Abstract
PTEN is a candidate tumour suppressor gene and frequently mutated in multiple cancers, however, not in pancreatic cancer. Recently, it has been demonstrated that PTEN expression is regulated by TGF-β1. Using TGF-β1 transgenic mice (n=7) and wildtype littermates (n=6), as well as pancreatic tissues obtained from organ donors (n=10) and patients with pancreatic cancer (n=10), we assessed the expression of PTEN by means of immunohistochemistry and semiquantitative PCR analysis. In addition, PANC-1 cells were treated with TGF-β1 in vitro and the levels of PTEN mRNA were determined in these cells. In human pancreatic cancers PTEN mRNA levels were significantly decreased (P<0.05). In addition, in the pancreas of TGF-β1 transgenic mice the expression of PTEN was significantly reduced (P<0.01), as compared to wildtype littermates and incubation of PANC-1 cells with TGF-β1 decreased PTEN mRNA levels after 24 h. Inasmuch as TGF-β1 decreases PTEN expression in human pancreatic cancer cells and human pancreatic cancers overexpress TGF-β1, the reduced expression of PTEN in pancreatic cancer may be mediated by TGF-β1 overexpression. Thus, although PTEN is not mutated in pancreatic cancers, the reduction of its expression may give pancreatic cancer cells an additional growth advantage.
British Journal of Cancer (2002) 86, 257–262. DOI: 10.1038/sj/bjc/6600031 www.bjcancer.com
© 2002 The Cancer Research Campaign
Keywords: pancreas, TGF beta, transformation, tumour, MMAC1
Pancreatic cancer is an aggressive cancer with an increasing incidence and poor survival (Warshaw and Fernandez-Del Castillo, 1992; Hahn and Schmiegel, 1998). Less than 5% of patients with pancreatic adenocarcinoma survive more than 5 years. The reason for this poor survival is, among others, the presence of advanced stages, the poor therapeutic options and the current incomplete knowledge concerning the pathogenesis and biology of the tumour (Warshaw and Fernandez-Del Castillo, 1992; Hahn and Schmiegel, 1998). Recently, the molecular biology and the pathogenesis of this cancer has been studied extensively, leading to the identification of K-ras mutations or the frequent inactivation of tumour suppressor genes, such as p53, p16 or Smad4 (Warshaw and Fernandez-Del Castillo, 1992; Hahn and Schmiegel, 1998). However, the exact molecular mechanisms of its pathogenesis remain largely unknown. We and others have reported the frequent overexpression of oncogenes and growth factors, such as EGF, FGF, VEGF and PDGF in pancreatic cancers along with the overexpression of their respective receptors (Korc et al, 1992; Ebert et al, 1995; Kornmann et al, 1997; Fujimoto et al, 1998). Furthermore, growth factors of the TGF-β superfamily are also overexpressed in this tumour and TGF-β1 overexpression is associated with poor survival in these patients (Friess et al, 1993). Although, TGF-β1 exerts an inhibitory effect on cancer cell growth in vitro, it also stimulates the synthesis of the extracellular matrix and has been implicated in the regulation of cell differentiation, angiogenesis, immunosuppression and fibrosis (Massagué, 1996; Korc, 1998; Löhr et al, 2001).
Recently, PTEN, a tumour suppressor gene which is located on chromosome 10q23, was identified by positional cloning (Steck et al, 1997; Li et al, 1997b). Several groups have reported loss of heterozygosity, mutation or deletion of the gene in different cancers, including glioblastoma, prostate, lung and breast carcinoma and altered expression of PTEN was also detected in various precancerous lesions (Steck et al, 1997; Li et al, 1997b; McMenamin et al, 1999; Perren et al, 1999; Sano et al, 1999; Mutter et al, 2000). PTEN has homology to a protein tyrosine phosphatase and has the activity of a dual-specificity phosphatase (Myers et al, 1997). In addition, recent studies indicate that PTEN may function as a mediator of the phosphatidylinositol (PI3′) kinase pathway (Maehama and Dixon, 1998). These findings, along with the description of germline mutations and deletions of PTEN in two hereditary cancer predisposition diseases (Liaw et al, 1997; Marsh et al, 1997; Nelen et al, 1997), i.e. Cowden Disease and the Bannayan-Zonana-syndrome, point to a role of PTEN as a tumour suppressor gene in the pathogenesis of malignant tumours. However, PTEN mutations or deletions do not seem to be present in pancreatic cancer (Sakurada et al, 1997; Okami et al, 1998). Since PTEN expression is regulated by TGF-β1 (Li et al, 1997a), we assessed PTEN mRNA levels in human pancreatic cancers by RT–PCR analysis and immunohistochemistry. In addition, we determined the mRNA levels of PTEN in a model of TGF-β1 overexpressing transgenic mice which develop pancreatic fibrosis and studied the mRNA levels of PTEN in a pancreatic cancer cell line following incubation with TGF-β1.
