, c11, c21, c31, c22, c32, c33) to all phenotypes were dropped in the first reduced model (Model 2) which resulted in an improvement in fit compared to the full model (AIC=−9.65, −11.44 and −2.78 for ND, AD and MD, respectively). The best fitting trivariate models (model 4, Tables 1a–1c) for ND, AD and MD (AIC=−13.44, −15.44 and −6.78, respectively) did not allow for shared environmental pathways (Figure 1, c11, c21, c31, c22, c32, c33), did not allow for genetic factors common (Figure 1, a32) to combat and the outcome of interest (i.e. ND, AD, MD) and did not allow for unique environmental factors common (Figure 1, e32) to combat and the outcome of interest.
Table 1.
| Table 1a Cholesky model for lifetime co-occurrence of posttraumatic stress disorder (PTSD), combat and nicotine dependence (ND) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | ||||||||||||
| Factors common to | Factors common to | Factors specific to | χ2 | d.f | Δ χ2* | Δd.f | p-value | AIC | ||||
| PTSD | combat | ND | combat | ND | ND | |||||||
| 1 | A C E | A C E | A C E | A C E | A C E | A C E | 18.67 | 15 | -- | -- | -- | -- |
| 2 | A E | A E | A E | A E | A E | A E | 21.01 | 21 | 2.35 | 6 | 0.89 | −9.65 |
| 3 | A E | A E | A E | A E | E | A E | 21.01 | 22 | 2.35 | 7 | 0.94 | −11.65 |
| 4 | A E | A E | A E | A E | -- | A E | 21.01 | 23 | 2.35 | 8 | 0.97 | −13.44 |
| 5 | A E | A E | A E | E | -- | A E | 66.52 | 24 | 42.42 | 9 | <0.001 | 18.52 |
| Table 1b Cholesky model for lifetime co-occurrence of posttraumatic stress disorder (PTSD), combat and alcohol dependence (AD) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | ||||||||||||
| Factors common to | Factors common to | Factors specific to | χ2 | df | Δ χ2* | Δd.f | p-value | AIC | ||||
| PTSD | combat | AD | combat | AD | AD | |||||||
| 1 | A C E | A C E | A C E | A C E | A C E | A C E | 14.04 | 15 | -- | -- | -- | -- |
| 2 | A E | A E | A E | A E | A E | A E | 14.60 | 21 | 0.56 | 6 | 0.99 | −11.44 |
| 3 | A E | A E | A E | A E | E | A E | 14.60 | 22 | 0.56 | 7 | 0.99 | −13.44 |
| 4 | A E | A E | A E | A E | -- | A E | 14.60 | 23 | 0.56 | 8 | 1.00 | −15.44 |
| 5 | A E | A E | A E | E | -- | A E | 58.89 | 24 | 48.60 | 9 | <0.001 | 10.89 |
| Table 1c Cholesky model for lifetime co-occurrence of posttraumatic stress disorder (PTSD), combat and major depression (MD) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | ||||||||||||
| Factors common to | Factors common to | Factors specific to | χ2 | df | Δ χ2* | Δ df | p-value | AIC | ||||
| PTSD | combat | MD | combat | MD | MD | |||||||
| 1 | A C E | A C E | A C E | A C E | A C E | A C E | 32.42 | 15 | -- | -- | -- | -- |
| 2 | A E | A E | A E | A E | A E | A E | 41.64 | 21 | 9.22 | 6 | 0.16 | −2.78 |
| 3 | A E | A E | A E | A E | E | A E | 41.64 | 22 | 9.22 | 7 | 0.237 | −4.78 |
| 4 | A E | A E | A E | A E | -- | A E | 41.64 | 23 | 9.22 | 8 | 0.324 | −6.78 |
| 5 | A E | A E | A E | E | -- | A E | 72.26 | 24 | 41.03 | 9 | <0.001 | 23.03 |
Δ χ2 = χ2 (sub-model) - χ2 (full model). Δ df = df (sub-model) – df (full model). boldface indicates best fitting model
Δ χ2 = χ2 (sub-model) - χ2 (full model). Δdf = df (sub-model) – df (full model). Bold text indicates best fitting model
Δ χ2 = χ2 (sub-model) - χ2 (full model). Δ df = df (sub-model) – df (full model). Bold text indicates best fitting model
Table 2 a, b, c shows the variance component estimates and 95% confidence intervals for PTSD, combat, ND, AD and MD under the best fitting models. Genetic variance in risk for ND, AD and MD, that overlapped with PTSD and combat, was 30%, 20% and 21%, respectively. The unique environmental variance that overlapped with PTSD and combat was 3%, 1% and 9%, respectively. The remaining variance in risk for ND, AD and MD was due to disorder specific genetic contributions to ND (48%), AD (36%) and MD (12%), and disorder specific unique environmental contributions to ND (36%), AD (42%) and MD (58%).
