Abstract
Recent studies suggest that sustained morphine-mediated paradoxical pain may play an important role in the development of analgesic tolerance. The intracellular signal transduction pathways involved in sustained opioid mediated augmentation of spinal pain neurotransmitter (such as calcitonin gene-related peptide (CGRP)) release are not fully clarified. Cyclic AMP (cAMP)-dependent protein kinase (PKA) plays an important role in the modulation of presynaptic neurotransmitter release. Moreover, we have shown earlier that sustained opioid agonist treatment leads to a Raf-1-dependent sensitization of adenylyl cyclase(s) (AC superactivation), augmenting forskolin-stimulated cAMP formation upon opioid withdrawal (cAMP overshoot). Therefore, in the present study we examined the role of Raf-1 in sustained morphine-mediated regulation of cAMP formation and basal CGRP release in vitro, in cultured neonatal rat dorsal root ganglion (DRG) neurons. We found that sustained morphine treatment significantly augments intracellular cAMP production as well as basal CGRP release from cultured neonatal rat DRG neurons. The selective PKA inhibitor, H-89, attenuates the sustained morphine-mediated augmentation of basal CGRP release, indicating that the cAMP/PKA pathway plays an important role in regulation of CGRP release from sensory neurons. Since our present data also demonstrated that selective Raf-1 inhibitor, GW 5074, attenuated both the cAMP overshoot and the augmentation of CGRP release mediated by sustained morphine in neonatal rat DRG neurons, we suggest that Raf-1-mediated sensitization of the intracellular cAMP formation may play an important role in sustained morphine-mediated augmentation of spinal pain neurotransmitter release.
Keywords: Morphine, CGRP, Raf-1, cAMP, PKA, Dorsal root ganglion neurons
1. Introduction
The development of antinociceptive tolerance limits the therapeutic use of opioid analgesics in the clinical management of pain. The molecular mechanisms leading to antinociceptive tolerance are not fully understood. It has been suggested that the upregulation of spinal pain neurotransmitter, such as calcitonin gene-related peptide (CGRP) release after sustained opioid analgesic treatment may cause a paradoxical pain sensitization that is manifested as analgesic tolerance in vivo (Trang et al. 2005; Ossipov et al. 2005).
The intracellular signal transduction pathways leading to sustained morphine-mediated augmentation of spinal pain neurotransmitter release are not entirely clear. However, cAMP-dependent protein kinase (PKA) is known to play a major role in the regulation of presynaptic neurotransmitter synthesis and release (Carruthers et al., 2001; Supowit et al., 1995; Leenders and Shen, 2005; Rathee et al., 2002). On the other hand, sustained opioid agonist treatment was shown to upregulate the cAMP signal transduction cascade (adenylyl cyclase (AC) superactivation) in multiple regions of the nervous system (Nestler and Aghajanian, 1997), including the primary sensory dorsal root ganglion (DRG) neurons (Crain and Shen, 1998; Terwilliger et al., 1991). However, the contribution of the upregulated cAMP cascade to sustained morphine-mediated augmentation of spinal pain neurotransmitter release still needs to be addressed.
Since we have found earlier that a Raf-1 inhibitor, 3-(3,5-dibromo-4-hydroxybenzylidene)-5-iodo-1,3-dihydroindol-2-one (GW 5074), attenuates long-term opioid mediated AC superactivation in recombinant model cell lines (Varga et al., 2002; Yue et al., 2006), we hypothesize that Raf-1 mediated AC superactivation upon sustained opioid treatment, via activation of PKA, may contribute to sustained opioid-mediated augmentation of pain neurotransmitter release. In the present study we tested the effect of sustained morphine treatment on intracellular cAMP production and basal CGRP release in primary cultured DRG neurons. The selective PKA inhibitor, N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H-89) was used to determine the role of cAMP/PKA pathway in sustained morphine mediated CGRP release. Moreover, the role of Raf-1 in sustained morphine-mediated AC superactivation and augmentation of basal CGRP release in DRG neurons was also investigated. Our findings indicate that Raf-1-mediated sensitization of the cAMP/PKA pathway plays an important role in sustained morphine-mediated augmentation of spinal pain neurotransmitter CGRP release.
