Abstract
Objective
To examine incidence rates and antecedents of MCI and AD among diverse elders without dementia at the initial visit. To examine the characteristics of elders with MCI who reverted to normal on follow-up.
Methods
2364 Caribbean Hispanics, African Americans, or non-Hispanic Whites, age 65 or older, free of dementia at initial evaluation and followed every 18 to 24 months. Incidence rate of MCI and AD was determined by examination of neurological, medical, psychiatric, and neuropsychological function.
Results
Over 10,517 personyears, 21% of normal elders progressed to MCI (annual incidence rate = 5.1%, 95% CI = 4.6%–5.6%). Of those with MCI initially, 21.8% were subsequently diagnosed with AD (annual incidence rate = 5.4%; 95% CI = 4.7%–6.3%), 47% remained unchanged, and 31% reverted to normal. Those with MCI were 2.8 times more likely to develop AD than normal elders. MCI with impairment in memory and at least one other cognitive domain was associated with highest risk of progression to AD and was also least likely to revert to normal at follow-up. Consistent diagnosis of MCI, or incident probable or possible AD was 60% sensitive and 94% specific for the pathological diagnosis of AD.
Interpretation
Impaired memory and language were useful predictors of transition to AD. Reversion to normal from MCI was frequent, but those with impairment in more than one cognitive domain were more likely to progress or remain impaired than those with single domain impairment. Clinical diagnosis of MCI does not always predict AD neuropathology.
INTRODUCTION
The term mild cognitive impairment (MCI) describes the transitional state between normal aging and Alzheimer’s disease (AD) 1–3 or dementia 4. Implementation of the criteria for MCI, as well as determination of the rates of progression from MCI to AD or dementia, has been an area of great interest, in part because identifying the earliest signs of dementia will be crucial for interventions to prevent or slow progression of decline in AD and for research on AD and other dementias5.
The prevalence of MCI, as well as progression rates to AD or dementia vary depending on multiple factors such as implementation of MCI criteria, recruitment source, age at the initial assessment, and length of follow-up. Annual progression from MCI to dementia ranges from 12 to 17% 1,3,6 in clinic-based studies, whereas lower progression rates have been observed in population- or community-based studies (4 to 15%7–13 per year). Furthermore, in population-based studies, there appears to be more frequent occurrence of “reversion to normal” among elderly with MCI than in clinical cohorts, ranging from 14% to 40% 8,9,11,13,14.
In addition to the type of cohort, the other key methodological factors differing across studies of MCI include 1) whether MCI diagnoses are assigned on a case-by-case basis in a consensus conference of expert clinicians or assigned purely objectively using neuropsychological, functional, and medical data; 2) whether the diagnoses were made based on data collected before formal criteria for MCI were published, and thus may not be entirely suitable for the application of Peterson criteria for MCI 2) the extent to which non-demented elders with memory deficits are distinguished from those with cognitive deficits in non-memory domains; 3) the extent to which those with isolated deficits in one cognitive domain are distinguished from those with impairment in multiple cognitive domains; 4) the test score “cutoff” (and thus the extent of impairment) used to define cognitive impairment; 5) the use of norms that adjust for age and other background factors such as years of school, sex, and race/ethnicity; 6) the extent to which subjective memory complaints are considered as a requirement for the diagnosis of MCI; 7) whether intact functional capacity was required for a diagnosis of MCI; and finally, 8) whether follow-up diagnosis is made with the knowledge of prior diagnostic status.
Few longitudinal studies of MCI have been conducted among elderly from diverse racial or ethnic groups 14, or from those from other linguistic, or educational 8 backgrounds. Progression rates to AD may differ among ethnically and educationally diverse elders with MCI because the cognitive tests used to classify “objective” cognitive impairment have poor specificity in these groups15,16. Furthermore, compared to non-Hispanic Whites, African Americans and Hispanics are more likely to have hypertension and diabetes 17,18, and the impact of these conditions on the incidence rates of MCI and on the progression to dementia from MCI is unknown.
In this study we determined the incidence rates of MCI within a large population-based cohort of ethnically, linguistically, and educationally diverse elders without dementia or cognitive impairment at the beginning of the study. We also compared the incidence rates of AD and dementia among elders with or without MCI at baseline and compared rates of progression across MCI subtypes and determined the antecedents for progression. We also examined the proportion and characteristics of elderly with MCI who reverted to normal on follow-up and compared these data to individuals who remained MCI or progressed to AD at follow-up. Among a small subsample of elders who died and donated brain tissue, we performed a preliminary exploration of the relationship of MCI classification to presence of AD neuropathology and final neuropathological diagnoses.
METHOD
The Columbia University Institutional Review Board approved this project. All individuals discussed the study with a trained research assistant and provided written informed consent before their baseline visit.
Sampling Plan and Participants
Participants were Medicare recipients age 65 or older residing in three contiguous census tracts in Northern Manhattan, New York, in the neighborhoods of Washington/Hamilton Heights and Inwood who were asked to participate in a longitudinal study of aging, cognitive function, and dementia. The population from which participants were drawn was comprised of individuals from several countries of origin representing three broadly defined ethnic categories (i.e., Caribbean Hispanic, African American, and non-Hispanic White of European ancestry). Participants were excluded if they did not speak English or Spanish. The study combined longitudinal data from two recruitment efforts in this community, one beginning in1992 and the other in 1999. The sampling strategies and recruitment outcomes of these two cohorts are detailed in prior publications 19–21. Re-evaluations occurred during follow-up waves that were spaced approximately 18 to 30 months apart. Beginning in 2002, participants in both cohorts was asked whether they were interested in brain donation.
As shown in Figure 1, combining the 1992 and 1999 cohorts resulted in a group of 4308 potential participants for this study. Data was used from only those participants who had sufficient neuropsychological, functional, medical and neurological information to determine the presence or absence of MCI using published criteria 19. We found that 3830 (89%) participants had sufficient data. We computed the incidence rates for MCI and dementia in a sample excluding 615 individuals with prevalent dementia at the initial visit and 785 elders without longitudinal data. Among the 785 without longitudinal data, 38% refused further visits, 24% died, 20% could not be located, 11% moved out of the area, 6% could not be scheduled for a visit, and 1% were lost for some other reason. Therefore, the “overall” rate of follow-up in this sample was 76% including deaths. The follow-up rate among people who were alive at the time of follow-up was 81%. For the current analyses, we further excluded 66 (2.7%) of the 2430 participants who were non-demented at first visit and were seen for at least one follow-up but had insufficient data required for a diagnosis of dementia or MCI at any follow-up visit. Participants in the current study (n = 2364) had an average age of 75.8 years (SD = 6.4) and an average years of school of 10.0 years (SD = 4.8). They were significantly younger and better educated than those excluded because of missing data or lack of follow-up (n = 1329; 36%), whose average age was 76.6 years (SD = 6.8) and an average years of school of 9.2 years (SD = 4.4). The groups did not differ with respect to racial composition, but there were significantly more women (68.6%) in the final sample than among those who were excluded (64.9%). Using a summary measure of medical burden, we found that those who were excluded did not have more medical illnesses than the final sample. As compared to those in the final sample, elders without follow-up were significantly more likely to score less than 1.5 standard deviations below the demographically matched normative sample on neuropsychological composite scores assessing memory (25% vs. 17%), executive function (21.5% vs. 13%), visuospatial function (21.5% vs., 17.4%) and language function (23.1% vs. 17.5%). The average time between follow-up visits in the final sample was 24.3 months (SD = 6.4). Of the sample of 2364 elders, 823 were re-evaluated once, 901 were re-evaluated twice, 192 received three re-evaluations, 174 were re-evaluated four times, 165 were re-evaluated six times, 89 were re-evaluated seven times, 9 were re-evaluated eight times, and 11 were re-evaluated nine times.
