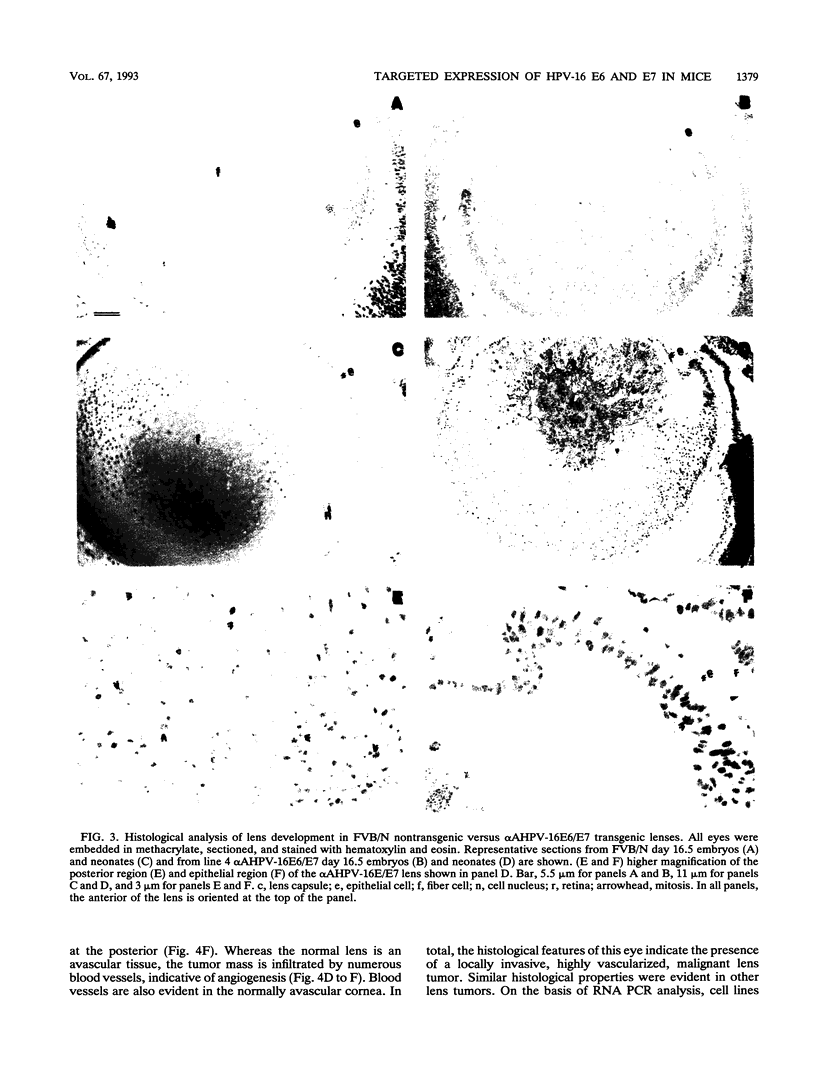
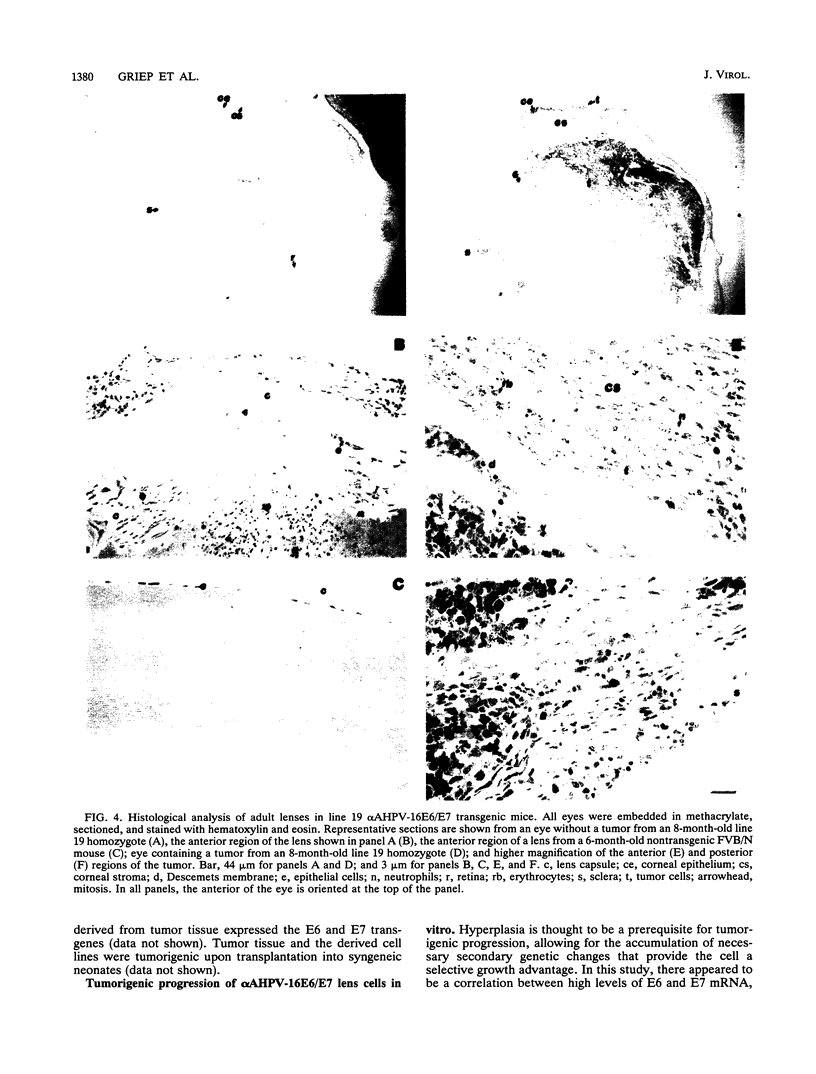
Abstract
The human papillomavirus type 16 (HPV-16) E6 and E7 oncogenes are thought to play a role in the development of most human cervical cancers. These E6 and E7 oncoproteins affect cell growth control at least in part through their association with and inactivation of the cellular tumor suppressor gene products, p53 and Rb. To study the biological activities of the HPV-16 E6 and E7 genes in epithelial cells in vivo, transgenic mice were generated in which expression of E6 and E7 was targeted to the ocular lens. Expression of the transgenes correlated with bilateral microphthalmia and cataracts (100% penetrance) resulting from an efficient impairment of lens fiber cell differentiation and coincident induction of cell proliferation. Lens tumors formed in 40% of adult mice from the mouse lineage with the highest level of E6 and E7 expression. Additionally, when lens cells from neonatal transgenic animals were placed in tissue culture, immortalized cell populations grew out and acquired a tumorigenic phenotype with continuous passage. These observations indicate that genetic changes in addition to the transgenes are likely necessary for tumor formation. These transgenic mice and cell lines provide the basis for further studies into the mechanism of action of E6 and E7 in eliciting the observed pathology and into the genetic alterations required for HPV-16-associated tumor progression.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Transgenic models of tumor development. Science. 1991 Nov 22;254(5035):1161–1167. doi: 10.1126/science.1957168. [DOI] [PubMed] [Google Scholar]
- Banks L., Edmonds C., Vousden K. H. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene. 1990 Sep;5(9):1383–1389. [PubMed] [Google Scholar]
- Beebe D. C., Silver M. H., Belcher K. S., Van Wyk J. J., Svoboda M. E., Zelenka P. S. Lentropin, a protein that controls lens fiber formation, is related functionally and immunologically to the insulin-like growth factors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2327–2330. doi: 10.1073/pnas.84.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D., Vassalli J. D., Combépine C., Godeau F., Nagamine Y., Reich E., Kocher H. P., Duvoisin R. M. Cloning, nucleotide sequencing and expression of cDNAs encoding mouse urokinase-type plasminogen activator. Eur J Biochem. 1985 Apr 15;148(2):225–232. doi: 10.1111/j.1432-1033.1985.tb08829.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain C. G., McAvoy J. W. Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr Eye Res. 1987 Sep;6(9):1165–1169. doi: 10.3109/02713688709034890. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Lee I. C., Ross S. R. Requirement for the simian virus 40 small tumor antigen in tumorigenesis in transgenic mice. Mol Cell Biol. 1988 Aug;8(8):3382–3390. doi: 10.1128/mcb.8.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Wrede D., Tidy J., Scholefield J., Crawford L., Vousden K. H. Status of c-myc, p53 and retinoblastoma genes in human papillomavirus positive and negative squamous cell carcinomas of the anus. Oncogene. 