Table 2.
| Table 2a Variance component estimates* for posttraumatic stress disorder (PTSD), combat and nicotine dependence (ND) from best fitting model | ||||||
|---|---|---|---|---|---|---|
| Factors common to | Factors common to | Factors specific to ND | ||||
| PTSD | combat | ND | combat | ND | ||
| 0.33 | 0.05 | 0.30 | .30 | .48 | ||
| A | (0.22, 0.45) | (0.02, 0.10) | (0.06, 0.23) | (0.24, 0.36) | - | (0.38, 0.57) |
| C | - | - | - | - | - | - |
| 0.67 | .10 | .03 | 0.55 | 0.36 | ||
| E | (0.55, 0.78) | (0.06, 0.16) | (0.01, 0.07) | (0.48, 0.62) | - | (0.30, 0.42) |
| Table 2b Variance component estimates* for posttraumatic stress disorder (PTSD), combat and alcohol dependence (AD) from best fitting model | ||||||
|---|---|---|---|---|---|---|
| Factors common to | Factors common to | Factors specific to AD | ||||
| PTSD | combat | AD | combat | AD | ||
| 0.36 | 0.04 | 0.20 | 0.31 | 0.36 | ||
| A | (0.25, 0.48) | (0.01, 0.08) | (0.11, 0.33) | (0.25, 0.40) | - | (0.23, 0.46) |
| C | - | - | - | - | - | - |
| 0.64 | .11 | .01 | 0.54 | - | 0.42 | |
| E | (0.53, 0.76) | (0.07, 0.17) | (0.002,0.04) | (0.47, 0.61) | (0.36, 0.48) | |
| Table 2c Variance component estimates* for posttraumatic stress disorder (PTSD), combat and major depression (MD) from best fitting model | ||||||
|---|---|---|---|---|---|---|
| Factors common to | Factors common to | Factors specific to MD | ||||
| PTSD | combat | MD | combat | MD | ||
| 0.34 | 0.02 | 0.21 | 0.32 | 0.12 | ||
| A | (0.22, 0.46) | (0.00, 0.05) | (0.09, 0.37) | (0.26, 0.38) | - | (0.00, 0.25) |
| C | - | - | - | - | - | - |
| 0.66 | .13 | .09 | 0.53 | - | 0.58 | |
| E | (0.54, 0.78) | (0.08, 0.20) | (0.04, 0.18) | (0.45, 0.61) | (0.46, 0.70) | |
A- additive genetic, C-shared environment, E-non-shared environment
4. Discussion
Our results indicate that combat exposure alone is not independently associated with DSM-III-R lifetime diagnoses of ND, AD, or MD. Rather, the association between combat and the outcomes is accounted for by genetic and environmental contributions to PTSD. After accounting for variance in common with PTSD and combat, there were no genetic or unique environmental contributions common to combat and ND, combat and AD, and combat and MD. The majority of the overlapping vulnerability was genetic, although non-shared environmental influences common to PTSD were also statistically significant. Specifically, genetic influences common to PTSD explained 30% of the variance in ND, 20% of the variance in AD, and 21% of the variance in MD. Significant non-shared environmental influences common to PTSD explained only 1% of the variance in AD, 3% of the variance in ND, and 9% of the variance in MD. Disorder-specific genetic influences explained a substantial portion of variance for AD (36%) and ND (48%). Such influences were not significant for MD; the majority of the variance in MD was explained by disorder-specific non-shared environmental influences (58%). Disorder-specific non-shared environmental influences were also important components of the variance in AD (42%) and ND (36%).