Figure 1.
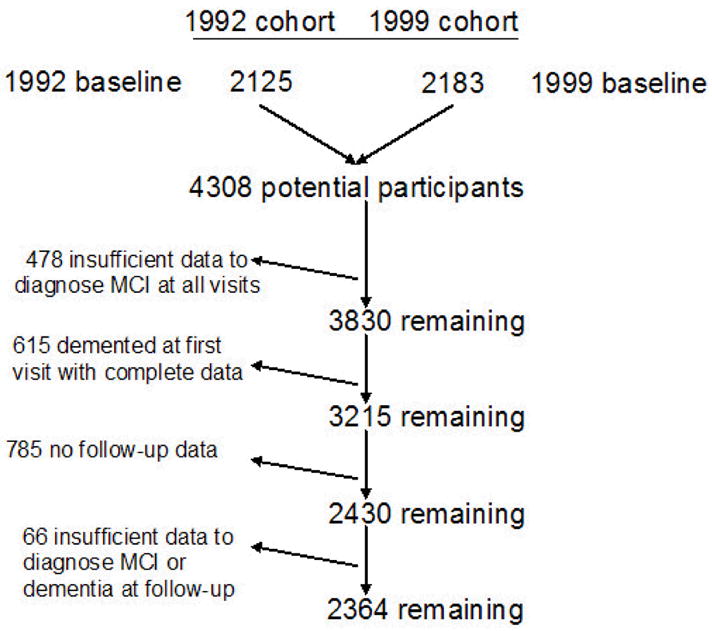
Derivation of the sample for the current study from two community-based samples recruited in 1992 and 1999.
Of the 2364 participants in the study, 388 (16.4%) died at follow-up and an autopsy was obtained in 27 (7%) of those who died. As compared to the full sample of 2337 participants who were not autopsied, the autopsied participants were significantly older at the initial assessment (76.1 ± 6.2 vs. 79.3 ± 6.8; t(1, 2362) = 2.7, p =.008) and had more years of school (12.5 ± 4.7 vs. 9.9 ± 4.8; t(1, 2362) = 2.7, p =.007) but did not differ with respect to ethnic composition, sex distribution, or proportion of elders who met criteria for MCI.
Assessment Procedures
Racial/ethnic group
Ethnic group was determined by self-report using the format of the 2000 US Census 22. All individuals were first asked to report their race (i.e., American Indian/Alaska Native, Asian, Native Hawaiian or other Pacific Islander, Black or African American, or White), then, in a second question, were asked whether they were Hispanic.
Medical and neurological evaluation
At the initial visit and each follow-up, a physician recorded medical history and medications in a semi-structured format. Neurological and physical examinations were performed, including assessment of extrapyramidal signs and functional status.
Psychiatric status
Presence of current depression was determined by asking nine questions that correspond with DSM-IV23 criteria for major depressive episode. Current depressive symptoms were assessed using ten items from the Center for Epidemiological Studies-Depression Scale 24,25. Presence of past major depressive episodes, as well as current or past anxiety disorders, psychosis, hallucinations, delusions, psychiatric hospitalizations, psychiatric medications, and alcohol or drug dependence was also assessed. Elders with depression or other psychiatric disorders were not excluded from the study.
Assessment of activities of daily living
Items from a Disability and Functional Limitations Scale 26,27 were used to elicit self or observer ratings of instrumental activities of daily living, such as using the telephone, preparing meals, handling money, and completing chores. This instrument has the flexibility to be completed using information from the participant or a collateral, who is a family member, friend, or other person identified by the participant. A summary measure was created, compiling complaints from 6 domains (using the phone, cooking, shopping, handling finances, making change for purchases, and correctly taking medications). Based on a cutoff capturing 95% of the normative sample, participants were considered to be functionally intact if they or their caregivers reported difficulty on fewer than three of these items.
Memory complaints
Perceived difficulty with memory was assessed with eleven items from the Disability and Functional Limitations Scale, described above, and the Blessed Functional Activities Scale 28. Participants were asked whether they had memory difficulties in general, as well as difficulties in specific areas such as memory for names. Participants were considered to have memory complaints if they indicated that they had problems on one or more of the items.
Neuropsychological battery
The neuropsychological measures used were selected to assess cognitive functions that are typically affected in dementia and have been shown to effectively distinguish between normal aging and dementia in this community 29. The evaluation included measures of learning and memory, orientation, abstract reasoning, language, and visuospatial ability. Specific ability areas and tests administered include verbal list learning and memory (Selective Reminding Test [SRT] 30), nonverbal memory (multiple choice version of the Benton Visual Retention Test [BVRT] 31), orientation (items from the Mini Mental State Examination [MMSE] 32), verbal reasoning (Similarities subtest of the Wechsler Adult Intelligence Scale - Revised [WAIS-R] 33) nonverbal reasoning (Identities and Oddities subtest of the Mattis Dementia Rating Scale 34), naming (15-item version of the Boston Naming Test 35), letter fluency (Controlled Word Association 36), category fluency (animals, food, and clothing, using procedures from the Boston Diagnostic Aphasia Examination [BDAE] 37), repetition (high-frequency phrases of the BDAE 37), auditory comprehension (first six items of the Complex Ideational Material subtest of the BDAE 37), visuoconstruction (Rosen Drawing Test 38), and visuoperceptual skills (multiple choice matching of figures from the BVRT 31). Norms for these tests in this population were developed based on age, years of school, sex, and ethnicity and were previously described 19.
Consensus Diagnosis
After each clinical assessment, a group of physicians and neuropsychologists reviewed the functional, medical, neurological, psychiatric, and neuropsychological data and reached a consensus regarding the presence or absence of dementia using DSM-III-R criteria 39. For follow-up evaluations, this group was shielded from the prior consensus diagnoses. If dementia was diagnosed, the etiology was determined using published research criteria for probable and possible AD 40, vascular dementia 41, Lewy Body Dementia 42, and other dementias. Severity of dementia was rated using the Clinical Dementia Rating Scale (CDR) 43. Only those who were not diagnosed with dementia were considered for a diagnosis of MCI.
MCI Diagnostic criteria
MCI criteria were retrospectively applied among nondemented individuals after the consensus conference for each visit. Consistent with standard criteria 2, for all subtypes of MCI, those considered for MCI were required to have: 1) a memory complaint (defined above) 2) objective impairment in at least one cognitive domain based on the average of the scores on the neuropsychological measures within that domain and a 1.5 SD cutoff using corrections for age, years of education, ethnicity, and sex and based on the previously established norms, 3) essentially preserved activities of daily living (defined above), and 4) no diagnosis of dementia at the consensus conference. A fifth criterion for amnestic MCI is “preserved general cognitive function”. For the MCI subtypes with isolated impairment in one cognitive domain, this criterion was met if neuropsychological test scores in other cognitive domains were not impaired. In other words, cognitive criteria for MCI–amnestic was met if elders were not impaired on the composite scores for visuospatial, language, and executive function.
