Abstract
Due to advanced disease at the time of diagnosis the prognosis of oesophageal cancer is generally poor. As mass screening for oesophageal cancer is neither feasible nor reasonable, high-risk groups should be identified and surveilled. The aim of this study was to define the risk of oesophageal cancer in patients with (previous) head and neck cancer. A total of 148 patients with (previous) head and neck cancer were prospectively screened for oesophageal cancer by video-oesophagoscopy and random oesophageal biopsies. Even in a macroscopically normal looking oesophagus, four biopsy specimens were taken every 3 cm throughout the entire length of the squamous oesophagus. Low- or high-grade squamous cell dysplasia was detected histologically in 10 of the 148 patients (6.8%). All but one dysplasias were diagnosed synchronously with the head and neck cancers. In addition, oesophageal squamous cell carcinoma was diagnosed in 11 of the 148 patients (7.4%). Most invasive cancers (63.6%) occurred metachronously. The risk of squamous cell neoplasia of the oesophagus is high in patients with (previous) head and neck cancer. Surveillance is recommended in this high-risk group.
British Journal of Cancer (2002) 86, 239–243. DOI: 10.1038/sj/bjc/6600018 www.bjcancer.com
© 2002 The Cancer Research Campaign
Keywords: surveillance, secondary malignancy, squamous cell cancer, larynx, pharynx, oral cavity
Heavy consumption of alcohol and tobacco is responsible for synchronous and metachronous primary malignancies in the upper aerodigestive tract. When a second oesophageal malignancy develops in a patient with previous head and neck cancer (HNC), the prognosis is determined by the oesophageal cancer, and unfortunately it is poor (Cooper et al, 1989; Leon et al, 1999). This is due to the advanced tumour stage at which oesophageal cancer becomes symptomatic. In patients with both HNC and oesophageal cancer, the former often develops first, and there are more metachronous than synchronous cases (Cooper et al, 1989; Makuuchi et al, 1996; Agrawal and Wenig, 1998; Leon et al, 1999). Screening for oesophageal squamous cell cancer has therefore been advocated for HNC patients (Cooper et al, 1989; Makuuchi et al, 1996; Horiuchi et al, 1998).
In analogy to the well-studied colorectal carcinogenesis, Mandard et al (2000) recently suggested a model of genetic steps in the development of squamous cell cancer of the oesophagus. The sequence of histopathological changes includes esophagitis, atrophy, mild to severe dysplasia, carcinoma in situ and, finally, invasive oesophageal cancer. The concept of a stepwise carcinogenesis implies the efficacy of surveillance in patients at high risk for squamous cell cancer of the oesophagus. Patients who survived HNC are known to develop a second neoplasm with a risk of 3–7% per year (Cooper et al, 1989; Sturgis and Miller, 1995). Prospective studies in Japan and recently in Brazil detected oesophageal squamous cell neoplasia in 5.1–11.8% of HNC patients (Shiozaki et al, 1990; Makuuchi et al, 1996; Tincani et al, 2000). General surveillance of HNC patients is not yet recommended in Western countries, mainly due to a lack of adequate data from this part of the world (Agrawal and Wenig, 1998; Deleyiannis and Weymuller, 1998).
In this study we used high-resolution video-oesophagoscopy to prospectively screen 148 HNC patients for oesophageal squamous neoplasia. To detect early histological changes even in a macroscopically normal looking oesophagus, we took multiple biopsies according to a systematic endoscopic biopsy protocol. The high rate of oesophageal neoplasia we observed confirms previous data from Japan and argues for a more general surveillance of HNC patients. As chromoendoscopy with Lugol staining only moderately improves the diagnostic accuracy of video-endoscopy (Meyer et al, 1997) in oesophageal squamous cell neoplasia, we applied Lugol chromoendoscopy only to assess the mucosal extension of early neoplastic lesions and to search for any additional neoplastic foci.
MATERIALS AND METHODS
Patients
A prospective study involving 148 patients with HNC was carried out at the University Hospital Benjamin Franklin in Berlin from May 2000 to August 2001. An oesophagogastroduodenoscopy with systematic oesophageal biopsies was suggested to all patients who presented with a previous history or first diagnosis of head and neck cancer (oral cavity, larynx, oro- or hypopharynx) to either the ENT Department, the Department of Maxillofacial Plastic Surgery or the Department of Gastroenterology. Patients with oesophageal varices or bleeding disorders were excluded. The study was approved by the Ethics Committee of the University Hospital Benjamin Franklin, Free University Berlin.
