Abstract
Diets high in fat are associated with an increased risk of prostate cancer, although the molecular mechanism is still unknown. We have previously reported that arachidonic acid, an omega-6 fatty acid common in the Western diet, stimulates proliferation of prostate cancer cells through production of the 5-lipoxygenase metabolite, 5-HETE (5-hydroxyeicosatetraenoic acid). We now show that 5-HETE is also a potent survival factor for human prostate cancer cells. These cells constitutively produce 5-HETE in serum-free medium with no added stimulus. Exogenous arachidonate markedly increases the production of 5-HETE. Inhibition of 5-lipoxygenase by MK886 completely blocks 5-HETE production and induces massive apoptosis in both hormone-responsive (LNCaP) and -nonresponsive (PC3) human prostate cancer cells. This cell death is very rapid: cells treated with MK886 showed mitochondrial permeability transition between 30 and 60 min, externalization of phosphatidylserine within 2 hr, and degradation of DNA to nucleosomal subunits beginning within 2–4 hr posttreatment. Cell death was effectively blocked by the thiol antioxidant, N-acetyl-l-cysteine, but not by androgen, a powerful survival factor for prostate cancer cells. Apoptosis was specific for 5-lipoxygenase—programmed cell death was not observed with inhibitors of 12-lipoxygenase, cyclooxygenase, or cytochrome P450 pathways of arachidonic acid metabolism. Exogenous 5-HETE protects these cells from apoptosis induced by 5-lipoxygenase inhibitors, confirming a critical role of 5-lipoxygenase activity in the survival of these cells. These findings provide a possible molecular mechanism by which dietary fat may influence the progression of prostate cancer.
Cancer of the prostate is the most commonly diagnosed malignancy among men in the United States and Europe, killing thousands every year (1). Metastatic prostate cancer responds initially to androgen withdrawal therapy, but hormone resistance always develops (2). Chemotherapeutic agents currently available have little or no impact on the survival of the patients with hormone-refractory prostate cancer. For this reason, metastatic prostate cancer almost always has a fatal outcome. Although the incidence of the localized, latent form of prostate cancer is the same globally regardless of ethnic origin, there is significant variation in the occurrence of metastatic disease between Western countries and Eastern countries, suggesting involvement of environmental factors in metastatic progression. The underlying molecular mechanism involved in the progression phase of the disease is an active area of current research.
Epidemiological studies and experiments with animal models have repeatedly suggested a link between fat content in the diet and the risk of metastatic prostate cancer (3–6). In addition, there are now multiple studies on prostate cancer cell lines, suggesting a role for arachidonic acid and its precursor, linoleic acid, in prostate cancer growth and metastasis (7–9). Recently, we have documented that arachidonic acid can directly stimulate in vitro growth of both hormone-responsive and -nonresponsive human prostate cancer cells, which suggests a causal link between dietary fat and prostate cancer progression (10). Despite these reports, the underlying molecular mechanism by which arachidonic acid may contribute to the progression of advanced prostate cancer remains obscure.
Arachidonic acid can be metabolized to produce a host of proinflammatory substances, called eicosanoids, that act as potent autocrine and paracrine regulators of cell biology (11). These substances are known to modulate diverse physiologic and pathologic responses, including growth and invasiveness of tumor cells as well as suppression of immune surveillance (12–14). Release of arachidonic acid and formation of eicosanoids have also been implicated in the action of a number of cytokines, including epidermal growth factor, platelet derived growth factor, and bombesin (15–18). The specific eicosanoid responsible for mitogenesis varies with the cytokine and the cell lineage involved, and has included prostaglandin E2 (PGE2) as well as several lipoxygenase products.
In addition to their role in regulating mitogenesis, various eicosanoids can either trigger or block apoptosis. As with mitogenesis, the specific eicosanoid involved in triggering or blocking apoptosis is cell lineage-dependent. For example, synthesis of PGE2 plays a central role in the apoptosis required for egg release during ovulation (19). In contrast, PGE2 blocks activation-induced apoptosis in CD4+/CD8+ T lymphocytes (20). In addition, both FAS and tumor necrosis factor receptor activation are associated with arachidonic acid release and eicosanoid formation in certain cell lineages (21). A recent paper showed that arachidonic acid suppresses ceramide-induced cell death in prostate cancer cells and that this suppression depends on formation of lipoxygenase products (22). Previously, we have demonstrated that arachidonic acid stimulates mitogenesis of human prostate cancer cells in vitro. This mitogenesis is blocked if further metabolism of arachidonic acid through 5-lipoxygenase is interrupted. Moreover, MK886, a specific inhibitor of 5-lipoxygenase, not only blocked the growth stimulation by arachidonic acid but, at a concentration of 10 μM, killed more than 90% of the cells in culture (10).