2. Materials and methods
2.1. Primary culture of neonatal rat DRG neurons
The animal study protocol is approved by Institutional Animal Care and Use Committee of the University of Arizona. DRGs from newborn (1-3 day old) Sprague-Dawley rats were dissected aseptically from all spinal levels. The ganglia were digested with 0.1% collagenase (Sigma, St. Louis, MO) for 3-5 min and 0.25% trypsin (Invitrogen, San Diego, CA) for 10 min. The DRGs were then triturated through a fire-polished Pasteur pipette in Neurobasal A medium containing 0.1 mg/ml DNase I (Sigma, St. Louis, MO) and 5 mM MgSO4, centrifuged and re-suspended in Neurobasal A medium containing B27 supplement (Invitrogen, San Diego, CA); (Neurobasal A/B27 medium) and 250 ng/ml Nerve growth factor (NGF; Sigma, St. Louis, MO). 24 well plates were seeded with 1.6×104 cells per well. Anti-mitotic drugs [uridine (150μM) and 5-fluo-deoxy-uridine (50μM); Sigma, St. Louis, MO)] were added and the cells were allowed to differentiate for 7-9 days at 37°C in a CO2 incubator.
2.2. Drug treatments
The stock solutions of the drugs were prepared as follows: GW 5074 (10 mM, Sigma, St. Louis, MO) and H-89 (1 mM, Millipore Corporation, Billerica, MA) were dissolved in DMSO; morphine sulfate (1 mM, National Institute of Drug Abuse, Bethesda, MD) and naltrexone (10 mM, Tocris, Ellisville, MO) were dissolved in distilled water. All the drugs were further diluted in Neurobasal A/B27 medium. 24 hours before the experiment, the DRG neurons were gently washed and NGF and anti-mitotic drugs were withdrawn from the culture medium. On the day of the experiment, the DRG neurons were pre-incubated (1 h) in the presence or absence of either PKA inhibitor H-89 (1 μM; inhibitor concentration was selected from an H-89 dose-response curve; S. Tumati, unpublished observations) or Raf-1 inhibitor GW5074 [10 μM; inhibitor concentration was adopted from our previous study (Varga et al. 2003a)] at 37 °C. After pretreatment, the cells were incubated (24 h) with saturating concentration of morphine (1 μM) in the presence (Mor+GW group or Mor+H-89 group) or absence (Mor group) of the appropriate kinase inhibitor. To test the involvement of opioid receptors, DRG cells were treated with the nonselective opioid receptor antagonist naltrexone (10 μM) in the presence (Mor+NTX group) or absence (NTX group) of morphine for 24 hours. Control cells were incubated in Neurobasal A/B27 medium in the absence (Control group) or presence (GW or H-89 group) of the kinase inhibitors.
2.3. Measurement of basal CGRP release
CGRP release from the pretreated samples was assayed in duplicate, using an enzyme immunoassay (EIA) kit (Cayman Chemicals, Ann Arbor, MI). Briefly, after drug pretreatment, the cells were gently washed twice with Phenol Red-free Neurobasal A medium (Invitrogen, San Diego, CA) followed by a 10 min incubation in fresh Phenol Red-free Neurobasal A medium at 37°C in a CO2 incubator. The medium was collected, and released CGRP was measured according to the manufacturer's instructions, by measuring the absorbance of the final reaction product at λ=412 nm. Standard CGRP dose-absorbance curves were measured in parallel with each assay.
2.4. Forskolin stimulated cAMP formation
Intracellular cAMP formation was determined as described by Rubenzik et al. (2001). Briefly, after drug treatments, the DRG neurons were washed three times with Neurobasal A medium. Then a phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine ((IBMX); 4 mM; Sigma, St. Louis, MO) was added to each well. cAMP formation was stimulated with a water-soluble forskolin analogue [7-deacetyl-7-(O-N-methylpiperazino)-γ-butyryl forskolin × 2HCl, Calbiochem, San Diego, CA] (1 μM) for 20 min at 37°C. Cells were lysed and 50 μl supernatant of the cell lysates were incubated with [3H]cAMP (4 nM; Perkin Elmer, Boston, MA) and protein kinase A (30 μg/ml; Sigma, St. Louis, MO) at 4°C for 2h. cAMP standards were run in parallel with each assay. After incubation, activated charcoal (26 mg/ml; NORIT, Amersfoort, The Netherlands) was added to adsorb free [3H]cAMP. Radioactivity was counted in EcoLite scintillation fluid (ICN Pharmaceuticals, Costa Mesa, CA) using Beckman LS6000SC scintillation counter.