The 148 patients ranged in age from 34 to 89 years (average 61.0 years). Forty-two patients were women. The primary head and neck cancer was located in either the oral cavity (n=59), oropharynx (n=48), hypopharynx (n=23) or larynx (n=18 patients). In cases with multiple metachronous primary malignancies, the first head and neck carcinoma was regarded as the index tumour. When multiple head and neck tumours were diagnosed simultaneously, the tumour that caused the presenting complaint was chosen as the index lesion.
The oesophageal neoplasm was classified as synchronous if diagnosed within 6 months after the diagnosis of HNC and metachronous if diagnosed after a period of 6 months. Seventy-five of the 148 HNC patients were oesophagoscoped within 6 months after the diagnosis of HNC.
Endoscopy
All endoscopies were performed using a high-resolution videoendoscopes (GIF-XQ 140, Olympus Optical Co. (Europa) GmbH, Hamburg, Germany). Biopsy specimens were obtained with the 7.5 mm open span biopsy forceps (K02 22 V-A, Endo-Flex, Voerde, Germany). Biopsy specimens were taken every 3 cm throughout the entire length of the squamous oesophagus, starting 3 cm above the gastrooesophageal junction. Four biopsies were taken from each level and placed in different bottles so that the locations could be separately documented. Even in a macroscopically normal looking oesophagus, 20 to 28 biopsies were sampled according to this systematic endoscopic biopsy protocol. In addition, multiple specimens were taken of any endoscopic abnormality.
Histology
The final diagnosis was based on an independent review of the cases by two to three observers. If dysplasia was found, the microsections were evaluated by three different pathologists. All observers agreed on the diagnosis of cancer or dysplasia in the patients described in this study. In specimens where there was no complete agreement concerning the grade of dysplasia, two out of three was considered a consensus.
Oesophageal carcinomas if restricted to the submucosal layer were classified superficial and as intraepithelial if restricted to the epithelial layer (Endo et al, 1986; Sugimachi et al, 1988; Mori et al, 1993; Schlemper et al, 2000c). Low- and high-grade dysplasia (synonym: intraepithelial neoplasias; Gabbert et al, 2000) was defined as unequivocal neoplastic transformation according to criteria published previously (Lewin and Appelman, 1995).
RESULTS
Oesophageal squamous cell cancer (ESCC)
Among the 148 patients examined, 14 invasive oesophageal squamous cell cancers were detected in 11 patients (7.4%; Table 1). Three of the 11 patients even had two oesophageal cancers each; in addition to two oesophageal cancers two of the three patients suffered from oesophageal dysplasia at a separate third location. In four of the 11 patients, HNC and oesophageal cancer were diagnosed synchronously. Interestingly, the patients related their clinical symptoms only to the head and neck region. The other seven patients had metachronous oesophageal cancers that developed 10, 13, 27, 29, 41, 74 and 114 months after the (first) head and neck cancer. These patients did not present with dysphagia. The oesophageal cancers were preceded by 15 primary head and neck cancers (mean of 1.4 HNC per ESCC-patient). In contrast, the HNC patients in whom no oesophageal dysplasia or cancer was detected had suffered from a mean of 1.1 HNC per patient.
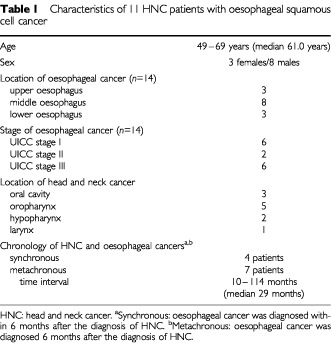
Table 1. Characteristics of 11 HNC patients with oesophageal squamous cell cancer.
Apart from the 148 study patients, seven further HNC patients presenting with dysphagia during the study period were diagnosed as suffering from squamous cell cancer of the oesophagus. Since these seven patients complained of dysphagia, they were not included in the group of 148 study patients. All seven patients suffered from stenotic stage III squamous cell cancer of the oesophagus. The intervals between the diagnosis of (the first) HNC and oesophageal cancer were 2, 3, 5, 7, 8, 9 and 13 years. The seven patients had developed 10 HNC prior to the now presented oesophageal cancers.