MATERIALS AND METHODS
The following products were purchased: Taq polymerase from Gibco-BRL (Eggenstein, Germany). Oligonucleotides were purchased from MWG-Biotech. (Ebersberg, Germany). All other chemicals and reagents were of molecular biology grade and were purchased from Sigma Chemical (Deisenhofen, Germany).
Transgenic mice
Transforming growth factor beta-1 overexpressing transgenic mice were generated by the introduction of the rat insulin II gene promotor fused to the murine TGF-β1 cDNA. The fusion fragment was inserted in the EcoRI site of pBS containing the human growth hormone (Sanvito et al, 1995). Seven transgenic animals were used for this study; control animals were of the BL6 strain. Transgenesis was determined on tail DNA by dot blot or Southern hybridization. All animals were maintained on laboratory chow and tap water. Animals were housed and treated in accordance with appropriate guidelines. After 3–9 months animals were sacrificed, the pancreas was removed and tissues were snap frozen in liquid nitrogen. In all transgenic mice the pancreas exhibited massive fibrosis, as previously described (Sanvito et al, 1995).
Tissue samples
Pancreatic cancer tissues (six female, four male) were obtained from patients undergoing pancreatic surgery. Normal pancreatic tissues were obtained from 10 individuals (five female) through an organ donor programme. The median age of the patients with pancreatic cancer was 61.5 years (range, 48–73 years). The median age of the organ donors was 42 years (range, 37–47 years). Immediately following surgical removal, all tissue samples were either fixed in Bouin's solution or frozen in liquid nitrogen. All cancer tissue samples were graded independently by a pathologist, and classified histologically as adenocarcinoma of the exocrine pancreas. This study was approved by the Ethics Committee of the University of Berne, Switzerland.
Cell line
PANC-1 cells were cultured in DMEM medium (Gibco-BRL, Gaithersburg, MA, USA) supplemented with 10% foetal calf serum (FCS, Gibco-BRL), penicillin (100 U ml−1), and streptomycin (100 U ml−1) at 37°C in 5% CO2 atmosphere. To analyze the effect of TGF-β1 on cell morphology pancreatic cancer cell lines were grown to 70% confluence in DMEM containing 10% FCS. Afterwards, cells were washed twice in serum free medium, starved for 24 h in serum free medium and finally treated for 12 and 24 h with 10 ng ml−1 TGF-β1 (R&D, Wiesbaden, Germany) or medium alone (Geng et al, 1999). Thereafter total RNA was extracted using the RNAclean kit (AGS, Heidelberg, Germany) as indicated by the manufacturer.
RNA extraction
Total RNA was extracted from the human pancreatic tissues and the pancreas of the transgenic mice by the acid guanidinium-thiocyanate method, as previously described (Ebert et al, 1994).