2.5. Data analysis
One sample t tests were used to compare normalized mean values from each treatment group to the control group (100%). One-way ANOVA tests, followed with Newman-Keuls Multiple Comparison Tests, were subsequently performed to evaluate statistical differences between treatment groups. Unpaired t tests were used to evaluate the statistical difference between control and morphine treated groups in the CGRP release experiments. Statistical evaluations were performed using GraphPad Prism 4.0 software.
3. Results
3.1. Sustained morphine treatment leads to AC superactivation in cultured neonatal rat DRG neurons
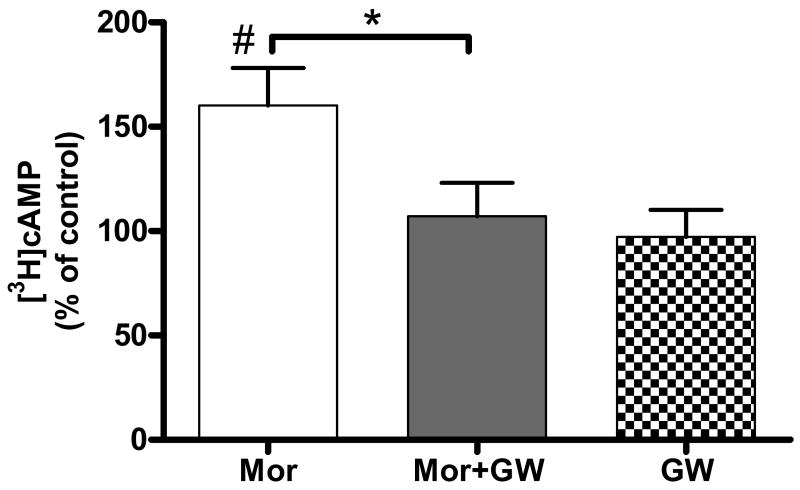
Forskolin (1 μM) -stimulated cAMP production in control neonatal rat DRG neurons was 6.2 ± 1.4 pmol/50μl sample (n=7). Sustained (24 h) treatment of the cells with morphine (1 μM) augmented forskolin-stimulated cAMP formation to 9.8 ± 2.5 pmol/50μl sample, that is 160 ± 18% of the control group (P < 0.05, n=7; Fig. 1).
Fig. 1.
Sustained morphine treatment augments forskolin-stimulated cAMP formation in cultured neonatal rat DRG neurons in a Raf-1-dependent manner. Treatment of cultured neonatal rat DRG neurons with 1 μM morphine (Mor) for 24 h augmented forskolin (1 μM)-stimulated cAMP formation to 160 ± 18% of control (#P < 0.05, one-sample t test, n=7). Pretreatment (1 h) of the cells with the selective Raf-1 inhibitor, GW5074 (10 μM), followed by a 24 h co-incubation with 1 μM morphine (Mor+GW) dramatically attenuated sustained morphine-mediated adenylyl cyclase superactivation to 107 ± 16% of the control (*P < 0.05, one way ANOVA, n=7). Treatment of DRG neurons with 10 μM GW alone caused no statistical difference in forskolin-stimulated cAMP formation relative to the medium-treated control (GW, 97 ± 13% of control, P > 0.05; one-sample t test, Fig. 1). The results (mean ± SEM) are expressed as the percentage of cAMP formation in control DRG neurons.
3.2. The selective Raf-1 inhibitor, GW5074 attenuates sustained morphine-mediated AC superactivation in cultured neonatal rat DRG neurons
As shown in Fig. 1, pretreatment (1 h) of the neonatal rat DRG neurons with the selective Raf-1 inhibitor, GW5074 (10 μM) attenuated sustained (24h) morphine-mediated augmentation of forskolin-stimulated cAMP formation by 88% (7.1 ± 2.0 pmol/50μl, p < 0.05 relative to morphine treated group, n=7). Importantly, forskolin-stimulated cAMP formation was not significantly different between control and GW5074 pretreated DRG cells (5.9 ± 1.5 pmol/50μl, 97 ± 13% of the control, P > 0.05; Fig. 1), indicating that the kinase inhibitor in itself had no effect on the activity of adenylyl cyclase enzymes. In addition, radioligand binding assays have demonstrated (X. Yue, unpublished observations) that GW5074 does not compete for [3H]DAMGO binding sites in recombinant Chinese Hamster Ovary cells expressing the human μ-opioid receptor, indicating that GW 5074 does not act by antagonizing acute opioid signaling.