Oesophageal squamous cell dysplasia (ESCD)
Even in a macroscopically normal looking oesophagus we sampled multiple biopsies according to the outlined systematic endoscopic biopsy protocol. Oesophageal squamous cell dysplasia (eight low- and four high-grade dysplasias) was detected in 10 of the 148 (6.8%) patients biopsied according to that protocol (Table 2). In nine of the 10 patients, HNC and squamous cell dysplasia (= intra-epithelial neoplasia) of the oesophagus were diagnosed synchronously. Altogether 13 head and neck cancers had occurred in the 10 patients with oesophageal dysplasia (mean 1.3 HNC per patient). All patients found to suffer from dysplasias or early oesophageal cancer were further examined by chromoendoscopy with Lugol dye solution (Shiozaki et al, 1990; Endo et al, 1986; Sugimachi et al, 1988) and by endoscopic ultrasound (Ziegler et al, 1991). Lugol chromoendoscopy identified all but one dysplastic lesions and proved very helpful in assessing the mucosal extension of early neoplastic lesions (Figure 1); additional neoplastic foci were not detected by Lugol staining. There were no major complications related to the endoscopic examinations or the biopsies taken.
Table 2. Characteristics of 10 HNC patients with oesophageal squamous cell dysplasiaa.
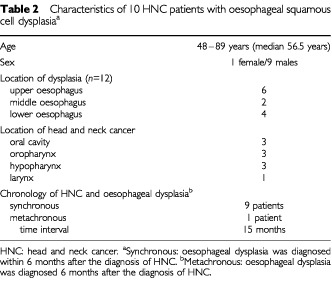
Figure 1.
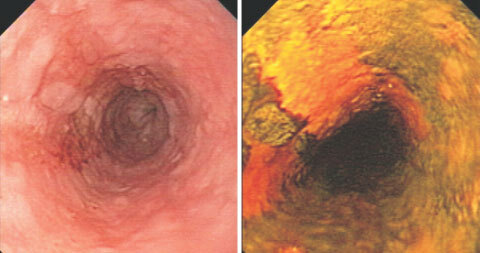
Oesophageal squamous cell cancer in a HNC patient. Left panel: Videoendoscopic image of a T1No squamous cell cancer at 25 cm from the incisors. Twenty-nine months ago the patient had been treated for a squamous cell cancer of the oral cavity. Right panel: The same tumour after staining with Lugol dye solution to delineate the tumour margins.
DISCUSSION
Oesophageal cancer is a disease with a dramatically different geographic incidence. In high-risk areas like Linxian, China, mass surveillance programmes for the malignancy have been implemented to reduce the high mortality rate of 161 per 100 000 (Wu et al, 1982). In most Western countries, the incidence of oesophageal squamous cell cancer is less than 6 per 100 000 (Fleischer and Haddad, 1999; Gabbert et al, 2000). The rarity of this neoplasm does not justify implementing a costly endoscopic surveillance programme for the general population in low-risk areas. However, even in low-risk areas, surveillance may be warranted for high-risk groups. High-risk groups for oesophageal squamous cell cancer include male heavy drinkers and smokers, patients with corrosive esophagitis, achalasia or with (previous) head and neck cancer (Makuuchi et al, 1996).
The incidence of a second neoplasm in patients who survive HNC is 3–7% per year (Cooper et al, 1989; Sturgis and Miller, 1995; Ina et al, 1994). Nevertheless, surveillance of HNC patients has thus far not been generally recommended. This is mainly due to a lack of adequate data, the difficulty of early diagnosis and the lack of safe and effective therapeutic options (Agrawal and Wenig, 1998; Deleyiannis and Weymuller, 1998). Here we provide evidence that 11 patients out of a group of 148 patients with (previous) HNC suffered from oesophageal squamous cell cancer. This high frequency may have been biased by the referral practice to our hospital which provides care for HNC patients in a population of about 750 000 people in the southwestern part of Berlin. In referral centers in Germany, as many as 25–35% of the newly diagnosed oesophageal squamous cell cancers of the oesophagus are now reported in HNC patients. Many patients with previous HNC may be used to some degree of odyno- or dysphagia so that they are hardly alert to any symptoms of early oesophageal cancer. This may explain why oesophageal cancer is generally diagnosed late even in patients who have already experienced the sequelae of HNC (Cooper et al, 1989; Leon et al, 1999). Thus, in our study, six of the 11 patients with oesophageal cancer already had a stage III tumour disease.