Our findings are largely consistent with those of Breslau et al. [12,13] who found that it is PTSD and not the traumatic event, per se, that increased risk for psychiatric comorbidity. Taken together, our findings suggest that the co-occurrence of PTSD and these other disorders is partly explained by a shared diathesis. This diathesis appears to represent a genetically mediated vulnerability to developing psychopathology following exposure to life-threatening events. The presence of a significant common non-shared environmental pathway from PTSD to ND, AD, and MD is consistent with PTSD symptoms having a direct, albeit small, effect on risk of these disorders [32]. Significant common non-shared environmental influences can also reflect common unmeasured environmental risk factors or shared measurement error.
Our data do not support the position of Summerfield [37,38] who argues that PTSD is an arbitrary definition given to a range of normal responses to war trauma and should be conceptualized as a sociological phenomenon rather than a psychiatric illness. Though we have reported evidence for common vulnerability factors, interpretation of this finding must consider the evidence for specific genetic contributions to PTSD and comorbid affective and substance use disorder. The present study and previous models of illicit drug dependence and PTSD [15] and models of PTSD, generalized anxiety disorder and panic disorder [39] include genetic variance that is specific to PTSD and genetic variance that is specific to comorbid substance use and anxiety disorders. Comorbid psychiatric disorders that are associated with combat trauma are not completely explained by genetic vulnerability shared with PTSD. Overall, our current and previous reports suggest at least 39% of the genetic variance in PTSD is not shared with these other disorders.
Xian et al. [15] reported no genetic variance specific to AD in a common factor model of PTSD, AD and drug dependence (DD). When compared with this previous finding, the present results suggest there is a specific genetic factor to AD not accounted for by PTSD, which can be detected when variance is not portioned into that due to DD. Koenen et al. [9] reported 62% of the covariance in PTSD and ND is due to common genetic factors which leaves a substantial proportion of genetic variance specific to ND as we found in the present analyses. Fu et al. [16] analyses of conduct disorder, MD and PTSD is consistent with the present analyses in that both allow for a common genetic factor to PTSD and MD
Approximately 1.4 million men are in active duty in the US armed forces and this number is likely to increase [33]. Given the continuing conflicts in Iraq and Afghanistan, active duty military personnel are at high risk of combat exposure and PTSD [34]. Combat exposure is one of the traumas with a high conditional risk of developing PTSD among males in the general population [35]. Thus, disentangling the etiology of the association among combat exposure, PTSD, and other disorders remains an important public health goal.
Our findings, and those of others [12,13,36], indicate that it is not traumatic events themselves but the development of PTSD following such events that is associated with increased risk of developing other mental disorders. These findings imply that intervention efforts with demonstrated efficacy in trauma-exposed populations [40] could be focused on preventing the development and adverse secondary consequences of PTSD among the minority of trauma victims most at risk for the disorder, rather than on prevention among trauma victims more generally. Among traumatized populations, identifying those at greatest risk for subsequent substance use and affective disorders may be possible by collecting family and personal histories of psychiatric disorders that are often comorbid with PTSD.
4.1. Limitations
Our findings are subject to several limitations. First, the sample consisted entirely of male Vietnam era veterans and results may not generalize to non-combat PTSD. The relationship between PTSD, trauma and ND, AD, and MD may not generalize to civilians, females or other male cohorts. Our assessment of psychiatric diagnoses, trauma exposure, PTSD and their dates of onset were undertaken retrospectively. If individuals who had PTSD were more likely than those who did recall a history of other disorders or vice versa, this would inflate our associations. It is possible that pre-existing psychopathology contributed to the ND, AD and MD outcomes. Because minimum psychiatric health was required for service in the military, AD and MD would be less likely than ND to precede combat exposure. However, it is possible that ND onset prior to combat. Our assessments are also based on DSM-III-R and not DSM-IV and other measures of PTSD are available. Replication with DSM-IV assessments and other measures of PTSD is warranted in future data collection with VET Registry members.