In order to cast the widest net to determine prevalence of MCI and to determine which individuals were more likely to progress to dementia, the original Petersen criteria1,2, which focus on memory impairment, were expanded to include mutually exclusive subtypes based on cognitive features. Our first subtype, MCI–amnestic (MCI-A), corresponded most closely to the original definition used by Petersen and colleagues 1,2,. Memory impairment was defined as a score < 1.5 SD below demographically corrected mean on an average composite measure comprising the following learning and memory measures: 1) total recall from the SRT 2) delayed free recall from the SRT, and 3) recognition from the BVRT. Performance on composite scores from all other cognitive domains (i.e., executive, language, and visuospatial) was required to be within normal limits (score had to be greater than or equal to 1.5 SD below the demographically corrected mean). Other MCI subtypes were classified allowing for impairment in a single non-memory domain if performance on composite scores from all other cognitive domains was within norms. MCI- Executive Function (MCI-E) was assigned if impairment was demonstrated on an average composite measure comprising the following measures: 1) Letter Fluency; 2) Category Fluency, and 3) the WAIS-R Similarities subtest. MCI- Language (MCI-L) was defined as isolated impairment on an average composite measure comprising: 1) Boston Naming Test; 2) BDAE Repetition, and the 3) BDAE Comprehension test. MCI- Visuospatial (MCI-V) was assigned if impairment was demonstrated on an average composite score comprising: 1) Rosen Drawing and 2) BVRT matching. As described in prior studies of MCI 9,54,55 cognition could also be impaired in multiple cognitive domains. MCI - Multiple Cognitive Domains with memory impairment (MCI-MCDM) was diagnosed if there was objective impairment on the memory domain composite score and if there was impairment on at least one other cognitive domain. MCI - Multiple Cognitive Domains without memory impairment (MCI-MCDN) was assigned if there was impairment in two or more of the three non-memory domains, and if the memory domain composite score was within norms. Again, classification into the six subtypes was mutually exclusive.
Neuropathology
The brains were harvested as soon as possible after death, weighed fresh, and processed according to an upgraded version of a published protocol. 44. Briefly, each brain was divided following a sagittal cut through the corpus callosum. One half was extensively dissected and blocks were frozen fresh for further investigations. The other half was immersed in buffered, 10 percent formalin solution and processed for thorough neuropathological evaluation. For microscopical examination, at least eighteen, standardized representative blocks were obtained; additional blocks were selected as per the findings on gross examination or documented symptoms. Seven-micrometer thick paraffin sections from all blocks were stained with Luxol-fast-blue and counterstained with hematoxylin and eosin for general survey. Selected sections were stained with Bielschowsky for evaluation of axons, neuritic plaques, and neurofibrillary and glial tangles; and antibodies against β-amyloid for vascular and parenchymal deposits; phosphorylated tau (AT8) for neuronal and glial tangles; ubiquitin for ubiquitinated cytoplasmic, nuclear or axonal aggregates; α-synuclein for Lewy bodies, Lewy neurites, and glial tangles; or other antibodies as indicated by findings, or history. The mean number of neuritic plaques in five random, 100 x fields per slide was recorded using Bielschowsky-stained slides or β-amyloid labeled sections from seven blocks.
A diagnosis of Alzheimer disease was assigned according to the criteria of The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) 45. The likelihood that dementia was due to the Alzheimer changes (neuronal loss, presence of neurofibrillary tangles of Alzheimer and of neuritic plaques) was assessed according to the criteria proposed by ‘The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathologic Assessment of Alzheimer’s Disease’ 46. Furthermore, a Braak and Braak stage was assigned to reflect the extent of involvement of the neurofibrillary tangles of Alzheimer 47. A brain was assigned to the category of Alzheimer disease Lewy body variant (ADLBV), if there was documented dementia, neuronal loss with neuritic plaques, and neurofibrillary tangles that occurred in numbers of diagnostic significance for AD; and with cortical and subcortical Lewy bodies. The subcortical areas with Lewy bodies included substantia innominata, amygdala, hypothalamus, substantia nigra pars compacta, and nucleus coeruleus. Other causes of dementia were diagnosed according to standard neuropathologic criteria available at the time of autopsy.
Data Analyses
Demographic and follow-up characteristics of participants who were classified as having MCI were compared to those without MCI using t-tests and chi-square analyses. Among those without MCI at baseline, age-specific incidence rates of MCI were calculated within four age groups (65–69, 70–74, 75–79, 80+ years), and 95% CIs about these rates (assuming a Poisson distribution), were calculated separately for the entire population, for men and women, by ethnic group, and by years of education, split at the median (0–11 years versus 12+ years). A Cox proportional hazards model was performed to examine multiple predictors of time to first MCI diagnosis; these predictors were demographic (age, years of education, race/ethnicity, sex), genetic (presence of at least one APOE-ε4 allele), membership in the 1992 or 1999 cohort, and baseline medical/psychiatric (history of stroke, hypertension, diabetes, heart disease, or psychiatric illness)variables.. In these analyses, time to first MCI diagnosis represents the date of progression from normal status to MCI and was calculated as the number of days between the initial neuropsychological evaluation and the neuropsychological evaluation in which MCI was first diagnosed. For those who did not develop MCI, time was calculated as the number of days between the initial neuropsychological evaluation and the last neuropsychological evaluation. Age-specific incidence rates of AD were also calculated among those with and without MCI at baseline. Another Cox proportional hazards model was performed, this time predicting time to AD diagnosis. In this model, the demographic, genetic, and medical predictors were added as a first set, and the additional contribution of MCI status at baseline was tested by including these variables as a second set of predictors. To determine the individual antecedents of progression to AD, we again used Cox proportional hazards model to predict time to first diagnosis of AD, with demographic (age, education, sex, race/ethnicity, 1992/1999 cohort) and medical/psychiatric (history of hypertension, diabetes, heart disease, stroke, or psychiatric illness) variables entered as a first step, and then allowing individual components of the MCI classification at the initial visit to enter the model using a forward stepwise procedure. We performed univariate ANOVAs (examining any significant differences using Tukey’s post-hoc tests) and chi-square analyses to compare the characteristics of participants with MCI at baseline who reverted to normal at follow-up versus those who remained classified as MCI or progressed to AD. We also calculated the sensitivity, specificity, and accuracy of MCI or AD using the postmortem diagnosis as the gold standard. The pathological diagnosis of Alzheimer’s disease was made if the neuropathologist found sufficient AD changes in the brain 46,47. In some cases, Alzheimer’s changes were present but were infrequent and did not cross the standard threshold for neuropathological diagnosis of AD. We evaluated the sensitivity and specificity of three clinical states for neuropathological diagnosis of AD: 1) diagnosis of prevalent MCI or incident MCI or incident AD without reversion to normal at follow-up; 2) prevalent or incident impairment in any neuropsychological domain (regardless of AD or MCI diagnosis) without reversion to normal at follow-up; and 3) prevalent or incident neuropsychological impairment in memory (regardless of AD or MCI diagnosis) that did not revert to normal at follow-up.