3.3. Sustained morphine treatment augments basal CGRP release from cultured neonatal rat DRG neurons
Interestingly, sustained (24 h) morphine (1 μM) treatment also significantly augmented basal CGRP release from neonatal rat DRG neurons. Basal CGRP release in control DRG neurons was 641 ± 38 pg/ml. Sustained morphine (1 μM) treatment augmented basal CGRP release to 905 ± 52 pg/ml, which is 141% of the control. (P < 0.001, n=13, unpaired t test. Data are presented as mean ± SEM).
3.4. A selective PKA inhibitor (H-89) attenuates sustained morphine-mediated augmentation of basal CGRP release from cultured neonatal rat DRG neurons
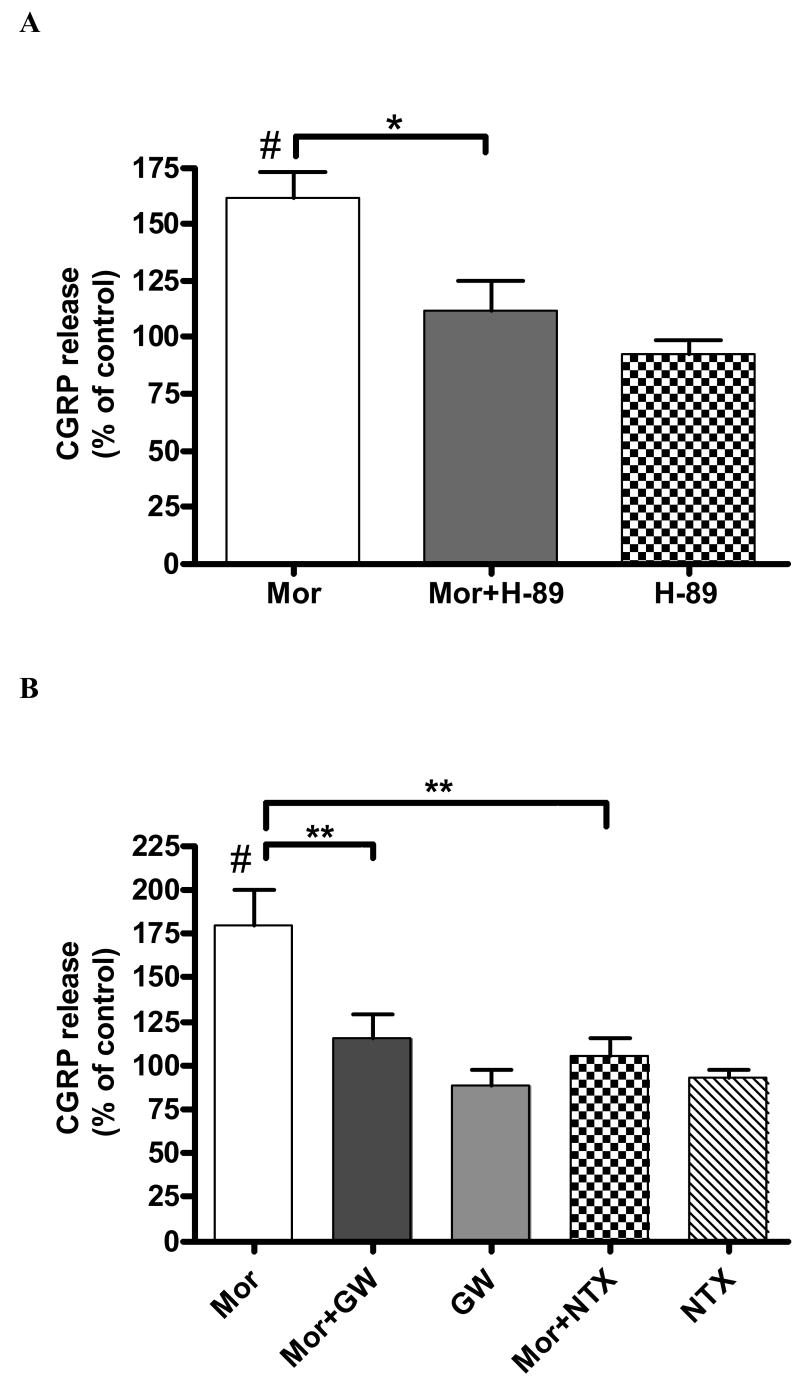
In order to examine the role of cAMP-dependent protein kinase (PKA) in sustained morphine mediated augmentation of basal CGRP release, we pre-incubated (1 h) cultured neonatal rat DRG neurons with a selective PKA inhibitor H-89 (1 μM). Pretreatment (1 h) of the cells with 1 μM H-89 followed by 24 h co-incubation with 1 μM morphine attenuated basal CGRP release to 111 ± 9% of control group. This is an 80% reduction relative to the group treated with morphine alone (162±13% relative to the control) (P < 0.05, n=3; Fig. 2A). Importantly, as shown in Fig. 2A, pre-treatment with H-89 alone had no effect on basal CGRP release (P > 0.05, n=3) from neonatal rat DRG neurons, indicating that the effect of the kinase inhibitor is not due to cytotoxicity.
