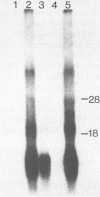
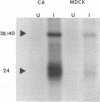
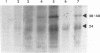
Abstract
Borna disease virus (BDV) infects cells of the nervous system in a wide range of species. Previous work suggests that there are differences in BDV replication in neuronal cells and glial cells. Many neurons are lysed by the immunopathologic response to BDV; lysis of dentate gyrus neurons in the absence of encephalitis is seen in rats inoculated with BDV as neonates. In contrast, persistently BDV-infected astrocytes increase over the course of BDV infection. Therefore, we compared BDV replication in neuronal (SK-N-SH and SK-N-SHEP) and astrocytic (C6) cell lines. While SK-N-SH cells produced more infectious virions per cell, the C6 cells contained more BDV proteins and RNA. BDV sequences in the supernatants of both cell types were identified, despite low titers of infectious virus, suggesting the release of incomplete virions into the medium. C6 cells secreted a factor or factors into the medium that enhanced the production of BDV proteins and RNA in other cell lines. In addition, nerve growth factor treatment produced the same enhancement. Thus, BDV replication in certain neural cells in vitro may be linked to the production of cell-specific factors which affect viral replication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenander A. T., de Vellis J. Glial-released proteins: III. Influence on neuronal morphological differentiation. Brain Res. 1981 Nov 9;224(1):117–127. doi: 10.1016/0006-8993(81)91121-5. [DOI] [PubMed] [Google Scholar]
- Baker D. L., Reddy U. R., Pleasure D., Thorpe C. L., Evans A. E., Cohen P. S., Ross A. H. Analysis of nerve growth factor receptor expression in human neuroblastoma and neuroepithelioma cell lines. Cancer Res. 1989 Aug 1;49(15):4142–4146. [PubMed] [Google Scholar]
- Bangham C. R., Kirkwood T. B. Defective interfering particles: effects in modulating virus growth and persistence. Virology. 1990 Dec;179(2):821–826. doi: 10.1016/0042-6822(90)90150-p. [DOI] [PubMed] [Google Scholar]
- Bednarik D. P., Folks T. M. Mechanisms of HIV-1 latency. AIDS. 1992 Jan;6(1):3–16. doi: 10.1097/00002030-199201000-00001. [DOI] [PubMed] [Google Scholar]
- Briese T., de la Torre J. C., Lewis A., Ludwig H., Lipkin W. I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Duchala C. S., Griffin J. W., Kincaid A. L., Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987 Nov;61(11):3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Duchala C. S., Narayan O. Borna disease. An immunopathologic response to viral infection in the CNS. Ann N Y Acad Sci. 1988;540:661–662. doi: 10.1111/j.1749-6632.1988.tb27204.x. [DOI] [PubMed] [Google Scholar]
- Carbone K. M., Moench T. R., Lipkin W. I. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol. 1991 May;50(3):205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- Carbone K. M., Park S. W., Rubin S. A., Waltrip R. W., 2nd, Vogelsang G. B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991 Nov;65(11):6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Trapp B. D., Griffin J. W., Duchala C. S., Narayan O. Astrocytes and Schwann cells are virus-host cells in the nervous system of rats with Borna disease. J Neuropathol Exp Neurol. 1989 Nov;48(6):631–644. doi: 10.1097/00005072-198911000-00005. [DOI] [PubMed] [Google Scholar]
- Clements G. B., Kennedy P. G. Modulation of herpes simplex virus (HSV) infection of cultured neuronal cells by nerve growth factor and antibody to HSV. Brain. 1989 Oct;112(Pt 5):1277–1294. doi: 10.1093/brain/112.5.1277. [DOI] [PubMed] [Google Scholar]
- Coutlee F., Rubalcaba E. A., Viscidi R. P., Gern J. E., Murphy P. A., Lederman H. M. Quantitative detection of messenger RNA by solution hybridization and enzyme immunoassay. J Biol Chem. 1990 Jul 15;265(20):11601–11604. [PubMed] [Google Scholar]
- Coutlée F., Yang B. Z., Bobo L., Mayur K., Yolken R., Viscidi R. Enzyme immunoassay for detection of hybrids between PCR-amplified HIV-1 DNA and a RNA probe: PCR-EIA. AIDS Res Hum Retroviruses. 1990 Jun;6(6):775–784. doi: 10.1089/aid.1990.6.775. [DOI] [PubMed] [Google Scholar]
- Danner K., Heubeck D., Mayr A. In vitro studies on Borna virus. I. The use of cell cultures for the demonstration, titration and production of Borna virus. Arch Virol. 1978;57(1):63–75. doi: 10.1007/BF01315638. [DOI] [PubMed] [Google Scholar]
- Danner K., Mayr A. In vitro studies on Borna virus. II. Properties of the virus. Arch Virol. 1979;61(4):261–271. doi: 10.1007/BF01315012. [DOI] [PubMed] [Google Scholar]