In order to detect early lesions even in a macroscopically normal looking and staining oesophagus (Fagundes et al, 1999), we took multiple biopsies according to a systematic endoscopic biopsy protocol and found that 6.8% (10 out of 148) of the patients biopsied had oesophageal squamous cell dysplasias (synonym: intraepithelial neoplasias; Gabbert et al, 2000; Schlemper et al, 2000a, 2000b, 2000c). This high percentage compares well or even surmounts the rates observed in previous studies (Makuuchi et al, 1996; Meyer et al, 1997; Fagundes et al, 1999; Tincani et al, 2000; Petit et al, 2001) and provides strong evidence for the efficacy of the screening protocol used in this study. Various alternative screening strategies have been described. These include brush-capsule cytology (Leoni-Parvex et al, 2000), biannual oesophageal endoscopy without Lugol staining (Petit et al, 2001), Lugol chromoendoscopy with (Fagundes et al, 1999) or without random biopsies (Shiozaki et al, 1990; Makuuchi et al, 1996), (auto)fluorescence endoscopy (Haringsma et al, 2001) or tri-modal spectroscopy (Georgakoudi et al, 2001). These different strategies have, however, not yet been compared in controlled clinical trials.
Due to its low price and general availability Lugol staining has probably been used most commonly. Drawbacks of Lugol staining are the low yield of neoplasia in small unstained areas (<1% ESCC risk in unstained areas of <5 mm) and the missing of normal staining intra-epithelial neoplasias. By taking two random biopsies in the middle of the oesophagus, Fagundes et al (1999) observed intra-epithelial neoplasias in 7 out of 165 (4.2%) alcoholic smokers despite a normal Lugol stain of the squamous oesophagus. Therefore, randomized controlled studies are indicated to compare the efficacy and cost effectiveness of the various strategies.
Since mucosal surfaces in the upper aerodigestive tract, lungs, and the oesophagus are exposed to the same carcinogens, multiple anatomical sites in the oesophagus may be at risk for the simultaneous or sequential development of dysplastic and malignant lesions. This multifocal large-field carcinogenesis is reflected by the finding that 11 patients with oesophageal cancer had suffered from a mean of 1.4 primary HNC, 10 patients with oesophageal dysplasia from a mean of 1.3 HNC and the remaining patients from a mean of 1.1 primary HNC. This suggests that patients with multiple HNC have an even higher risk of developing or already harbouring oesophageal dysplasia or cancer. Oesophageal cancer was multifocal in 27.3% of our patients. In addition, we observed a similar rate of oesophageal dysplasia and oesophageal squamous cell cancer (6.8 vs 7.4%). All but one oesophageal squamous cell dysplasias were detected synchronously, but cancer was mostly (63.6%) diagnosed metachronously.
Thus, our findings imply that squamous cell dysplasia precedes oesophageal squamous cell cancer. This supports the concept of a stepwise development of oesophageal squamous cell cancer recently put forward by Mandard et al (2000). Although most second oesophageal squamous cell cancers appear to develop as independent neoplasms, it has recently been reported that a clonal population of neoplastic cells from the oro- or hypopharynx may be capable of travelling substantial distances to give rise to second tumours in the oesophagus (Califano et al, 1999).
The introduction of endoscopic oesophageal mucosal resection (EEMR) has revolutionized the treatment of intra-epithelial oesophageal neoplasia and early cancer (Makuuchi, 1996; Ell et al, 2000). In experienced hands, this therapeutic endoscopic technique has a very low morbidity and mortality and is an attractive alternative to oesophageal resection in certain situations (Makuuchi et al, 1996; Horiuchi et al, 1998; Ell et al, 2000). The more general availability of EEMR offers HNC patients not only an earlier diagnosis of oesophageal cancer but also an effective minimal invasive treatment option if oesophageal squamous cell neoplasia is diagnosed early. Photodynamic therapy (with haematoporphyrins) has also been reported to eradicate early squamous cell cancer in HNC patients (Savary et al, 1998). Thus, surveillance of oesophageal squamous cell cancer is recommended in HNC patients (Makuuchi et al, 1996). In addition, the survival benefit achieved by screening for early oesophageal cancer (Horiuchi et al, 1998) underlines the need for a more general implementation of surveillance in HNC patients.