Polymerase chain reaction
Oligonucleotide primers were purchased from MWG-Biotech. (Ebersberg, Germany). cDNAs were synthesized from total RNA (1 μg sample−1) isolated from human and mouse pancreatic tissues and the cancer cell line, using oligodeoxythymidylate and reverse transcriptase. Following inactivation, 1 μl of the reaction mixture were incubated in buffer containing 0.2 mM concentrations of dATP, dCTP, dGTP, dTTP, 0.2 μM concentrations each of oligonucleotide primers, 3 mM MgCl2 and a 10× buffer consisting of 200 mM Tris-HCl (pH 8.0), 500 mM KCl, and 1 U Taq polymerase. PCR primers were designed to amplify a region spanning from 117 to 741 of the PTEN gene (F1, 5′-CAGAAAGACTTGAAGGCGTAT-3′ and B1, 5′-AACGGCTGAGGGAACTC-3′), as previously described (Sano et al, 1999). The 624 bp fragment contains a NsiI restriction site allowing to distinguish it from the PTEN pseudogene (Sano et al, 1999). The primers designed to amplify enolase comprise a region from 77 to 532 bp of the coding region of the enolase gene (E1, 5′-TGGCAGGACTTCAGA-3′; E2, 5′-AGTGGCTAGAAGTTCACC-3′). For semiquantitative analysis of PTEN mRNA levels in the pancreas of transgenic and wildtype mice, the primer F1 was used in conjunction with a different antisense primer (H1, 5′-TCTAGGGCCTCTTGTGCCTTT-3′) for the amplification of a 622 bp fragment of mouse PTEN (Sano et al, 1999). Again, digestion with NsiI allowed differentiation from the PTEN pseudogene (Sano et al, 1999). In addition, primers specific for rat glyceraldehyde-3-phosphate dehydrogenase (GAP) mRNA (G1,G2) were also added to the reaction in order to assess the PTEN mRNA levels in the murine pancreas semiquantitatively. Primers were chosen as previously reported (Ebert et al, 1999): G1, 5′-GCTGGATCCTTCATTGACCTCAACTAG-3′; G2, 5′-CGAGAATTCATACCAGGAAATGAGC-3′. PCR amplification was performed after an initial denaturation of 3 min at 94°C, followed by 40 cycles of 45 s at 94°C, 45 s at 54°C and 1 min at 72°C, and finally 10 min of final elongation at 72°C. The PCR products were treated with NsiI at 37°C for 2 h and then separated on 1.5% agarose gel (Ebert et al, 1994). The level of PTEN mRNA was analyzed densitometrically from the agarose gel and was standardized to the respective enolase or GAP mRNA level. The PTEN:enolase or PTEN:GAP ratio was calculated and was analyzed by the t-test (Siegel, 1956).
Immunohistochemistry
The presence of human PTEN was assessed using paraffin-embedded tissue sections obtained from 10 patients with pancreatic cancer undergoing pancreatic surgery. The human tissues were fixed in Bouin's solution and paraffin embedded. The anti-PTEN antibody (n-19) is an affinity-purified goat polyclonal antibody raised against a peptide mapping at the amino-terminus of human PTEN and was used at a dilution of 1:800 (Santa Cruz, CA, USA) (Steck et al, 1997; Sano et al, 1999). In addition, sections were also incubated with the anti-PTEN antibody C-20, which is a goat polyclonal antibody raised against a peptide mapping at the carboxy-terminus of human PTEN (Santa Cruz, CA, USA). Paraffin sections (4 μm thick) were deparaffinized and rehydrated. For negative controls, the primary antibodies were omitted and/or preimmune serum was used. Furthermore, the anti-PTEN antibody n-19 was also incubated with its respective blocking peptide which resulted in no specific immunoreactivity in the immunohistochemical analysis, demonstrating the specificity of the anti-PTEN antibody (Figure 1). Endogenous peroxidase activity was inhibited by immersing the sections in 0.3% H2O2 for 30 min. The sections were incubated with the antiserum at 37°C for 1 h and washed with PBS buffer. The reaction was detected using the standard streptavidin-peroxidase technique (LSAB kit, DAKO Hamburg, Germany). The analysis was performed according to the manufacturer's recommendations and all reactions were performed at 23°C. Finally, the sections were counterstained with Mayer's hematoxylin (Ebert et al, 1994).
Figure 1.
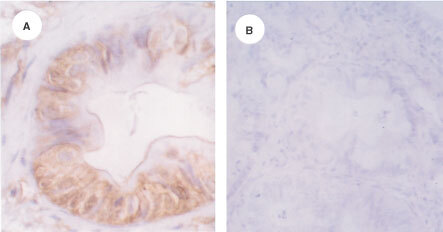
Immunohistochemical analysis of PTEN expression in pancreatic cancer. (A) In human pancreatic cancers PTEN immunoreactivity was present in some of the cancer cells. (B) Sections incubated with the anti-PTEN antibody and the blocking peptide exhibited no PTEN immunoreactivity.
Statistical analysis
The t-test was used to determine statistical difference. A P value of less than 0.05 was considered statistically significant (Siegel, 1956).