- Dawson G. J., Elizan T. S., Mowshowitz S. L., Cohen R. Virus-host cell interaction in rat C6 glial cell cultures persistently infected with herpes simplex virus. Intervirology. 1983;19(2):95–104. doi: 10.1159/000149343. [DOI] [PubMed] [Google Scholar]
- Deas J., Erecińska M. Effect of tunicamycin, an inhibitor of protein glycosylation, on the high-affinity transport of acidic amino acid neurotransmitters in C6 glioma cells. Brain Res. 1989 Mar 27;483(1):84–90. doi: 10.1016/0006-8993(89)90037-1. [DOI] [PubMed] [Google Scholar]
- Doerig C., Pizer L. I., Wilcox C. L. Detection of the latency-associated transcript in neuronal cultures during the latent infection with herpes simplex virus type 1. Virology. 1991 Jul;183(1):423–426. doi: 10.1016/0042-6822(91)90159-9. [DOI] [PubMed] [Google Scholar]
- Duchala C. S., Carbone K. M., Narayan O. Preliminary studies on the biology of Borna disease virus. J Gen Virol. 1989 Dec;70(Pt 12):3507–3511. doi: 10.1099/0022-1317-70-12-3507. [DOI] [PubMed] [Google Scholar]
- Fenton E. L. Tissue culture assay of nerve growth factor and of the specific antiserum. Exp Cell Res. 1970 Mar;59(3):383–392. doi: 10.1016/0014-4827(70)90645-2. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog S., Kompter C., Frese K., Rott R. Replication of Borna disease virus in rats: age-dependent differences in tissue distribution. Med Microbiol Immunol. 1984;173(4):171–177. doi: 10.1007/BF02122108. [DOI] [PubMed] [Google Scholar]
- Herzog S., Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168(3):153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- Kennedy P. G., Clements G. B., Brown S. M. Differential susceptibility of human neural cell types in culture to infection with herpes simplex virus. Brain. 1983 Mar;106(Pt 1):101–119. doi: 10.1093/brain/106.1.101. [DOI] [PubMed] [Google Scholar]
- Lipkin W. I., Travis G. H., Carbone K. M., Wilson M. C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig T. H., Becht H., Groh L. Borna disease (BD), a slow virus infection. Biological properties of the virus. Med Microbiol Immunol. 1973;158(4):275–289. doi: 10.1007/BF02121414. [DOI] [PubMed] [Google Scholar]
- Löve A., Norrby E., Kristensson K. Measles encephalitis in rodents: defective expression of viral proteins. J Neuropathol Exp Neurol. 1986 May;45(3):258–267. [PubMed] [Google Scholar]
- Mayr A., Danner K. Production of Borna virus in tissue culture. Proc Soc Exp Biol Med. 1972 Jun;140(2):511–515. doi: 10.3181/00379727-140-36492. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Thibault K. J., Hatalski C. G., Lipkin W. I. Sequence similarity between Borna disease virus p40 and a duplicated domain within the paramyxovirus and rhabdovirus polymerase proteins. J Virol. 1992 Nov;66(11):6572–6577. doi: 10.1128/jvi.66.11.6572-6577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. The switch between latency and replication of Epstein-Barr virus. J Infect Dis. 1990 May;161(5):833–844. doi: 10.1093/infdis/161.5.833. [DOI] [PubMed] [Google Scholar]
- Morita S., Takeoka Y., Imai H., Yamamoto H., Suehiro S., Ueda S., Katoh S. Differential action of nerve growth factor, cyclic AMP and neurotropin on PC12h cells. Cell Struct Funct. 1988 Jun;13(3):227–234. doi: 10.1247/csf.13.227. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M., Bovolenta P. Growth factors and growth factor receptors in the hippocampus. Role in plasticity and response to injury. Prog Brain Res. 1990;83:341–355. doi: 10.1016/s0079-6123(08)61261-3. [DOI] [PubMed] [Google Scholar]
- Notter M. F., Hansen J. T., Okawara S., Gash D. M. Rodent and primate adrenal medullary cells in vitro: phenotypic plasticity in response to coculture with C6 glioma cells or NGF. Exp Brain Res. 1989;76(1):38–46. doi: 10.1007/BF00253621. [DOI] [PubMed] [Google Scholar]
- Ohuchi M., Ohuchi R., Mifune K., Ishihara T., Ogawa T. Characterization of the measles virus isolated from the brain of a patient with immunosuppressive measles encephalitis. J Infect Dis. 1987 Sep;156(3):436–441. doi: 10.1093/infdis/156.3.436. [DOI] [PubMed] [Google Scholar]
- Ojika K., Appel S. H. Neurotrophic effects of hippocampal extracts on medial septal nucleus in vitro. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2567–2571. doi: 10.1073/pnas.81.8.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli G., Ludwig H. Increase of virus yields and releases of Borna disease virus from persistently infected cells. Virus Res. 1985 Feb;2(1):29–33. doi: 10.1016/0168-1702(85)90057-7. [DOI] [PubMed] [Google Scholar]