Fig. 2.
The selective PKA inhibitor, H-89 (A) and the selective Raf-1 inhibitor, GW5074 (B) attenuate sustained morphine-mediated augmentation of basal CGRP release from neonatal rat DRG neurons. 24 h incubation of cultured DRG neurons with 1μM morphine (Mor) augmented basal CGRP release to 162 ± 13% (A) and 179 ± 24% (B) relative to control (#P < 0.05 in both experiments, one-sample t test; n=3 and n=4, respectively). A. Pretreatment (1 h) of the cells with 1 μM H-89, followed by 24 h co-incubation in the presence of 1 μM morphine (Mor+H-89) attenuated morphine-mediated CGRP release to 111 ± 9% of the control (*P < 0.05, one way ANOVA, n=3). Treatment of the DRG neurons with 1 μM H-89 alone (H-89) caused no difference in basal CGRP release relative to control (92 ± 9% relative to control, P > 0.05; one-sample t test, n=3). B. Pretreatment (1 h) of the cells with 10 μM GW5074, followed by 24 h co-incubation with 1 μM morphine (Mor+GW) attenuated sustained morphine-mediated CGRP release to 115 ± 14% of the control (**P < 0.01 compared with Mor group, one way ANOVA, n=3). Sustained morphine-mediated CGRP release was completely prevented by co-incubation with the opioid antagonist, naltrexone (100 ± 12% relative to control; Mor+NTX; **P < 0.01, compared with Mor group; one way ANOVA, n=3). Treatment of the DRG neurons with GW 5074 (GW) or naltrexone (NTX) alone caused no difference in basal CGRP release relative to control (89 ± 11% of GW group and 93 ± 6% of NTX group relative to control, P > 0.05 for both groups; one-sample t test, n=3). The results (mean ± SEM) are expressed as the percentage of basal CGRP release from control DRG neurons.
3.5. A selective Raf-1 inhibitor (GW5074) attenuates sustained morphine-mediated augmentation of basal CGRP release from neonatal rat DRG neurons
Sustained morphine treatment augmented basal CGRP release to 179 ± 24% relative to control (P< 0.05, n = 4). On the other hand, after pretreatment (1 h) of the cells with 10 μM GW5074 followed by 24 h co-incubation with 1 μM morphine, basal CGRP release was reduced to 115 ± 14% of the control (P < 0.01 compared with Mor group, n = 3; Fig. 2B). The nonselective opioid receptor antagonist, naltrexone (10 μM) completely prevented morphine-mediated augmentation of basal CGRP release (100 ± 12% relative to control; P < 0.01 compared with Mor group, n=3; Fig. 2B), indicating that opioid receptor stimulation is necessary for the regulation of CGRP release by sustained morphine in cultured neonatal rat DRG neurons. Treatment with GW5074 (10 μM) or naltrexone (10 μM) alone had no effect on basal CGRP release from DRG cells relative to control (P > 0.05, n=3 in both groups; Fig. 2B), indicating that their effects on CGRP release are not due to cytotoxicity.