RESULTS
In human pancreatic cancers RT–PCR analysis revealed the presence of PTEN mRNA in all samples (Figure 2A). PTEN immunoreactivity was also detected in some of the cancer cells (Figure 1A), whereas some cancer cells were devoid of PTEN immunoreactivity. Immunohistochemical analysis of pancreatic cancer tissue sections after incubation of the primary antibody with the blocking peptide exhibited no specific immunoreactivity (Figure 1B).
Figure 2.
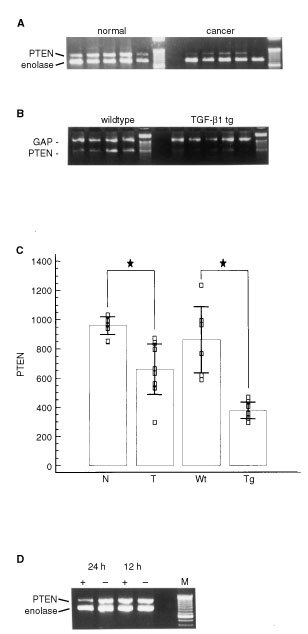
RT–PCR analysis of PTEN mRNA levels in a pancreatic cancer cell line, in human and murine pancreas. (A) Semiquantitative analysis revealed decreased expression of PTEN mRNA in pancreatic cancers (right) as compared to the normal pancreas (left). Last lane, DNA ladder. (B) In transgenic mice overexpressing TGF-β1 (TGF-β1 tg) PTEN mRNA levels were also decreased as compared to wildtype littermates (wildtype). Last lane, DNA ladder. (C) Densitometric analysis confirmed a significant reduction of PTEN mRNA levels in pancreatic tumours (T) as compared to the normal pancreas (N) and in TGF-β1 transgenic mice (Tg) as compared to wildtype mice (Wt). Mean ±s.d.; □, PTEN mRNA levels as determined by densitometric analysis and standardization against their respective enolase mRNA or GAP mRNA levels; ★, P<0.05. (D) Incubation of PANC-1 cells with TGF-β1 led to a significant reduction of PTEN mRNA levels after 24 h, however not after 12 h. +, addition of TGF-β1; −, control without agonist addition; M, DNA ladder.
In order to determine the levels of PTEN mRNA in the pancreas of individuals without pancreatic disease and in the pancreas of patients with pancreatic adenocarcinoma we coamplified cDNA fragments encoding PTEN mRNA and enolase mRNA. In comparison with the PTEN mRNA levels in the normal pancreas obtained from organ donors without malignant disease of the pancreas, we found a significant reduction of PTEN mRNA levels in the cancer tissues (P<0.01) (Figure 2A,C).
Since PTEN expression is regulated in part by TGF-β1 which is overexpressed in pancreatic cancer cells, we studied the levels of PTEN mRNA in the pancreas of transgenic mice overexpressing TGF-β1. These mice exhibit massive fibrosis of the exocrine pancreas through overexpression of TGF-β1 under the control of a rat insulin II gene promotor. Using semiquantitative RT–PCR, we determined the PTEN mRNA levels in the pancreas of seven transgenic and six wildtype mice (Figure 2B). All mice expressed PTEN mRNA and GAP mRNA in the pancreas, as determined by RT–PCR analysis of cDNAs generated from total RNA. After quantification of PTEN mRNA levels using semiquantitative RT–PCR we detected a significant reduction of PTEN mRNA levels in the pancreas of the TGF-β1 overexpressing mice as compared to the normal pancreas (P<0.05) (Figure 2B,C).
Finally, in order to demonstrate a direct effect of TGF-β1 on PTEN mRNA levels in pancreatic cancer cells in vitro, we incubated PANC-1 cancer cells with TGF-β1 (10 ng ml−1) for 12 and 24 h. Semiquantitative RT–PCR analysis was performed via coamplification of PTEN and enolase mRNA. PANC-1 cells expressed abundant PTEN mRNA (Figure 2D). Using this semiquantitative RT–PCR analysis we observed no reduction of PTEN mRNA levels after 12 h of TGF-β1 treatment (+) as compared to the untreated control (−). However, after 24 h incubation with TGF-β1 a more than 60% reduction of PTEN mRNA levels was observed in PANC-1 cancer cells (Figure 2D, left).