Role of the funding source
The funding source had no role in study design, in the collection, analysis, and interpretation of data, in the writing of this manuscript. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Sample characteristics
The mean age of the 2364 participants was 76.1 years (SD = 6.2 years), and they had an average of 10.0 years (SD = 4.8) of education. The cohort was 28.4% non-Hispanic White, 32.6% non-Hispanic Black, and 39.0% Hispanic, and 68.6% were women. Only 8% of the Hispanics were interviewed and tested in English. Information for the Disability and Functional Limitations scale was primarily provided by the participant (97%). The functional instrument used primarily information from an informant among the remaining 3% of the sample; this small group of elders were significantly more likely to have significant functional complaints and memory complaints, and were more likely to be classified as having MCI. Informants were present (but may not have been the primary source for the Disability and Functional Limitations instrument) during the initial interview for 6.7% of the participants, and over the course of all visits, an informant was present for 18% of the participants. APOE genotype was available for 2067 (87%) of the cohort.. At the initial visit, 74.2% of the participants reported a history of hypertension, 36.3% heart disease, 23.1% diabetes, and 8.7% reported a history of stroke. An average of 2.3 follow-up evaluations was performed with a mean duration of follow-up of 4.7 years (SD = 2.8). There were no differences in the initial age of participants across the 1992 and 1999 cohorts. Compared to the 1992 cohort, education (in years) was higher in the 1999 cohort (8.6 years vs. 10.9 years; t(2362) = 11.9, p <.001). There were more Hispanics in the 1992 cohort (47.3%) than the 1999 cohort (33.4%) and fewer non-Hispanic Whites (20.3%) in the 1992 cohort as compared to the 1999 cohort (33.8%). Participants in the 1999 cohort were more likely to report stroke (9.8%) and heart disease (39.2%) than the 1992 cohort (7.1% and 32.1%, respectively), but the prevalence of hypertension and diabetes was similar. The prevalence of MCI at first visit was higher in the 1992 cohort (26.9%) than in the 1999 cohort (21.8%; χ2 (1, n = 2364) = 7.9, p =.005). The length of follow-up was twice as long on average among the 1992 cohort (6.7 years) versus the 1999 cohort (3.3 years).
Incidence Rates for MCI
Over 7504.9 person-years of follow-up, there were 379 incident MCI (Table 1). Table 2 compares the incidence rates of the 170 cases (2.3%; 95% CI = 1.9%–2.6%) in which memory was impaired (MCI-amnestic and MCI-MCDM combined) and 209 cases (2.8%; 95% CI = 2.4%–3.2%) in which memory was not impaired (MCI-executive, MCI-visuospatial, MCI-language, and MCI-MCDN combined) by age, years of school, race/ethnicity, and sex. The annual incidence rate of MCI-amnestic (1.4%) was significantly higher than that of and MCI-MCDM (0.87%; incidence rate difference = 0.53, 95% CI, 0.19–0.87%). A Cox proportional hazards model with time to first diagnosis of MCI (regardless of the subtype) as the outcome revealed that as compared to those age 65–69, those age 70–74 (RR = 1.6, 95% CI: 1.1–2.4), age 75–79 (RR = 1.9, 95% CI: 1.3–2.8), and 80 and older (RR = 2.5, 95% CI: 1.7–3.6) were at higher risk for developing MCI. As compared to non-Hispanic Whites, older adults who self-identified as Black (RR = 1.4, 95% CI: 1.0–1.8) or Hispanic (RR = 1.4, 95% CI: 1.0–1.9) were also at higher risk of developing MCI. Elders with a history of a diagnosis of hypertension were also at higher risk for developing MCI (RR = 1.4, 95% CI: 1.1–1.9). In this model, sex, education, cohort, history of heart disease, diabetes stroke, and psychiatric illness were not significant predictors of incident MCI. Among the smaller subsample of 1572 participants with APOE data and without MCI at the initial visit, older age and hypertension remained risks for incident MCI, but race/ethnicity was no longer a significant predictor of progression to MCI. Presence of the APOE-ε4 allele was not associated with higher risk of developing MCI. We also performed Cox proportional hazards models with time to first diagnosis of 1) MCI with memory impairment (MCI-amnestic and MCI-MCDM combined) and 2) MCI without memory impairment in the entire sample of people without MCI at the initial visit (n = 1800). Older age and hypertension increased risk for developing MCI with memory impairment, while heart disease was protective (RR = 0.70, 95% CI: 0.50–0.98). Fewer than 12 years of education was the only significant risk for developing incident MCI without memory impairment (RR = 1.4, 95% CI: 1.0–1.9).
Table 1.
Incident cases of MCI among non-demented elders who did not have MCI at first visit (n = 1800, 7504.9 personyears)
| MCI subtype | Number of incident MCI cases | Rate per 100 personyears | 95% CI (lower) | 95% CI (higher) |
|---|---|---|---|---|
| MCI with memory impairment | 170 | 2.3% | 1.9% | 2.6% |
| MCI - amnestic | 105 | 1.4% | 1.1% | 1.7% |
| MCD with memory | 65 | 0.9% | 0.7% | 1.1% |
| MCI without memory impairment | 209 | 2.8% | 2.4% | 3.2% |
| MCI - executive | 19 | 0.3% | 0.1% | 0.4% |
| MCI - language | 67 | 0.9% | 0.7% | 1.1% |
| MCI - visuospatial | 85 | 1.1% | 0.9% | 1.4% |
| MCD without memory | 38 | 0.5% | 0.1% | 0.7% |
| All MCI subtypes | 379 | 5.1% | 4.6% | 5.6% |
Table 2.