4. Discussion
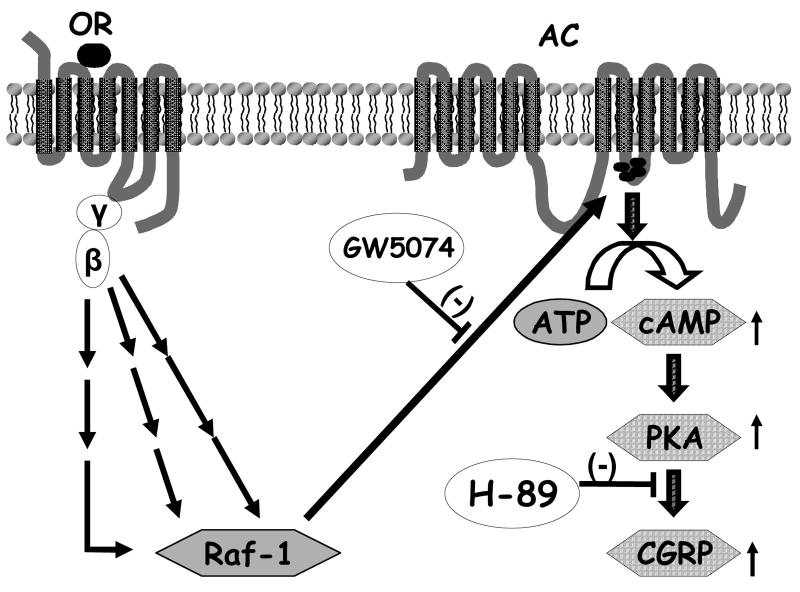
In the present study, we demonstrate that a. sustained (24 h) morphine treatment significantly augments intracellular cAMP production and basal CGRP release from cultured neonatal rat DRG neurons; b. sustained morphine-mediated augmentation of basal CGRP release from cultured DRG neurons is sensitive to the selective PKA inhibitor, H-89; and that c. both sustained morphine-mediated AC superactivation and augmentation of basal CGRP release are attenuated by the selective Raf-1 inhibitor, GW5074 in cultured primary sensory neurons. In summary, we suggest the putative molecular mechanisms shown in Fig. 3, to interpret our data.
Fig. 3.
Putative role of Raf-1-mediated AC superactivation in the regulation of CGRP release from DRG neurons by sustained morphine treatment. Sustained stimulation of opioid receptor (OR) by morphine in DRG neurons liberates Gi/o protein βγ-subunits. Free βγ-subunits activate multiple intracellular effectors that converge at activation of Raf-1 (Varga et al., 2003a), leading to phosphorylation and sensitization of adenylyl cyclase (AC). Our present data indicate that such upregulation of the cAMP signal transduction cascade may regulate CGRP release by activation of cAMP-dependent protein kinase (PKA) in cultured sensory neurons.
Long-term morphine treatment leads to multiple intracellular compensatory adaptations and neuroplastic changes. Thus, sustained morphine treatment was shown to enhance the content and release of excitatory neurotransmitters, including the pronociceptive neurotransmitter CGRP in the dorsal horn of the spinal cord (Menard 1995a, 1995b; Ma et al. 2000; Gardell et al. 2002; Trang et al. 2005). Indeed, supporting the above observation, in the present work we demonstrate that 24 h morphine treatment significantly augments basal CGRP release from cultured neonatal rat primary sensory neurons (141% of control).
The increase in the spinal CGRP content observed following sustained morphine treatment was shown to coincide with a decline in antinociception and a decrease in morphine potency, as reflected by an increase in its ED50 value (Powell et al., 2000). Furthermore, the development of tolerance to spinally infused morphine could be attenuated by co-administration of competitive CGRP receptor antagonists, suggesting that activation of CGRP receptors in the dorsal horn contributes to the induction of opioid analgesic tolerance (Menard et al., 1996; Powell et al., 2000, 2003). However, the underlying mechanisms that lead to the augmentation of spinal CGRP expression and release in response to sustained opioid exposure are still to be explored.
Activation of the cAMP transduction pathway is known to modulate presynaptic neurotransmitter synthesis and release (Carruthers et al., 2001, Supowit et al., 1995, Leenders and Sheng, 2005). Thus, it was shown earlier that treatment with a non-hydrolyzable cAMP analogue (dibutyryl cAMP) upregulates CGRP mRNA and peptide concentrations in cultured rat DRG neurons (Supowit et al., 1995). In addition, a direct AC activator, forskolin, was reported to stimulate CGRP release from trigeminal sensory neurons. Forskolin-stimulated CGRP release from the sensory neurons was blocked by a selective PKA inhibitor myr-PKI14-22 (Carruthers et al., 2001), suggesting a crucial role for cAMP-dependent protein kinase (PKA) in the regulation of CGRP release from cultured sensory neurons. On the other hand, it was found earlier that sustained opioid agonist treatment upregulates the cAMP signal transduction cascade (AC superactivation) in primary sensory DRG neurons (Crain et al., 1998; Terwilliger et al., 1991). In the present study we tested the role of sustained morphine-mediated AC superactivation in the augmentation of spinal CGRP release from primary sensory neurons. We demonstrate, for the first time, that sustained morphine treatment augments basal CGRP release from neonatal rat DRG neurons in a PKA-dependent manner, indicating that sustained morphine-mediated upregulation of the cAMP signal transduction cascade may regulate basal CGRP release by the activation of cAMP-dependent protein kinase.