DISCUSSION
The tumour suppressor gene PTEN, located on chromosome 10q23, encodes a protein tyrosine phosphatase and is frequently deleted and mutated in various malignancies, including glioblastomas, breast and prostate cancer (Steck et al, 1997; Li et al, 1997b; Sano et al, 1999; Perren et al, 2000). Furthermore, previous studies have reported frequent loss of heterozygosity at chromosome 10q23 in endometrial and thyroid cancers (Simpkins et al, 1998). This gene is considered to act as a tumour suppressor gene in the pathogenesis of multiple cancers for several reasons: (i) This gene is frequently mutated in tumours arising in patients with Cowden disease and the Bannayan-Zonana syndrome, which are both characterized as hereditary syndromes leading to multiple hamartomas in the case of Cowden disease and both exhibit an increased risk of developing breast, thyroid and skin cancer (Liaw et al, 1997; Lynch et al, 1997; Marsh et al, 1997; Nelen et al, 1997). (ii) PTEN has been shown to act against phosphatidylinositol phosphates, pointing to a role of PTEN as a mediator of the phosphatidylinositol kinase pathway (Myers et al, 1997; Maehama and Dixon, 1998). (iii) Expression of PTEN is associated with growth inhibition and inhibition of transformation of epithelial cells and PTEN exerts an inhibitory effect on the regulation of cell spreading and migration. (iv) Furthermore, it has been shown to induce apoptosis in vitro and in vivo (Stambolic et al, 1998; Davies et al, 1999). In summary, the various biological aspects of PTEN function support a role of PTEN as a tumour suppressing gene in vivo and in vitro.
While we and others have identified a high frequency of growth factor overexpression in pancreatic cancer, the molecular mechanisms of growth factor mediated pancreatic tumorigenesis remain largely unknown (Korc et al, 1992; Ebert et al, 1995; Fujimoto et al, 1998; Löhr et al, 2001). TGF-β1, the most prominent and well characterized member of the large TGF-β superfamily, is highly expressed in pancreatic cancers (Friess et al, 1993). Although it exerts a growth inhibitory effect on pancreatic cancer cells in vitro, its overexpression is associated with poor survival in patients with advanced pancreatic cancer. In addition, the two other mammalian isoforms of TGF-β, i.e. TGF-β2 and TGF-β3, are also overexpressed in pancreatic cancer (Friess et al, 1993; Satoh et al, 1998) and human pancreatic cancers also express high levels of TGF-β receptor II (TβR-II). In contrast, TβR-I is not aberrantly expressed in pancreatic cancers (Korc, 1998).
The molecular mechanism of the resistance of pancreatic cancers to the growth inhibitory action of TGF-βs, however, is not fully understood. TGF-β1 overexpression may result from the decreased expression of its receptors (Korc, 1998). Furthermore, TβR-II might be inactivated by mutation which has been reported in colon and gastric cancers of the replication error positive phenotype. For instance, in the MiaPaCa-2 pancreatic cancer cell line low levels of TβR-II expression and mutation of the kinase domain have been reported (Korc, 1998). In another study, mutations of the polyadenine tract of TβR-II and mutations in the kinase domain of the TβR-II gene have been identified (Korc, 1998). In addition, the inactivation of down-stream targets of TGF-β induced signalling pathways, such as Smad4 which is mutated or deleted in a high percentage of pancreatic cancers, may lead to a subsequent induction of TGF-β1 expression in pancreatic cancers (Hahn and Schmiegel, 1998). Nonetheless, the overexpression of TGF-β1 in pancreatic cancer has an important role in the pathogenesis of this malignancy (Friess et al, 1993; Löhr et al, 2001). Through the enhancement of tumour angiogenesis, the induction of the epithelial-mesenchymal interaction, and the suppression of an adequate immune response TGF-β1 may contribute to pancreatic tumour development (Friess et al, 1993; Massagué, 1996; Löhr et al, 2001). Recently, several molecular alterations which are induced by TGF-β1 in pancreatic cancer cells in vitro have been identified. Thus, the induction of PDGFs and cyclin D1 in pancreatic cancer cells by TGF-β1 has been reported (Ebert et al, 1995; Kornmann et al, 1999). Our current study may further help to understand how this growth factor may contribute to pancreatic cancer pathogenesis, despite its growth inhibitory effect on pancreatic