Rate of progression to MCI (memory and non-memory impairment types) per 100 person-years (95% CI) according to age, education, ethnicity, and gender among elders who did not have MCI at baseline (n = 1800)
| Total N | person years | # incident MCI - memory | rate per 100 personyears(95% CI) | # incident MCI–non- memory | rate per 100 personyears(95% CI) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| 65–69 | 261 | 1277.3 | 14 | 1.1% (0.5–1.7) | 22 | 1.7% (1.0–2.4) |
| 70–74 | 588 | 2588.1 | 55 | 2.1% (1.6–2.7) | 67 | 2.6% (2.0–3.2) |
| 75–79 | 478 | 1968.6 | 44 | 2.2% (1.6–2.9) | 67 | 3.4% (2.6–4.2) |
| 80+ | 473 | 1674.8 | 57 | 3.4% (2.5–4.3) | 53 | 3.2% (2.3–4.0) |
| Education | ||||||
| 0–11 | 1231 | 3741.5 | 94 | 2.5% (2.0–3.0) | 130 | 3.5% (2.9–4.1) |
| 12+ | 1133 | 3767.2 | 76 | 2.0% (1.6–2.5) | 79 | 2.1% (1.6–2.6) |
| Ethnicity | ||||||
| White | 671 | 2140.6 | 38 | 1.8% (1.2–2.3) | 41 | 1.9% (1.3–2.5) |
| African American | 771 | 2470.8 | 57 | 2.3% (1.7–2.9) | 72 | 2.9% (2.3–3.6) |
| Hispanic | 922 | 2897.3 | 75 | 2.6% (2.0–3.2) | 96 | 3.3% (2.7–4.0) |
| Sex | ||||||
| Men | 743 | 2253.6 | 53 | 2.4% (1.7–3.0) | 62 | 2.8% (2.1–3.4) |
| Women | 1621 | 5255.1 | 117 | 2.2% (1.8–2.6) | 147 | 2.8% (2.4–3.2) |
Incidence rate of AD and dementia among participants with and without MCI
Over 10517.4 personyears of follow-up, there were 309 cases of incident AD. As shown in Table 3, the incidence rates for AD differed by the presence of MCI. While 10.3% (186 participants of 1800) without MCI at their initial visit were diagnosed with AD at a follow-up visit, 21.8% (123 cases of 564) with MCI at the first visit were diagnosed with AD at follow-up. Using a Cox proportional hazards model predicting time to first diagnosis of AD among the entire sample of 2364 elders, we found that older participants with fewer than 12 years of school, African Americans and Hispanics, and those with history of diabetes or stroke were at higher risk of developing AD. With all demographic, medical, and psychiatric variables in the model, we found that those with MCI-MCDM, MCI-amnestic, MCI-language, and MCI-MCDN initially were more likely to develop AD as compared to elderly without MCI. Table 4 shows RR of developing AD among MCI subtypes as determined by a Cox proportional hazards model among the subset of 2,067 subjects with APOE genotypes. These results were essentially identical to that with the entire sample. Not shown in Table 4 is that as compared to those age 65–69, those age 70–74 (RR = 2.6, 95% CI: 1.4–4.8), age 75–79 (RR = 5.0, 95% CI: 2.7–9.3), and 80 and older (RR = 11.2, 95% CI: 6.0–20.1) were at higher risk for developing MCI. Participants with less than 12 years of school were 2.0 times (95% CI: 1.4–2.7) more likely to develop AD than those with 12 or more years of school. As compared to non-Hispanic Whites, older adults who self-identified as Black (RR = 2.3, 95% CI: 1.5–3.5) or Hispanic (RR = 2.4, 95% CI: 1.6–3.7) were at higher risk of developing AD, as were those with an APOE-ε4 allele (RR = 1.4, 95% CI: 1.0–1.7), and history of diabetes (RR = 1.5, 95% CI: 1.1–1.9) and stroke (RR = 2.3, 95% CI: 1.6–3.3). Sex, cohort, hypertension, heart disease, and psychiatric history, did not significantly influence risk of developing AD. Figure 2 demonstrates the cumulative hazard of developing AD among the MCI subtypes among participants with APOE-ε4 information. Finally, there were 18 incident cases of non-AD dementia: (5 vascular dementia, 2 Diffuse Lewy Body Disease, 2 tumor-related, 1 alcohol-related, 1 secondary to metabolic dysfunction, 1 secondary to a psychiatric syndrome, and 6 where the etiology of dementia could not be determined). There was no change in the results when time to first “all-cause” dementia diagnosis was used as the outcome in these analyses.
Table 3.
Rate of progression to AD per 100 person-years (95% CI) according to age, education, ethnicity, and gender among elders with and without MCI at baseline
| Total N | person- years | # of incident AD cases | rate per 100 person-years(95% CI) | |
|---|---|---|---|---|
| No MCI | 1800 | 8224.1 | 186 | 2.3% (1.9–2.6) |
| Age 65–69 | 261 | 1359.1 | 3 | 0.2% (0.0–0.4) |
| Age 70–74 | 588 | 2921.1 | 37 | 1.3% (0.9–1.7) |
| Age 75–79 | 478 | 2178.6 | 53 | 2.4% (1.8–3.1) |
| Age 80+ | 473 | 1765.3 | 93 | 5.3% (4.2–6.3) |
| MCI with memory impairment | 318 | 875.5 | 65 | 7.4% (5.7–9.2) |
| Age 65–69 | 26 | 126.2 | 4 | 3.2% (0.1–6.2) |
| Age 70–74 | 73 | 286.6 | 13 | 4.5% (2.1–6.9) |
| Age 75–79 | 70 | 227.9 | 22 | 9.7% (5.8–13.5) |
| Age 80+ | 77 | 234.7 | 26 | 11.1% (7.1–15.1) |
| MCI without memory impairment | 246 | 1417.9 | 58 | 4.1% (3.1–5.10) |
| Age 65–69 | 47 | 225.0 | 5 | 2.2% (0.3–4.2) |
| Age 70–74 | 91 | 417.3 | 12 | 2.9% (1.3–4.5) |
| Age 75–79 | 90 | 410.6 | 20 | 4.9% (2.8–7.0) |
| Age 80+ | 90 | 365.0 | 21 | 5.8% (3.4–8.1) |
Table 4.
Relative risk of incident AD associated with MCI status at baseline
| Diagnostic Category | Relative Risk | 95.0% CI (lower) | 95.0% CI (higher) |
|---|---|---|---|
| Not MCI | 1 | ||
| MCI-amnestic | 3.2 | 2.1 | 4.7 |
| MCI-executive | 0.7 | 0.2 | 2.8 |
| MCI-language | 2.0 | 1.1 | 3.5 |
| MCI-visuospatial | 1.2 | 0.7 | 2.0 |
| MCI-MCDM | 4.3 | 2.9 | 6.4 |
| MCI-MCDN | 1.9 | 1.1 | 3.0 |
Note: Other predictors in the Cox proportional hazards model predicting time to first diagnosis of AD were age, years of school, sex, race/ethnicity, cohort (1992 vs, 1999), presence of hypertension, diabetes, heart disease, stroke, and psychiatric history.
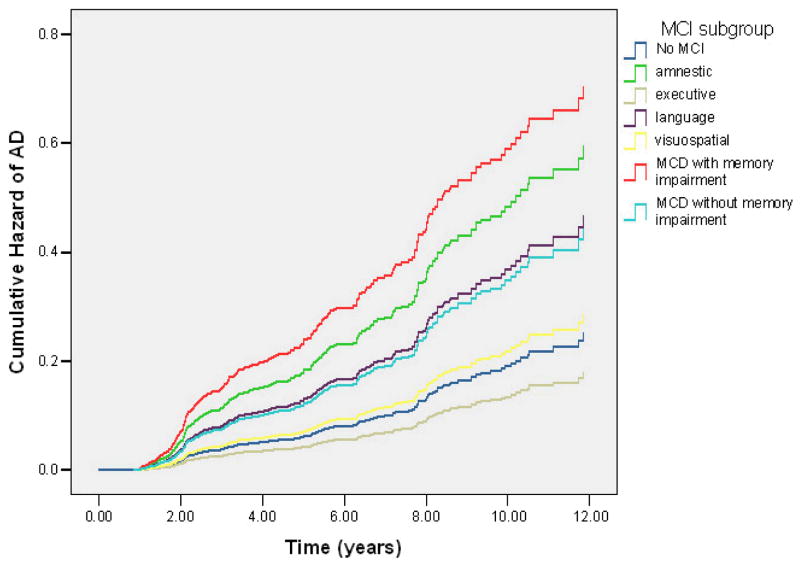
Figure 2.
Cumulative hazard of developing AD by MCI subtype among 2,364 elders without dementia at initial visit and at least one follow-up visit.
Antecedents of progression to AD from MCI
Characteristics of the initial assessment were evaluated as predictors of progression to AD. Adjusting for differences in demographics and medical factors, impairment on the memory composite score was the best predictor of progression to AD from MCI (RR = 3.00, 95% CI = 2.32–3.86), followed by isolated impairment in language (RR = 2.09, 95% CI = 1.61–2.71). Memory complaints entered the model as well, (RR = 1.66, 95% CI = 1.23–2.24), as did isolated impairment in visuospatial function (RR = 1.49, 95% CI = 1.14–1.94). These results did not change in the smaller sample when APOE-ε4 status was included in the first set of variables.