Previously we have investigated the molecular mechanisms of AC superactivation in recombinant Chinese Hamster Ovary (CHO) cell lines expressing human δ- or μ-opioid receptors. We found that sustained opioid agonist treatment leads to phosphorylation of an adenylyl cyclase isoenzyme (AC VI) in CHO cells expressing human δ-opioid receptor (Varga et al, 1999). Subsequently, we have demonstrated that multiple parallel signal transduction pathways converge at Raf-1 to mediate AC superactivation in the same CHO cells line (Varga et al., 2003a, 2003b). Similar to our data, Beazely et al. (2005a) have found recently that multiple signaling pathways merge at Raf-1 to potentiate forskolin-stimulated cyclic AMP accumulation in recombinant human embryonic kidney (HEK) cells overexpressing the AC VI isoenzyme. The same authors (Beazely et al., 2005b) have also shown that in HEK cells overexpressing the AC VI isoenzyme and the D2L dopamine receptor, GW 5074 attenuated sustained quinpyrole-mediated AC superactivation in response to PKC activators. Finally, Beazely et al. (2005b) also found that multiple pathways (non- conventional PKC isoforms and tyrosine kinases) are involved in sustained quinpyrole-induced sensitization of AC VI in these cells. Since Tan et al. (2001) and Ding et al. (2004) have demonstrated that Raf-1 directly phosphorylates multiple AC isoenzymes, leading to their sensitization toward stimulators, such as forskolin or activated Gs, we hypothesize that Raf-1-mediated phosphorylation and sensitization of AC isoenzymes may be a common mechanism of feedback-regulation of cellular cAMP production in response to sustained stimulation of Gi/o protein coupled receptors in mammalian cells, including primary sensory neurons.
In the present study we demonstrate that the selective Raf-1 inhibitor GW5074 dramatically reduces sustained morphine-mediated cAMP overshoot in cultured neonatal rat primary sensory neurons. Importantly, we also demonstrates that sustained morphine-mediated augmentation of basal CGRP release was significantly attenuated by Raf-1 inhibitor GW5074, indicting the critical role of Raf-1-mediated AC superactivation in sustained morphine-mediated augmentation of basal CGRP release from cultured DRG neurons. Collectively, our study demonstrates that Raf-1 mediated activation of the cAMP/PKA pathway (see Fig. 4) could be a major intracellular signal transduction pathway involved in the augmentation of pain neurotransmitter release from primary sensory neurons upon sustained opioid analgesic treatment.
Acknowledgments
This work was supported by grants from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beazely MA, Alan JK, Watts VJ. Protein kinase C and epidermal growth factor stimulation of Raf1 potentiates adenylyl cyclase type 6 activation in intact cells. Mol Pharmacol. 2005a;67:250–259. doi: 10.1124/mol.104.001370. [DOI] [PubMed] [Google Scholar]
- Beazely MA, Watts VJ. Activation of a novel PKC isoform synergistically enhances D2L dopamine receptor-mediated sensitization of adenylate cyclase type 6. Cell Signal. 2005b;17:647–653. doi: 10.1016/j.cellsig.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Carruthers AM, Sellers LA, Jenkins DW, Jarvie EM, Feniuk W, Humphrey PPA. Adenosine A1 Receptor-Mediated Inhibition of Protein Kinase A-Induced Calcitonin Gene-Related Peptide Release from Rat Trigeminal Neurons. Mol Pharmacol. 2001;59:1533–1541. doi: 10.1124/mol.59.6.1533. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Modulation of opioid analgesia, tolerance and dependence by Gs-coupled, GM1 ganglioside-regulated opioid receptor functions. Trends in Pharm Sci. 1998;19:358–365. doi: 10.1016/s0165-6147(98)01241-3. [DOI] [PubMed] [Google Scholar]
- Ding Q, Gros R, Gray ID, Taussig R, Ferguson SS, Feldman RD. Raf kinase activation of adenylyl cyclases: isoform-selective regulation. Mol Pharmacol. 2004;66:921–928. [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders AG, Sheng ZH. Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity. Pharmacol Ther. 2005;105:69–84. doi: 10.1016/j.pharmthera.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Zheng WH, Kar S, Quirion R. Morphine treatment induced calcitonin gene-related peptide and substance P increases in cultured dorsal root ganglion neurons. Neuroscience. 2000;99:529–539. doi: 10.1016/s0306-4522(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Menard DP, van Rossum D, Kar S, Jolicoeur FB, Jhamandas K, Quirion R. Tolerance to the antinociceptive properties of morphine in the rat spinal cord: alteration of calcitonin gene-related peptide-like immunostaining and receptor binding sites. J Pharmacol Exp Ther. 1995a;273:887–894. [PubMed] [Google Scholar]