Acknowledgments
We thank the nurses of the Central Endoscopy Unit, University Hospital Benjamin Franklin, FU Berlin, for their excellent support of this study. We are indebted to CM deVilliers, C Ell, M Hollstein, H Makuuchi, T Ponchon and M Stolte for reading the manuscript and helpful suggestions.
References
- AgrawalAWenigB1998Screening for simultaneous oesophageal primary tumors. Esophagoscopy vs esophagography Arch Otolaryngol Head Neck Surg 124930932 [DOI] [PubMed] [Google Scholar]
- CalifanoJLeongPKochWEisenbergerCSidranskyDWestraW1999Second oesophageal tumors in patients with head and neck squamous cell carcinoma: an assessment of clonal relationships Clin Cancer Res 518621867 [PubMed] [Google Scholar]
- CooperJPajakTRubinPTupchongLBradyLLeibelSLaramoreGMarcialVDavisLCoxJ1989Second malignancies in patients who have head and neck cancer: Incidence, effect on survival and implications based on the RTOG experience Int J Radiation Oncology Biol Phys 17449456 [DOI] [PubMed] [Google Scholar]
- DeleyiannisFWeymullerE1998Screening patients with head and neck cancer for oesophageal cancer. A lack of adequate data Arch Otolaryngol Head Neck Surg 124933. [DOI] [PubMed] [Google Scholar]
- EllCMayAGossnerLPechOGünterEMayerGHenrichRViethMMüllerHSeitzGStolteM2000Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's oesophagus Gastroenterology 118670677 [DOI] [PubMed] [Google Scholar]
- EndoMTakeshitaKYoshidaM1986How can we diagnose early stages of oesophageal cancer? Endoscopic diagnosis Endoscopy 181118 [DOI] [PubMed] [Google Scholar]
- FagundesRde BarrosSPuttenAMelloEWagnerMBassiLBombassaroMGobbiDSoutoE1999Occult dysplasia is disclosed by Lugol chromoendoscopy in alcoholics at high risk for squamous cell carcinoma of the oesophagus Endoscopy 31325328 [DOI] [PubMed] [Google Scholar]
- FleischerDHaddadN1999Neoplasma of the oesophagusInThe oesophagusIn Castell D, Richter J (eds)pp 235259Philadelphia : Lippincott [Google Scholar]
- GabbertHShimodaTHainautPNakamuraYFieldJInoueH2000Squamous cell carcinoma of the ooesophagusInPathology and Genetics of Tumours of the Digestive SystemHamilton S & Aaltonen L (eds)WHO classification of tumours.Lyon: IARC Press [Google Scholar]
- GeorgakoudiIJacobsonBvan DamJBackmanVWallaceMMüllerMZhangQBadizadeganKSunDThomasGPerelmanLFeldM2001Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett's esophagus Gastroenterology 12016201629 [DOI] [PubMed] [Google Scholar]
- HaringsmaJTytgatGYanoHIishiHTatsutaMOgiharaTWatan SatoNMarconNWilsonBClineR2001Autofluorescence endoscopy: feasibility of detection of GI neoplasia unapparent to white light endoscopy with an evolving technology Gastroint Endosc 53642650 [DOI] [PubMed] [Google Scholar]
- HoriuchiMMakuuchiHMachimuraTTamuraYSakaiM1998Survival benefit of screening for early oesophageal carcinoma in head and neck cancer patients Dig Endosc 10110115 [Google Scholar]
- InaHShibuyaHOhashiIKitagawaM1994The frequency of a concomitant early oesophageal cancer in male patients with oral and oropharyngeal cancer Cancer 7320382041 [DOI] [PubMed] [Google Scholar]
- LeonXQuerMDiezSOrusCLopez-PousaABurguesJ1999Second neoplasm in patients with head and neck cancer Head Neck 21204210 [DOI] [PubMed] [Google Scholar]
- Leoni-ParvexSMihaescuAPellandaAMonnierPBosmanF2000Esophageal cytology in the follow-up of patients with treated upper aerodigestive tract malignancies Cancer (Cancer Cytopathol) 901016 [PubMed] [Google Scholar]
- LewinKAppelmanH1995Tumors of the oesophagus and stomachInAtlas of tumor pathologyRosai J, Sobin L (eds)pp4398Washington: AFIP [Google Scholar]