1991 Jul;6(7):1251–1257. [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Dyson N., Bernards R., Friend S. H., Gooding L. R., Hassell J. A., Major E. O., Pipas J. M., Vandyke T., Harlow E. Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol. 1990 Mar;64(3):1353–1356. doi: 10.1128/jvi.64.3.1353-1356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Edmonds C., Vousden K. H. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989 Jun;63(6):2650–2656. doi: 10.1128/jvi.63.6.2650-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage J. R., Meyers C., Wettstein F. O. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J Virol. 1990 Feb;64(2):723–730. doi: 10.1128/jvi.64.2.723-730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griep A. E., Kuwabara T., Lee E. J., Westphal H. Perturbed development of the mouse lens by polyomavirus large T antigen does not lead to tumor formation. Genes Dev. 1989 Jul;3(7):1075–1085. doi: 10.1101/gad.3.7.1075. [DOI] [PubMed] [Google Scholar]
- Griep A. E., Westphal H. Differentiation versus proliferation of transgenic mouse lens cells expressing polyoma large T antigen: evidence for regulation by an endogenous growth factor. New Biol. 1990 Aug;2(8):727–738. [PubMed] [Google Scholar]
- Hanahan D. Transgenic mice as probes into complex systems. Science. 1989 Dec 8;246(4935):1265–1275. doi: 10.1126/science.2686032. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P., Vousden K. H., Hubbert N. L., Lowy D. R., Schiller J. T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Bedell M. A., McCance D. J., Laiminis L. A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990 Feb;64(2):519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin P. J., Kaur P., Smith P. P., Perez-Reyes N., Blanton R. A., McDougall J. K. Progression of human papillomavirus type 18-immortalized human keratinocytes to a malignant phenotype. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):570–574. doi: 10.1073/pnas.88.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewers R. J., Hildebrandt P., Ludlow J. W., Kell B., McCance D. J. Regions of human papillomavirus type 16 E7 oncoprotein required for immortalization of human keratinocytes. J Virol. 1992 Mar;66(3):1329–1335. doi: 10.1128/jvi.66.3.1329-1335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel J., Bossy-Wetzel E., Radvanyi F., Klagsbrun M., Folkman J., Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991 Sep 20;66(6):1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Howley P. M. Bovine papillomavirus type 1 E1 replication-defective mutants are altered in their transcriptional regulation. J Virol. 1988 Nov;62(11):4009–4015. doi: 10.1128/jvi.62.11.4009-4015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti C., Morrissey L. C., Grossman S. R., Androphy E. J. Transcriptional activation by the papillomavirus E6 zinc finger oncoprotein. EMBO J. 1990 Jun;9(6):1907–1913. doi: 10.1002/j.1460-2075.1990.tb08317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lindgren V., Sippola-Thiele M., Skowronski J., Wetzel E., Howley P. M., Hanahan D. Specific chromosomal abnormalities characterize fibrosarcomas of bovine papillomavirus type 1 transgenic mice. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5025–5029. doi: 10.1073/pnas.86.13.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Mahon K. A., Chepelinsky A. B., Khillan J. S., Overbeek P. A., Piatigorsky J., Westphal H. Oncogenesis of the lens in transgenic mice. Science. 1987 Mar 27;235(4796):1622–1628. doi: 10.1126/science.3029873. [DOI] [PubMed] [Google Scholar]