- Menard DP, van Rossum D, Kar S, Quirion R. Alteration of calcitonin gene related peptide and its receptor binding sites during the development of tolerance to mu and delta opioids. Can J Physiol Pharmacol. 1995b;73:1089–1095. doi: 10.1139/y95-156. [DOI] [PubMed] [Google Scholar]
- Menard DP, van Rossum D, Kar S, St Pierre S, Sutak M, Jhamandas K, Quirion R. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci. 1996;16:2342–2351. doi: 10.1523/JNEUROSCI.16-07-02342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–324. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Ma W, Sutak M, Doods H, Quirion R, Jhamandas K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br J Pharmacol. 2000;131:875–884. doi: 10.1038/sj.bjp.0703655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KJ, Quirion R, Jhamandas K. Inhibition of neurokinin-1-substance P receptor and prostanoid activity prevents and reverses the development of morphine tolerance in vivo and the morphine-induced increase in CGRP expression in cultured dorsal root ganglion neurons. Eur J Neurosci. 2003;18:1572–1583. doi: 10.1046/j.1460-9568.2003.02887.x. [DOI] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenzik M, Varga E, Stropova D, Roeske WR, Yamamura HI. Expression of alpha-transducin in Chinese hamster ovary cells stably transfected with the human delta-opioid receptor attenuates chronic opioid agonist-induced adenylyl cyclase superactivation. Mol Pharmacol. 2001;60:1076–1082. doi: 10.1124/mol.60.5.1076. [DOI] [PubMed] [Google Scholar]
- Supowit SC, Christensen MD, Westlund KN, Hallman DM, DiPette DJ. Dexamethasone and activators of the protein kinase A and C signal transduction pathways regulate neuronal calcitonin gene-related peptide expression and release. Brain Res. 1995;686:77–86. doi: 10.1016/0006-8993(95)00461-x. [DOI] [PubMed] [Google Scholar]
- Tan CM, Kelvin DJ, Litchfield DW, Ferguson SSG, Feldman RD. Tyrosine kinase-mediated serine phosphorylation of adenylyl cyclase. Biochemistry. 2001;40:1702–1709. doi: 10.1021/bi0015818. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Trang T, Quirion R, Jhamandas K. The spinal basis of opioid tolerance and physical dependence: Involvement of calcitonin gene-related peptide, substance P, and arachidonic acid-derived metabolites. Peptides. 2005;26:1346–1355. doi: 10.1016/j.peptides.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik M, Grife V, Sugiyama M, Stropova D, Roeske WR, Yamamura HI. Involvement of Raf-1 in chronic delta-opioid receptor agonist-mediated adenylyl cyclase superactivation. Eur J Pharmacol. 2002;451:101–102. doi: 10.1016/s0014-2999(02)02220-3. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik M, Stropova D, Sugiyama M, Grife V, Hruby VJ, Rice KC, Roeske WR, Yamamura HI. Converging protein kinase pathways mediate adenylyl cyclase superactivation upon chronic delta-opioid agonist treatment. J Pharmacol Exp Ther. 2003a;30:109–115. doi: 10.1124/jpet.103.049643. [DOI] [PubMed] [Google Scholar]
- Varga EV, Stropova D, Rubenzik M, Waite S, Roeske WR, Yamamura HI. Phosphorylation of adenylyl cyclase VI upon chronic delta-opioid receptor stimulation. Eur J Pharmacol. 1999;364:R1–3. doi: 10.1016/s0014-2999(98)00847-4. [DOI] [PubMed] [Google Scholar]
- Varga EV, Yamamura HI, Rubenzik MK, Stropova D, Navratilova E, Roeske WR. Molecular mechanisms of excitatory signaling upon chronic opioid agonist treatment. Life Sci. 2003b;74:299–311. doi: 10.1016/j.lfs.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Yue X, Varga EV, Stropova D, Vanderah TW, Yamamura HI, Roeske WR. Chronic morphine-mediated adenylyl cyclase superactivation is attenuated by the Raf-1 inhibitor, GW5074. Eur J Pharmacol. 2006;540:57–59. doi: 10.1016/j.ejphar.2006.04.033. [DOI] [PubMed] [Google Scholar]