- Rataul S. M., Hirano A., Wong T. C. Irreversible modification of measles virus RNA in vitro by nuclear RNA-unwinding activity in human neuroblastoma cells. J Virol. 1992 Mar;66(3):1769–1773. doi: 10.1128/jvi.66.3.1769-1773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. A., Spengler B. A., Biedler J. L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983 Oct;71(4):741–747. [PubMed] [Google Scholar]
- Sakihama K., Eizuru Y., Minamishima Y. Interaction of herpes simplex virus type 2 with a rat glioma cell line. Microbiol Immunol. 1988;32(9):933–947. doi: 10.1111/j.1348-0421.1988.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Sariola H., Saarma M., Sainio K., Arumäe U., Palgi J., Vaahtokari A., Thesleff I., Karavanov A. Dependence of kidney morphogenesis on the expression of nerve growth factor receptor. Science. 1991 Oct 25;254(5031):571–573. doi: 10.1126/science.1658930. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies S., Liebert U. G., Baczko K., ter Meulen V. Restricted expression of measles virus in primary rat astroglial cells. Virology. 1990 Aug;177(2):802–806. doi: 10.1016/0042-6822(90)90553-4. [DOI] [PubMed] [Google Scholar]
- Schädler R., Diringer H., Ludwig H. Isolation and characterization of a 14500 molecular weight protein from brains and tissue cultures persistently infected with borna disease virus. J Gen Virol. 1985 Nov;66(Pt 11):2479–2484. doi: 10.1099/0022-1317-66-11-2479. [DOI] [PubMed] [Google Scholar]
- Steiner I., Kennedy P. G. Herpes simplex virus latency in the nervous system--a new model. Neuropathol Appl Neurobiol. 1991 Dec;17(6):433–440. doi: 10.1111/j.1365-2990.1991.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Stitz L., Schilken D., Frese K. Atypical dissemination of the highly neurotropic Borna disease virus during persistent infection in cyclosporine A-treated, immunosuppressed rats. J Virol. 1991 Jan;65(1):457–460. doi: 10.1128/jvi.65.1.457-460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Johnson E. M., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Schweitzer J. B., Johnson E. M., Jr Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988 Feb;8(2):664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiedemann N., Presek P., Rott R., Stitz L. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J Gen Virol. 1992 May;73(Pt 5):1057–1064. doi: 10.1099/0022-1317-73-5-1057. [DOI] [PubMed] [Google Scholar]
- Thierer J., Riehle H., Grebenstein O., Binz T., Herzog S., Thiedemann N., Stitz L., Rott R., Lottspeich F., Niemann H. The 24K protein of Borna disease virus. J Gen Virol. 1992 Feb;73(Pt 2):413–416. doi: 10.1099/0022-1317-73-2-413. [DOI] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991 May;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Skaper S. D., Varon S. Neuronotrophic and neurite-promoting factors: effects on early postnatal chromaffin cells from rat adrenal medulla. Brain Res. 1985 Jan;349(1-2):117–129. doi: 10.1016/0165-3806(85)90137-3. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Vey J., Hofmann H. D., Müller T. H., Wilson A. J. C6 glioma cell-conditioned medium induces neurite outgrowth and survival of rat chromaffin cells in vitro: comparison with the effects of nerve growth factor. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2242–2246. doi: 10.1073/pnas.81.7.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dinter S., Flintoff W. F. Rat glial C6 cells are defective in murine coronavirus internalization. J Gen Virol. 1987 Jun;68(Pt 6):1677–1685. doi: 10.1099/0022-1317-68-6-1677. [DOI] [PubMed] [Google Scholar]
- VandeWoude S., Richt J. A., Zink M. C., Rott R., Narayan O., Clements J. E. A borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990 Nov 30;250(4985):1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- Weskamp G., Gasser U. E., Dravid A. R., Otten U. Fimbria-fornix lesion increases nerve growth factor content in adult rat septum and hippocampus. Neurosci Lett. 1986 Sep 25;70(1):121–126. doi: 10.1016/0304-3940(86)90449-0. [DOI] [PubMed] [Google Scholar]
- Wilcox C. L., Johnson E. M., Jr Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol. 1988 Feb;62(2):393–399. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Carbone K. M., Lipkin W. I. Molecular characterization of the Borna disease agent. Virology. 1990 Dec;179(2):853–856. doi: 10.1016/0042-6822(90)90154-j. [DOI] [PubMed] [Google Scholar]