Reversal of MCI
All follow-up visits of the 564 elders with MCI at the beginning of the study were examined. We found that 30.2% (n = 170) did not have MCI or dementia at any follow-up visit. About half of those with MCI initially (n = 264; 46.8%) still had MCI at a subsequent visit and did not revert to normal or progress to dementia.
Individuals with MCI at baseline who progressed to AD were older, less well educated, more likely to be Hispanic, and reported a history of stroke as compared with both of the groups that did not progress to dementia (Table 5). Those with MCI initially who reverted to normal at follow-up did not differ in age, years of school, sex, race/ethnicity, or presence of medical conditions or an APOE-ε4 allele from elders diagnosed with MCI at follow-up. Those without MCI or dementia at any follow-up visit were followed up for less time (3.4 years, an average of 1.7 visits) compared to those with MCI (4.7 years, an average of 2.3 visits) or dementia (5.8 years, an average of 2.9 visits) at follow-up.
Table 5.
Demographic and medical characteristics of elders with MCI at baseline (n = 564) by diagnostic outcome, surveying 1) all follow-up visits and 2) only first follow-up visit
| All follow-up visits | First follow-up only | |||||
|---|---|---|---|---|---|---|
| MCI to no MCI | MCI to MCI | MCI to AD | MCI to no MCI | MCI to MCI | MCI to AD | |
| N | 170 | 264 | 123 | 255 | 230 | 72 |
| mean age | 76.3 | 75.8 | 78.3 | 76.5 | 75.8 | 79.0 |
| mean education | 9.2 | 9.8 | 6.9 | 8.9 | 9.6 | 7.0 |
| % African American | 28.2 | 32.6 | 24.4 | 28.2 | 32.2 | 25.0 |
| % Hispanic | 43.5 | 37.9 | 58.5 | 42.0 | 38.0 | 40.1 |
| % women | 68.8 | 68.2 | 66.7 | 71.0 | 63.9 | 70.8 |
| % APOE-E4 allele | 28.1 | 29.3 | 28.6 | 29.6 | 28.1 | 27.9 |
| % stroke | 8.2 | 6.8 | 12.2 | 6.7 | 7.8 | 16.7 |
| % hypertension | 70.6 | 80.3 | 78.9 | 74.5 | 79.6 | 77.8 |
| % diabetes | 20.6 | 24.6 | 27.6 | 21.6 | 23.9 | 33.3 |
| % heart disease | 31.2 | 35.6 | 41.5 | 34.9 | 33.5 | 44.4 |
| Avg. years of f/u | 3.4 | 4.7 | 5.8 | 2.2 | 2.2 | 2.2 |
| Avg. number of f/u | 1.7 | 2.3 | 2.9 | 255 | 230 | 72 |
Note: Table does not include data on 7 elders with MCI at baseline who progressed to non-AD dementia.
To determine if our results were an artifact of length of follow-up, we limited the analyses to one follow-up only. At the first follow-up of the 564 elders with MCI at first visit, 45.2% (n = 255) did not have MCI or dementia, 40.8% (n = 230) still had MCI, 12.8% (n = 72) progressed to AD, and 1.2% (n = 7) progressed to non-AD dementia. Comparisons of background and medical variables among these groups demonstrated that persons with MCI initially who progressed to AD at the next visit were older, less well educated, and more likely to report a history of stroke as compared to both of the groups who did not progress, despite having equal years of follow-up. There were no significant differences between the consistent MCI group and the MCI-to-non-MCI group. Of those with MCI who reverted to normal, 21% no longer met functional complaint criteria, 35% no longer had memory complaints, and 67% no longer met the neuropsychological criteria at follow-up. There was considerable overlap, such that 48% reverted because they failed to meet cognitive criteria only, 12% due to functional criteria only, and 17% due to memory complaint criteria only, and the remainder reverted because they failed to meet multiple criteria. The most frequent cause of reversion was no longer meeting cognitive criteria.
We sought to determine whether there were differences in those who reverted to normal within each specific MCI subtype. Surveying all available follow-up visits, elders MCI with isolated impairment in one cognitive domain only were the most likely to revert to normal at follow-up (38.0% as a group), while those with MCI with impairment in multiple cognitive domains were least likely to revert to normal (19.3%; χ2 (1, n = 557) = 22.2, p <.001). Reversion to normal among those with cognitive impairment in multiple cognitive domains including memory did not differ statistically from the proportion reverting to normal with cognitive impairment in multiple non-memory domains. There was no difference in the proportion of elders with isolated cognitive impairment in memory, executive function, visuospatial skill, and language reverting to normal at follow-up.
Validity of antemortem diagnosis
Autopsies were obtained in a subsample (n = 27) who were 67% women, had a mean age of 79.3 years (SD = 6.8) at their initial visit, and a mean education of 12.5 years (SD = 4.7). They were 41% non-Hispanic White, 37% non-Hispanic Black, and 22% Hispanic. Prevalence of hypertension was 63%, diabetes was present in 29.6%, and 2 individuals (7.4%) reported clinical stroke. The mean interval between last clinical evaluation and death was 24.8 months (SD = 21.3).
Specificity was acceptable (94%) for neuropathologic AD for those diagnosed with prevalent or incident MCI (all subtypes) or incident AD without reversion to normal status, , but sensitivity was relatively low (60%). Sensitivity was the same (60%) when the clinical marker was stable neuropsychological impairment in any cognitive domain (regardless of MCI or AD status), but specificity was poor (59%). Memory complaints had a sensitivity of 70% for AD pathology but poor specificity (35%). Presence of stable neuropsychological impairment in any cognitive domain had perfect specificity (100%) for any brain pathology (not limited to AD), but sensitivity was low (62%). Three of the 27 participants showed reversion of an MCI diagnosis to normal cognitive status; two of these individuals had no detectable abnormality in brain tissue, and one had an asymptomatic infarct. False-negative findings included individuals with combined pathology as well as AD pathology alone. False-positive findings included primarily infarcts or vascular dementia, but also individuals with lobar atrophy, Parkinson disease, or brain tissue without recognized abnormality. Among those with AD pathology, time between last clinical evaluation was almost twice as long if clinical diagnosis did not include MCI or AD (34.7 months, SD = 31.4) as compared to those with a premortem diagnosis of AD or MCI (18.8 months, SD = 11.1), but this difference did not reach statistical significance.
DISCUSSION
This investigation describes the incidence rate of MCI and the progression of MCI to AD in a large population-based group of elders from diverse ethnic, linguistic, and educational backgrounds. We found that elders without MCI at the first visit who were above age 70 and had hypertension were at the highest risk for developing MCI. Our AD progression rates among those with MCI at first visit are comparable to other longitudinal studies of aging in White, non-Hispanic, well educated participants 7,8,11,13,48,49 and African Americans in Indiana 14. We also found that elders with MCI were at higher risk of developing AD at follow-up and that MCI subtypes that included memory impairment are at the highest risk for AD. We found that people classified as MCI were not always classified as MCI at follow-up, especially if impairment at first visit was limited to one isolated cognitive domain. Twenty-seven participants were autopsied which revealed that antemortem diagnosis of consistent MCI–that is MCI that did not revert to normal over time - or incident AD had a 60% sensitivity and 94% specificity for AD at postmortem examination.