- Matlashewski G., Schneider J., Banks L., Jones N., Murray A., Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 1987 Jun;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy J. W. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J Embryol Exp Morphol. 1978 Jun;45:271–281. [PubMed] [Google Scholar]
- Miskin R., Axelrod J. H., Griep A. E., Lee E., Belin D., Vassalli J. D., Westphal H. Human and murine urokinase cDNAs linked to the murine alpha A-crystallin promoter exhibit lens and non-lens expression in transgenic mice. Eur J Biochem. 1990 May 31;190(1):31–38. doi: 10.1111/j.1432-1033.1990.tb15541.x. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Mahon K. A., Miskin R., Dey A., Kuwabara T., Westphal H. Differentiation and oncogenesis: phenotypically distinct lens tumors in transgenic mice. New Biol. 1989 Nov;1(2):193–204. [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro G., Lee M., Morgan D., Defendi V. Evolution of in vitro transformation and tumorigenesis of HPV16 and HPV18 immortalized primary cervical epithelial cells. Am J Pathol. 1991 Jan;138(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Bagchi S., Barnes J. A., Raychaudhuri P., Kraus V., Münger K., Howley P. M., Nevins J. R. Analysis of trans activation by human papillomavirus type 16 E7 and adenovirus 12S E1A suggests a common mechanism. J Virol. 1991 Dec;65(12):6922–6930. doi: 10.1128/jvi.65.12.6922-6930.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Münger K., Yee C. L., Barnes J. A., Howley P. M. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol. 1992 Apr;66(4):2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E. J. Functional evidence for human tumour suppressor genes: chromosome and molecular genetic studies. Cancer Surv. 1992;12:5–24. [PubMed] [Google Scholar]
- Storey A., Pim D., Murray A., Osborn K., Banks L., Crawford L. Comparison of the in vitro transforming activities of human papillomavirus types. EMBO J. 1988 Jun;7(6):1815–1820. doi: 10.1002/j.1460-2075.1988.tb03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmeyer T., Reissmann P., Cordon-Cardo C., Hartmann M., Ackermann R., Slamon D. Correlation between retinoblastoma gene expression and differentiation in human testicular tumors. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6662–6666. doi: 10.1073/pnas.88.15.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kanda T., Sato H., Furuno A., Yoshiike K. Mutational analysis of human papillomavirus type 16 E7 functions. J Virol. 1990 Jan;64(1):207–214. doi: 10.1128/jvi.64.1.207-214.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Woodworth C. D., Waggoner S., Barnes W., Stoler M. H., DiPaolo J. A. Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Res. 1990 Jun 15;50(12):3709–3715. [PubMed] [Google Scholar]
- Yew P. R., Berk A. J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992 May 7;357(6373):82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]