cancer cells in vitro. In this study we found significantly decreased levels of PTEN mRNA in pancreatic cancers. Since two reports have demonstrated that PTEN mutations or deletions are not present in pancreatic cancers (Sakurada et al, 1997; Okami et al, 1998), other molecular mechanisms must contribute to its altered expression in pancreatic cancer. In a study by Li et al (1997a) the protein tyrosine phosphatase PTEN was found to be rapidly down-regulated by TGF-β1 in the HaCaT keratinocyte cell line. The incubation of TGF-β1 at a concentration of 2 ng ml−1 to actively growing HaCaT cells was associated with a marked reduction of PTEN mRNA levels occurring within 2 h after addition of the cytokine. While PTEN expression therefore seems to be regulated in part by TGF-β1 (Li et al, 1997a), we raised the hypothesis that the overexpression of TGF-β1 in pancreatic cancers may reduce the expression of PTEN which in turn may give these cells an additional growth advantage. First, we determined the levels of PTEN mRNA in human pancreatic cancers and found a significant reduction of PTEN expression in pancreatic cancer cells. In order to demonstrate that this reduction is mediated by TGF-β1, we analyzed the expression of PTEN in a transgenic model of TGF-β1 overexpressing mice. The pancreas of these transgenic mice exhibits massive fibrosis (Sanvito et al, 1995). Our analysis revealed a reduction of PTEN mRNA levels in the pancreas of these mice as compared to wildtype littermates. To further substantiate our findings, we incubated PANC-1 pancreatic cancer cells with TGF-β1 and found a dramatic reduction of PTEN mRNA levels in these cells. Interestingly, the reduction of PTEN mRNA levels in the PANC-1 cell line was present after 24 h incubation with TGF-β1 in contrast to the report by Li et al (1997a) who found a rapid decline of PTEN mRNA levels within 2 h after addition of TGF-β1. While we only analyzed the PTEN mRNA levels 12 and 24 h after incubation of PANC-1 cells with TGF-β1, we cannot exclude the possibility that a rapid reduction of PTEN mRNA levels had taken place earlier in the pancreatic cancer cells as well, which may have been followed by a restitution of PTEN mRNA levels and a second decrease 24 h after treatment of the cells. Furthermore, the concentrations of TGF-β1 in our study and that by Li et al (1997a) were different, so that a dose-dependent effect may also play a role in these findings. Nonetheless our studies confirm that PTEN mRNA levels are regulated and controlled at least in part by TGF-β1, which supports our hypothesis that the overexpression of TGF-β1 in pancreatic cancer may contribute to reduced PTEN mRNA levels in this malignancy. Thus, in conclusion our data strongly support the hypothesis that the decreased expression of this tumour suppressor in pancreatic cancers may result from the overexpression of TGF-β1.
Besides the regulation by TGF-β1, other molecular mechanisms may also contribute to the downregulation of PTEN expression in malignant tumours. Thus, the reduced expression of PTEN may result from hypermethylation of the PTEN promotor, the enhanced degradation of the transcript or the transcriptional inactivation of the gene (Lynch et al, 1997; Whang et al, 1998). For instance, in Cowden disease, nonsense-mediated degradation of the gene was reported (Liaw et al, 1997; Lynch et al, 1997; Nelen et al, 1997). In addition, in a study using prostate cancer tissues and cell lines, the treatment of the cells with the demethylating agent 5-azadeoxycytidine led to the restoration of PTEN mRNA levels, which reflects a possible role of hypermethylation of the promotor in the regulation of PTEN transcription (Whang et al, 1998). We did not study the methylation status of the PTEN promotor in our cancer samples. In fact, our study indicates that TGF-β1 contributes to reduced PTEN mRNA levels in pancreatic cancers.
Thus, despite the fact that PTEN inactivation through PTEN mutation or deletion is infrequent in pancreatic cancer, we raise the hypothesis that TGF-β1 overexpression may lead to reduced PTEN mRNA levels in pancreatic cancers which may give these cells an additional growth advantage and, thus, contributes to the aggressive phenotype of this cancer.
Acknowledgments
This study was supported by grants from the Matthias-Lackas-Stiftung and the Land Sachsen-Anhalt awarded to MPA Ebert. L Schandl was supported by the DAAD.