We also compared the incidence rates of MCI subtypes. Over an average follow-up of 4.7 years, incidence of amnestic MCI, MCI-visuospatial, and MCI-language were highest, while incidence of isolated MCI- executive was lowest. There are few epidemiological studies of MCI incidence. Because the mean age of the cohort, the length of follow-up, and the criteria used for MCI differ across studies 11, it is difficult to compare incidence rates in this study to others. Nevertheless, it appears that our incidence rates of MCI with memory impairment (2.3% annual incidence rate) are higher than studies conducted in Germany 50, France 49, or in the United States 11 but comparable to the Italian Longitudinal Study on Aging 8.
Being over 75 years of age was the most powerful predictor of progression from MCI to AD. However, MCI status at first visit was also useful in predicting who would go on to develop AD. Elders with MCI-MCDM had the highest progression rates and were about 4.3 times as likely to develop AD at follow-up as compared to elders without MCI. Furthermore, compared to elders without MCI, we found that those with amnestic MCI, MCI-language, and MCI-MCDN were at higher risk for developing AD at follow-up, but those with MCI-visuospatial and MCI-executive were not. Because progression to AD was less likely among those with MCI-visuospatial and MCI-executive, this suggests that elders in these categories are less likely to have underlying AD pathology. Therefore, our evaluation of incidence and antecedents of progression to MCI-visuospatial and MCI-executive is less meaningful than the analyses among elders with amnestic MCI, MCI-language, and MCI-MCDN. Evaluation of longitudinal outcomes of different subtypes of MCI can help guide future research to focus on those subtypes that are most likely to progress to AD. It follows that early biomarkers of risk for AD such as plasma Aβ, insulin levels, or volume of the hippocampus should show differences from normal controls among elders classified as having amnestic MCI, MCI-language, and MCI-MCDN.
We found that medical history of hypertension was associated with MCI, while history of diabetes and stroke were risk factors for developing AD. Possession of at least one APOE-ε4 allele did not predict progression to MCI and although significant, APOE-ε4 genotype was a weaker predictor of progression to AD than MCI with memory impairment. This finding may relate to the relatively old age of this cohort. Since examination of the relationship of these variables to MCI classification was exploratory, further study is needed to determine the cardiovascular and genetic correlates of different MCI subtypes and their interaction with memory function on risk of dementia.
In this study, African Americans and Hispanics, and those with less than a high school education, were not at higher risk for developing MCI; however, these groups were at higher risk of developing AD even when MCI status at initial visit was taken into account. This finding is likely related to the fact that our neuropsychological criteria for MCI used years of education and norms that were appropriate for the ethnic groups included in the study.
Examination of “reversion to normal” among participants with MCI was emphasized in this study. If assessed over the course of the entire follow-up period, 30.2% of our participants with MCI at their initial visit were not classified as MCI or demented subsequently. This proportion is comparable to other epidemiological studies, where 14% to 44% 7,11,14 of those with MCI at first visit did not have MCI at follow-up. Although we did not find any demographic or medical factors that were associated with instability of diagnosis, we found that MCI with impairment in multiple cognitive domains, with or without memory impairment, was less likely to revert to normal than MCI with impairment in one cognitive domain. This is not surprising since elders with impairment in multiple cognitive domains have poorer overall cognitive function and thus their neuropsychological test scores are less likely to be on the border of the cutoff. Of those who reverted to normal, most reverted due to cognitive criteria only, with relatively smaller proportions reverting due to functional criteria or memory complaint criteria only. If the cognitive criteria for MCI involve a single “cutoff” of any kind, it is possible that normal variability in cognitive test performance may lead to changes in classification. “Reversions” of this type may not actually represent transitions of one clinical state to another. Continued follow-up of elders who revert to normal should reveal whether these individuals are in very early stages of dementia (and therefore cognitive function may be expected to show mild fluctuation), whether fluctuation is inherent throughout the course of their follow-up, or whether fluctuation is a marker for “misdiagnosis” of cerebrovascular disease or normal age-related brain changes. We have preliminary support for the latter from our autopsy sample, in which none of the participants who reverted to normal after receiving a diagnosis of MCI were found to have AD at postmortem examination.
Compared to other clinical pathological studies 51, the sensitivity of antemortem MCI and incident AD diagnoses to AD pathology was relatively poor. We suspect this is at least partially explained by the fact that among those with neuropathological AD, the length time between last study visit and death was twice as long in those who were not diagnosed with AD or MCI clinically as compared to those who were. All of the autopsied elders in our study who were diagnosed with incident AD were classified as having MCI either at their first visit or a subsequent visit before their AD diagnosis. Specificity was high. Although the autopsy sample was comparable to the overall group with respect to age at baseline, sex, years of education, and ethnic group, a much larger cohort with autopsy is needed before drawing sweeping conclusions about the accuracy of MCI diagnosis with respect to neuropathology.
A limitation of this study is that our average follow-up is only 4.7 years. With longer follow-up, we will be able to compare progression to AD among elders without MCI at the initial visit who do and do not transition to MCI within the course of the study. Furthermore, it is possible that our conclusions about reversion to normal status after an initial classification as MCI would change with a longer follow-up period. It is also possible that those who died before we were able to complete the next follow-up developed MCI or dementia prior to their death; however, our data do not allow us to estimate the proportion of elders who may have progressed. In order to address this problem in future studies, when we discover that a participant has died, we are now administering the Dementia Questionnaire (52,53) to family members in order to inquire about cognitive status and functional decline prior to death.
One other potential limitation is that our cognitive battery did not include traditional measures of attention that would be able to distinguish impairment in this cognitive domain from other domains of cognitive function. It is likely that attentional processes are involved in performance on most if not all of the measures that were administered. To the extent that fluency, verbal list learning ad recall, and nonverbal memory tap into attentional processes more directly than measures of drawing, perhaps the cognitive domains of language and memory in this study are more “attention-loaded” than the visuospatial domain.
The utility of the subjective memory complaint criteria for MCI is controversial, as some studies have found that complaints do not improve upon the ability to predict progression to AD 11,12,50. We also found that when the criteria for MCI were allowed to predict progression to AD separately, neuropsychological impairment in memory conferred a 3-fold higher risk and impairment in language a 2-fold higher risk for incident AD. Presence of memory complaints and isolated visuospatial impairment reached statistical significance as a predictor of incident AD, albeit weaker predictors than memory and language impairment. Our autopsy data, although from a limited sample, showed that presence of consistent neuropsychological impairment in one or more cognitive domains was just as good a predictor of AD pathology as meeting full criteria for MCI or AD, In other words, in this limited sample, presence of a memory complaint did not improve the sensitivity of detection of AD neuropathology, Specificity of neuropsychological impairment was lower than that of MCI and/or AD, but this might be anticipated given that neuropsychological function may be affected by any brain disorder, not just AD. Specificity of neuropsychological impairment was perfect when the presence of any neuropathology was the gold standard. Furthermore, information from our autopsy sample supports the idea that neuropsychological impairment has better overall accuracy for the presence of AD pathology than presence of memory complaints, primarily because memory complaints are so prevalent in this age cohort (67% of our autopsied sample had consistent memory complaints), lowering their specificity for AD. These clinical and neuropathological results suggests that combining objective neuropsychological test scores and subjective complaint data are useful components of MCI classification, but that perhaps neuropsychological criteria should be given more weight than memory complaints.