- MakuuchiH1996Endoscopic mucosal resection for early oesophageal cancer. Indications and techniques Dig Endosc 8175179 [Google Scholar]
- MakuuchiHMachimuraTShimadaHMizutaniKChinoOKiseYNishiTTanakaHMitomiTHoriuchiMSakaiMGotohJSasakiJOsamuraY1996Endoscopic screening for oesophageal cancer in 788 patients with head and neck cancers Tokai Exp Clin Med 21139145 [PubMed] [Google Scholar]
- MandardAMHainautPHollsteinM2000Genetic steps in the development of squamous cell carcinoma of the oesophagus Mutation Research 462335342 [DOI] [PubMed] [Google Scholar]
- MeyerVBurtinPBourBBlanchiACalesPObertiFPersonBCroueADohnSBenoitRFabianiBBoyerJ1997Endoscopic detection of early oesophageal cancer in a high-risk population: does Lugol staining improve videoendoscopy? Gastrointest Endosc 45480484 [DOI] [PubMed] [Google Scholar]
- MoriMAdachiYMatsushimaTMatsudaHKuwanoHSugimachi1993Lugol staining pattern and histology of oesophageal lesions Am J Gastroenterol 88701705 [PubMed] [Google Scholar]
- PetitTGeorgesCJungGBorelCBronnerGFleschHMassardGVeltenMHaegelePSchraubS2001Systematic esophageal endoscopy screening in patients previously treated for head and neck squamous-cell carcinoma Ann Oncology 12643646 [DOI] [PubMed] [Google Scholar]
- SavaryJGrosjeanPMonnierPFontollietCWagnieresGBraichotteDvan den BerghH1998Photodynamic therapy of early squamous cell carcinomas of the esophagus: A review of 31 cases Endoscopy 30258265 [DOI] [PubMed] [Google Scholar]
- SchlemperRBorchardFDixonMKoikeMMuellerJStolteMWatanabeH2000aInternational comparability of the pathological diagnosis for early cancer of the digestive tract. Munich meeting J Gastroenterology 35Suppl102110 [PubMed] [Google Scholar]
- SchlemperRDawseySItabashiMIwashitaAKatoYKoikeMLewinKRiddellRShimodaTSipponenPStolteMWatanabeH2000bDifferences in diagnostic criteria for oesophageal squamous cell carcinoma between Japanese and Western pathologists Cancer 889961006 [DOI] [PubMed] [Google Scholar]
- SchlemperRRiddellRKatoYBorchardFCooperHDawseySDixonMFenoglio-PreiserCFlejouJGeboesHHattoriTHirotaTItabashiMIwafuchiAKimYKirchnerTKlimpfingerMKoikeMLauwersGLewinKOberhuberGOffnerFPriceARubioCShimizuMShimodaTSipponenPSolciaEStolteMWatanabeHYamabeH2000cThe Vienna classification of gastrointestinal epithelial neoplasia Gut 47251255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShiozakiHTaharaHKobayashiKYanoHTamuraSImamotoHYanoTOkuKMiyataMNishiyamaK1990Endoscopic screening of early oesophageal cancer with the Lugol dye method in patients with head and neck cancers Cancer 6620682071 [DOI] [PubMed] [Google Scholar]
- SturgisEMillerR1995Second primary malignancies in the head and neck cancer patient Ann Rhinol Otol Laryngol 104946954 [DOI] [PubMed] [Google Scholar]
- SugimachiKOhnoSMatsudaHMoriMKuwanoH1988Lugol-combined endoscopy detection of minute malignant lesions of the thoracic oesophagus Ann Surg 208179183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TincaniABrandaliseNAltemaniAScanaviniRValerioJLageHMolinaGMartinsA2000Diagnosis of superficial oesophageal cancer and dysplasia using endoscopic screening with a 2% Lugol dye solution in patients with head and neck cancer Head Neck 22170174 [DOI] [PubMed] [Google Scholar]
- WuYKHuangGJShaoLFZhangYDLinXS1982Honored guest address: progress in the study and surgical treatment of cancer of the oesophagus in China, 1940–1980 J Thorac Cardiovasc Surg 843033 [PubMed] [Google Scholar]
- ZieglerKSanftCZeitzMFriedrichMSteinHHäringRRieckenE-O1991Evaluation of endosonography in TN staging of ooesophageal cancer Gut 321620 [DOI] [PMC free article] [PubMed] [Google Scholar]