References
- DaviesMAKoulDDhesiHBermanRMcDonnellTJMcConkeyDYungKSteckPA1999Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN Cancer Res 5925512556 [PubMed] [Google Scholar]
- EbertMHoffmannJHaeckelCRutkowskiKSchmidRMWagnerMAdlerGSchulzHURoessnerAHoffmannWMalfertheinerP1999Induction of TFF1 gene expression in pancreas overexpressing transforming growth factor-α Gut 45105111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EbertMYokoyamaMKobrinMSFriessHLopezMEBüchlerMWKorcM1994Induction and expression of amphiregulin in human pancreatic cancer Cancer Res 5439593962 [PubMed] [Google Scholar]
- EbertMYokoyamaMKobrinMSFriessHBüchlerMWKorcM1995Induction of platelet-derived growth factors and overexpression of their receptors in pancreatic cancers Int J Cancer 62529535 [DOI] [PubMed] [Google Scholar]
- FriessHYamanakaYBüchlerMWEbertMBegerHGGoldLIKorcM1993Enhanced expression of transforming growth factor β isoforms in pancreatic cancer correlates with decreased survival Gastroenterology 10518461856 [DOI] [PubMed] [Google Scholar]
- FujimotoKHosotaniRWadaMLeeJUKoshibaTMiyamotoYTsujiSNakajimaSDoiRImamuraM1998Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived growth factor in human pancreatic cancer, and its relationship to angiogenesis Eur J Cancer 3414391447 [DOI] [PubMed] [Google Scholar]
- GengMEllenriederVWallrappCMüller-PillaschFSommerGAdlerG1999Identification of TGF-β1 target genes in pancreatic cancer cells by cDNA representational difference analysis Genes Chromosomes Cancer 26707910441008 [Google Scholar]
- HahnSASchmiegelWH1998Recent discoveries in cancer genetics of exocrine pancreatic neoplasia Digestion 59493501 [DOI] [PubMed] [Google Scholar]
- KorcMChandrasekarBYamanakaYFriessHBüchlerMBegerHG1992Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increase in the levels of epidermal growth factor and transforming growth factor-alpha J Clin Invest 9013521360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KorcM1998Role of growth factors in pancreatic cancer Surg Oncol Clin North Am 72541 [PubMed] [Google Scholar]
- KornmannMIshiwataTBegerHGKorcM1997Fibroblast growth factor-5 stimulates mitogenic signaling and is overexpressed in human pancreatic cancer: evidence for autocrine and paracrine actions Oncogene 1514171424 [DOI] [PubMed] [Google Scholar]
- KornmannMTangvoranuntakulPKorcM1999TGF-beta-1 up-regulates cyclin D1 expression in COLO-357 cells, whereas suppression of cyclin D1 levels is associated with down-regulation of the type I TGF-beta receptor Int J Cancer 83247254 [DOI] [PubMed] [Google Scholar]
- LiDMSunH1997aTEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta Cancer Res 5721242129 [PubMed] [Google Scholar]
- LiJYenCLiawDPodsypaninaKBoseSWangSIPucJMiliaresisCRodgersLMcCombieRBignerSHGiovanellaBCIttmannMTyckoBHibshooshHWiglerMHParsonsR1997bPTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer Science 27519431947 [DOI] [PubMed] [Google Scholar]
- LöhrMSchmidtCRingelJKluthMMullerPNizzeHJesnowskiR2001Transforming growth factor beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma Cancer Res 61550555 [PubMed] [Google Scholar]
- LiawDMarshDJLiJDahiaPLWangSIZhengZBoseSCallKMTsouHCPeacockeMEngCParsonsR1997Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome Nat Genet 166474 [DOI] [PubMed] [Google Scholar]
- LynchEDOstermyerEALeeMKArenaJFJiHDannJSwisshelmKSuchardDMacLeodPMKvinnslandSGjertsenBTHeimdalKLubsHMollerPKingMC1997Inherited mutation in PTEN that are associated with breast cancer, Cowden's disease, and juvenile polyposis Am J Genet 6112541260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MaehamaTDixonJE1998The tumour suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger phosphatidylinositol 3,4,5-triphosphate J Biol Chem 2731337513378 [DOI] [PubMed] [Google Scholar]