Table 6.
Diagnostic outcomes of elders with MCI at baseline by MCI subtype, surveying all follow-up visits.
| MCI Subtype | MCI to no MCI | MCI to MCI | MCI to AD | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| MCI - amnestic | 44 | 35.5 | 51 | 41.1 | 29 | 23.4 |
| MCI - executive | 20 | 44.4 | 23 | 51.1 | 2 | 4.4 |
| MCI - language | 24 | 34.8 | 28 | 40.6 | 17 | 24.6 |
| MCI - visuospatial | 39 | 40.6 | 39 | 40.6 | 18 | 18.8 |
| MCI - MCD with memor y impairment | 22 | 18.5 | 61 | 51.3 | 36 | 30.3 |
| MCI - MCD with memory impairment | 21 | 20.2 | 62 | 59.6 | 21 | 20.2 |
| MCI with memory impairment | 66 | 27.2 | 112 | 46.1 | 65 | 26.7 |
| MCI without memory impairment | 104 | 33.1 | 152 | 48.4 | 58 | 18.5 |
Note: Table does not include data on 7 elders with MCI at baseline who progressed to non-AD dementia.
Acknowledgments
This work was supported by National Institute on Aging grants P01-AG07232 (R. Mayeux), P50-AG08702 (R. Mayeux), R01-AG16206 (J. Manly), and the Charles S. Robertson Memorial Gift for Alzheimer’s Disease Research from the Banbury Fund.
Footnotes
CONFLICTS OF INTEREST: There are no conflicts of interest.
References
- 1.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Doody R, Kurz A, et al. Current concepts in Mild Cognitive Impairment. Archives Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Archives Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 4.Flicker C, Ferris S, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 5.Luis CA, Loewenstein DA, Acevedo A, et al. Mild cognitive impairment: directions for future research. Neurology. 2003;61:438–444. doi: 10.1212/01.wnl.0000080366.90234.7f. [DOI] [PubMed] [Google Scholar]
- 6.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological Prediction of Conversion to Alzheimer Disease in Patients With Mild Cognitive Impairment. Arch Gen Psy. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: A population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 8.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 9.Boyle PA, Wilson RS, Aggarwal NT, et al. Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 11.Ganguli M, Dodge HH, Shen C, et al. Mild cognitive impairment, amnestic type: An epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 12.Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. doi: 10.1212/01.wnl.0000089238.07771.c7. [DOI] [PubMed] [Google Scholar]
- 13.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al. Conversion to dementia from mild cognitive disorder: The Cache County Study. Neurology. 2006;67:229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 14.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment: Data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 15.Manly JJ, Jacobs DM, Sano M, et al. Cognitive test performance among nondemented elderly African Americans and Whites. Neurology. 1998;50:1238–1245. doi: 10.1212/wnl.50.5.1238. [DOI] [PubMed] [Google Scholar]
- 16.Manly JJ, Jacobs DM. Future directions in neuropsychological assessment with African Americans. In: Ferraro FR, editor. Minority and Cross-cultural Aspects of Neuropsychological Assessment. Lisse, Netherlands: Swets and Zeitlinger; 2001. pp. 79–96. [Google Scholar]
- 17.Sundquist J, Winkleby MA, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988–1994. Third National Health and Nutrition Examination Survey. JAGS. 2001;49:109–116. doi: 10.1046/j.1532-5415.2001.49030.x. [DOI] [PubMed] [Google Scholar]
- 18.Brancati FL, Kao WHL, Folsom AR, et al. Incident Type 2 Diabetes Mellitus in African American and White Adults: The Atherosclerosis Risk in Communities Study. JAMA: The Journal of the American Medical Association. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 19.Manly JJ, Bell-McGinty S, Tang MX, et al. Implementing Diagnostic Criteria and Estimating Frequency of Mild Cognitive Impairment in an Urban Community. Archives Neurol. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 20.Luchsinger JA, Tang M-X, Shea S, et al. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 21.Tang MX, Cross P, Andrews H, et al. Incidence of Alzheimer’s disease in African-Americans, Caribbean Hispanics and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 22.United States Office of Management and Budget. Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity (October 30, 1997). Standards for maintainig, collecting, and presenting federal data on race and ethnicity. United States Office of Management and Budget; 1997. 11-6-2000. [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 24.Radloff LL. The CES-D: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Kohout FJ, Berkman LF, Evans DA, et al. Two Shorter Forms of the CES-D Depression Symptoms Index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 26.Golden RR, Teresi JA, Gurland BJ. Development of indicator scales for the Comprehensive Assessment and Referral Evaluation (CARE) interview schedule. J Gerontol. 1984;39:138–146. doi: 10.1093/geronj/39.2.138. [DOI] [PubMed] [Google Scholar]
- 27.Gurland B, Kuriansky J, Sharpe L, et al. The Comprehensive assessment and Referral Evaluation (CARE)--rationale, development and reliability. International Journal of Aging and Human Development. 1977;8:9–42. doi: 10.2190/cl3j-0e20-97xx-mv5l. [DOI] [PubMed] [Google Scholar]
- 28.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of senile change in the cerebral grey matter of elderly subjects. Br J Psychol. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 29.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives Neurol. 1992;49:453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 30.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 31.Benton AL. The Visual Retention Test. New York, NY: The Psychological Corporation; 1955. [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental State’: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- 34.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Karasu TB, editors. Geriatric Psychiatry. New York, NY: Grune & Stratton; 1976. pp. 77–121. [Google Scholar]
- 35.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 36.Benton AL, Hamsher Kd. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- 37.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 38.Rosen W. The Rosen Drawing Test. Bronx, NY: Veterans Administration Medical Center; 1981. [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Revised Third. Washington, DC: American Psychiatric Press Inc; 1987. [Google Scholar]
- 40.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 41.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 42.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 43.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 44.Vonsattel J-PG, Aizawa H, Ge P, et al. An improved approach to prepare human brains for research. J Neuropath Exp Neuro. 1995;54:42–56. doi: 10.1097/00005072-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 46.The National Institute on Aging, and Regan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 47.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 48.Panza F, D’Introno A, Colacicco AM, et al. Current Epidemiology of Mild Cognitive Impairment and Other Predementia Syndromes. Am J Geriatr Psyhciatr. 2005;13:633–644. doi: 10.1176/appi.ajgp.13.8.633. [DOI] [PubMed] [Google Scholar]
- 49.Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- 50.Busse A, Bischkopf J, Reidel-Heller S, et al. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. Br J Psychiatr. 2003;182:449–454. [PubMed] [Google Scholar]
- 51.Galasko D, Hansen LA, Katzman R, et al. Clinical-neuropathological correlations in Alzheimer’s disease and related dementias. Archives Neurol. 1994;51:888–895. doi: 10.1001/archneur.1994.00540210060013. [DOI] [PubMed] [Google Scholar]
- 52.Kawas C, Segal J, Stewart WF, et al. A validation study of the Dementia Questionnaire. Archives Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 53.Ellis RJ, Jan K, Kawas C, et al. Diagnostic Validity of the Dementia Questionnaire for Alzheimer Disease. Archives Neurol. 1998;55:360–365. doi: 10.1001/archneur.55.3.360. [DOI] [PubMed] [Google Scholar]
- 54.Lopez OL, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Archives of Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 55.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]