4.2. Conclusions
The vulnerability for substance use disorder and MD following combat trauma is accounted for by genetic factors that overlap with PTSD. Based on the present analyses, common genetic vulnerability could contribute to the incidence of substance use disorders and MD among other non-Vietnam war veterans especially among those with who develop PTSD after combat exposure. Adequate health care resources should be in place to address not only PTSD but also those disorders that share common genetic vulnerability with this disorder.
Acknowledgements
This work was supported by NIH grants AA12640, AA11667, AA11998 and DA14363, DA020810. Dr. Koenen is supported in part by MH070627. Additional funding was provided by the National Institute on Drug Abuse, and the Robert Wood Johnson Foundation. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Drs. Scherrer and Xian had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis. 2001;189:99–108. doi: 10.1097/00005053-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Prigerson HG, Maciejewski PK, Rosenheck RA. Population attributable fractions of psychiatric disorders and behavioral outcomes associated with combat exposure among US men. Am J Public Health. 2002;92:59–63. doi: 10.2105/ajph.92.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulka RA, Schlenger WE, Fairbank JA, Hough RI, Jordan BK, Marmar CR, et al. Trauma and the Vietnam War Generation: Report of the findings from the National Vietnam Veterans Readjustment Study. New York: Brunner/Mazel; 1990. [Google Scholar]
- 4.Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshalt R. The psychological risks of Vietnam for U.S. Veterans: a revisit with new data and methods. Science. 2006;313:979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stellman SD, Stellman JM, Sommer JFJ. Social and behavioral consequences of combat and herbicide exposure in Vietnam among American Legionnaires. Environ Res. 1988;47:129–149. doi: 10.1016/s0013-9351(88)80038-0. [DOI] [PubMed] [Google Scholar]
- 6.Davidson JR, Kudler HS, Saunders WB, Smith RD. Symptom and comorbidity patterns in World War II and Vietnam veterans with posttraumatic stress disorder. Compr Psychiatry. 1990;31:162–170. doi: 10.1016/0010-440x(90)90020-s. [DOI] [PubMed] [Google Scholar]
- 7.Engdahl BE, Dikel TN, Eberly RE, Blank AS. Posttraumatic stress disorder in a community group of former prisoners of war: a normative response to severe trauma. Am J Psychiatry. 1997;154:1576–1581. doi: 10.1176/ajp.154.11.1576. [DOI] [PubMed] [Google Scholar]
- 8.Jelinek JM, Williams T. Post-traumatic stress disorder and substance abuse in Vietnam combat veterans: treatment problems, strategies and recommendations. J Subst Abuse Treat. 1984;1:87–97. doi: 10.1016/0740-5472(84)90031-x. [DOI] [PubMed] [Google Scholar]
- 9.Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- 10.Orsillo SM, Weathers FW, Litz BT, Steinberg HR, Huska JA, Keane TM. Current and lifetime psychiatric disorders among veterans with war zone-related posttraumatic stress disorder. J Nerv Ment Dis. 1996;184:307–313. doi: 10.1097/00005053-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toomey R, et al. Co-twin control study of the relationships among combat exposure, combat-related PTSD, and other mental disorders. J Traumatic Stress. 2003;16:433–438. doi: 10.1023/A:1025786925483. [DOI] [PubMed] [Google Scholar]
- 12.Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- 13.Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biol Psychiatr. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- 14.Lyons MJ, Goldberg J, Eisen SA, True W, Tsuang MT, Meyer JM. Do genes influence exposure to trauma? a twin study of combat. Am J Med Genet. 1993;48:22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- 15.Xian H, Chantarujikapong S, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, et al. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:91–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 16.Fu Q, Koenen KC, Miller MW, Heath AC, Bucholz KK, Lyons MJ, et al. Differential etiology of posttraumatic stress disorder with conduct disorder and major depression in male veterans. Biological Psychiatry. doi: 10.1016/j.biopsych.2007.04.036. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisen S, True WR, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin Registry: method of construction. Acta Genet Med Gemellol. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- 18.Eisen S, Neuman R, Goldberg J, Rice J, True WR. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg J, True WR, Eisen SA, Henderson WG, Robinette CD. The Vietnam Era Twin (VET) Registry: ascertainment bias. Acta Genet Med Gemellol. 1987;36:67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- 20.Henderson W, Eisen S, Goldberg J, True WR, Barnes JT, Vitek ME. Vietnam Twin Registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: what we have learned. Harv Rev Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- 22.Robins LN, Helzer JE, Cottler L, Goldring E. National Institute of Mental Health Diagnostic Interview Schedule Version III -Revised. St. Louis, MO: Department of Psychiatry, Washington University; 1988. [Google Scholar]
- 23.Koenen KC, Harley R, Lyons MJ, Wolfe J, Simpson JC, Goldberg J, et al. A twin registry study of familial and individual risk factors for trauma exposure and posttraumatic stress disorder. J Nerv Ment Dis. 2002;190:209–218. doi: 10.1097/00005053-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Janes SR, Goldberg J, Eisen S, True WR, Henderson W. Reliability and validity of a combat exposure index for Vietnam era veterans. J Clin Psychol. 1992;47:80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Neale MC, Boker SM, Xie G, Maes H. Mx: statistical modeling. VCU, Box 900126, Richmond, VA 23298. Department of Psychiatry. 2002 [Google Scholar]
- 26.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer; 1992. [Google Scholar]
- 27.Akaike H. Factor analysis and AIC. Psychometrics. 1987;52:317–333. [Google Scholar]
- 28.Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, et al. A registry-based twin study of depression in men. Arch Gen Psychiatry. 1988;55:468–472. doi: 10.1001/archpsyc.55.5.468. [DOI] [PubMed] [Google Scholar]
- 29.True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttranmatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 30.True WR, Xian H, Scherrer JF, Madden P, Bucholz KK, Heath AC, et al. Common vulnerability for nicotine and alcohol dependence. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- 31.Xian H, Scherrer JF, Eisen SA, Lyons MJ, Tsuang MT, True WR, et al. Nicotine dependence subtypes: association with smoking history, diagnostic criteria and psychiatric disorders in 5,440 regular smokers from the Vietnam Era Twin Registry. Addict Behav. 2007;32:137–147. doi: 10.1016/j.addbeh.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Purcell S, Koenen KC. Environmental mediation and the twin design. Behav Genet. doi: 10.1007/s10519-004-1484-9. in press. [DOI] [PubMed] [Google Scholar]
- 33.Segal D. America's military population. Popul Bulletin. 2004;59:1–40. [Google Scholar]
- 34.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. NEJM. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 35.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 36.Breslau N. Epidemiologic studies of trauma, posttraumatic stress disorder and other psychiatric disorders. Can J Psychiatry. 2002;47:923–929. doi: 10.1177/070674370204701003. [DOI] [PubMed] [Google Scholar]
- 37.Summerfield D. The psychological legacy of war and atrocity: The question of long-term transgenerational effects and the need for a broad view. J Nerv Ment Dis. 1996;184:375–377. doi: 10.1097/00005053-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Summerfield D. War, exile, moral knowledge and the limits of psychiatric understanding: a clinical case study of a Bosnian refugee in London. Int J Soc Psychiatry. 2004;49:264–268. doi: 10.1177/0020764003494004. [DOI] [PubMed] [Google Scholar]
- 39.Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psych Res. 2001;103:133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 40.Deahl MP, Srinivasan M, Jones N, Neblett C, Jolly A. Evaluating psychological debriefing: Are we measuring the right outcomes. J Traumatic Stress. 14:527–529. doi: 10.1023/A:1011160606866. [DOI] [PubMed] [Google Scholar]