- MarshDJDahiaPLZhengZLiawDParsonsRGorlinRJEngC1997Germline mutations in PTEN are present in Bannayan-Zonana syndrome Nat Genet 16333334 [DOI] [PubMed] [Google Scholar]
- MassaguéJ1996TGF-beta signaling: Receptors, transducers, and Mad proteins Cell 85947950 [DOI] [PubMed] [Google Scholar]
- McMenaminMESoungPPereraSKaplanILodaMSelersWR1999Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage Cancer Res 5942914296 [PubMed] [Google Scholar]
- MutterGLLinMCFitzgeraldJTKumJBBaakJPLeesJAWenigLPEngC2000Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers J Natl Cancer Inst 92924930 [DOI] [PubMed] [Google Scholar]
- MyersMPStolarovJPEngCLiJWangSIWiglerMHPersonsRTonksNK1997P-TEN, the tumor suppressor from human chromosome 10q23, is a dual specificity phosphatase Proc Natl Acad Sci USA 9490529057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NelenMRvan StaverenWCPeetersEAHasselMBGorlinRJHammHLindboeCFFrynsJPSijmonsRHWoodsDGMarimanECPadbergGWKremerH1997Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease Hum Mol Genet 613831387 [DOI] [PubMed] [Google Scholar]
- OkamiKWuLRigginsGCairnsPGogginsMEvronEHalachmiNAhrendtSAReedALHilgersWKernSEKochWMSidranskyDJenJ1998Analysis of PTEN/MMAC1 alterations in aerodigestive tract tumors Cancer Res 58509511 [PubMed] [Google Scholar]
- PerrenAKomminothPSaremaslaniPMatterCFeurerSLeesJAHeitzPUEngC2000Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells Am J Pathol 15710971103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PerrenAWenigLPBoagAHZieboldUThakoreKDahiaPLKomminothPLeesJAMulliganLMMutterGLEngC1999Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast Am J Pathol 15512531260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SakuradaASuzukiASatoMYamakawaHOrikasaKUyenoSOnoTOhuchiNFujimuraSHoriiA1997Infrequent genetic alterations of the PTEN/MMAC1 gene in Japanese patients with primary cancers of the breast, lung, pancreas, kidney, and ovary Jpn J Cancer Res 8810251028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanoTLinHChenXLangfordLAKoulDBondyMLHessKRMyersJNHongYKYungWKSteckPA1999Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis Cancer Res 5918201824 [PubMed] [Google Scholar]
- SanvitoFNicholsAHerreraPLHuarteJWohlwendAVassalliJDOrciL1995TGF-β1 overexpression in murine pancreas induces chronic pancreatitis and, together with TNF-α, triggers insulin-dependent diabetes Biochem Biophys Res Commun 21712791286 [DOI] [PubMed] [Google Scholar]
- SatohKShimosegawaTHirotaMKoizumiMToyotaT1998Expression of transforming growth factor beta1 (TGFbeta1) and its receptors in pancreatic duct cell carcinoma and in chronic pancreatitis Pancreas 16468474 [DOI] [PubMed] [Google Scholar]
- SiegelS1956Nonparametric statistics for behavioral sciences.New York: McGraw-Hill [Google Scholar]
- SimpkinsSBPeiffer-SchneiderSMutchDGGersellDGoodfellowPJ1998PTEN mutations in endometrial cancers with 10q LOH: additional evidence for the involvement of multiple tumor suppressors Gynecol Oncol 71391395 [DOI] [PubMed] [Google Scholar]
- StambolicVSuzukiAde la PompaJLBrothersGMMirtsosCSasakiTRulandJPenningerJMSiderovskiDPMakTW1998Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN Cell 952939 [DOI] [PubMed] [Google Scholar]
- SteckPAPershouseMAJasserSAYungWKLinHLigonAHLangfordLABaumgardMLHattierTDavisTFryeCHuRSwedlundBTengDHTavtigianSV1997Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15356362 [DOI] [PubMed] [Google Scholar]
- WarshawALFernandez-Del CastilloC1992Pancreatic carcinoma N Engl J Med 326455465 [DOI] [PubMed] [Google Scholar]
- WhangYEWuXSuzukiHReiterRETranCVessellaRLSaidJWIsaacsWBSawyersCL1998Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression Proc Natl Acad Sci USA 9552465250 [DOI] [PMC free article] [PubMed] [Google Scholar]