Based on this background information, we investigated the role of arachidonic acid metabolism in the survival of human prostate cancer cells. We report here that prostate cancer cells constitutively produce 5-HETE (5-hydroxyeicosatetraenoic acid), a product of arachidonate 5-lipoxygenase, in serum-free medium with no external stimulus. Moreover, 5-HETE production is dramatically increased following addition of exogenous arachidonic acid. Inhibition of 5-lipoxygenase blocks 5-HETE production and induces massive apoptosis in both hormone-responsive and -nonresponsive human prostate cancer cells. Finally, this apoptosis is prevented by simultaneous addition of the 5-HETE series of arachidonic acid metabolites, indicating a critical role of these metabolites in the survival of human prostate cancer cells.
MATERIALS AND METHODS
Cell Culture and Reagents.
PC3 and LNCaP human prostate cancer cells (both from American Type Culture Collection) were cultured in RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS) plus 100 μg/ml streptomycin and 100 units/ml penicillin. Cells were fed with fresh medium every third day and split at a confluence of ≈80%. Fatty acids and eicosanoids were bought either from Cayman Chemicals (Ann Arbor, MI) or Cascade Biochem Limited (Berkshire, U.K.). Inhibitors of eicosanoid biosynthesis were obtained from Biomol (Plymouth Meeting, PA). Rhodamine-123, Mitotracker Red, and Hoechst 33342 were purchased from Molecular Probes.
Membrane Morphology.
Prostate cancer cells (3 × 105) were plated in 60-mm tissue culture dishes (Falcon) in RPMI medium 1640 supplemented with 10% FBS and allowed to grow for 48 hr in a CO2 incubator. On the day of the experiment, the spent medium was replaced with fresh, serum-free RPMI medium 1640 and the cells were treated with 10 μM MK886 for various periods of time. Cellular morphology was observed by using a Zeiss Axioskop microscope at ×20. Photomicrographs were taken with Kodak 200 film.
Mitochondrial Permeability Transition (MPT).
Prostate cancer cells were plated on 22-mm glass coverslips and allowed to grow for 48 hr. On the day of the experiment, coverslips were placed in 6-well tissue culture plates in 1 ml of RPMI medium 1640, loaded with 5 μM Rhodamine-123, and incubated for 30 min at 37°C. Then the cells were washed and treated with 10 μM MK886 for 45 min at 37°C in serum-free RPMI medium 1640. At the end of the incubation periods the cells were washed, mounted on glass slides, and observed by fluorescent microscopy using a fluorescein short-pass filter set. Images were acquired by using a Zeiss Axioskop with ×63 Planapochromat oil-immersion objective equipped with a Sensys charge-coupled device camera (Photometrics, Tucson, AZ). Image analysis was performed by using iplab spectrum software (Signal Analytics, Vienna, VA). The acquired images were deconvoluted by using microtome software from VayTek, Inc., (Fairfield, IA) to eliminate background haze originating from out-of-focus planes.
Externalization of Phosphatidylserine.
Cells were cultured for 48 hr in RPMI medium 1640 supplemented with 10% FBS. The medium was then replaced with fresh serum-free RPMI medium 1640, and the cells were treated with 10 μM MK886 for various periods of time. A set of controls was run in which cells were treated with the plating medium containing solvent vehicle [0.02% dimethyl sulfoxide (DMSO)]. At the end of each incubation period the cells were washed and stained for fluorescent microscope analysis by an Apoptosis Detection Kit (R & D Systems), which is based on cell-membrane phospholipid asymmetry and annexin V binding. The assay method was supplied by the manufacturer.
Cell Death ELISA.
Programmed cell death was also monitored quantitatively by determining the formation of nucleosomes associated with apoptosis-induced cleavage of internucleosomal linker DNA. Cells were cultured in 60-mm tissue culture dishes (Falcon) for 48 hr and then treated either with the experimental agents or solvent vehicles for various periods of time. At the end of each incubation period the cells were harvested and lysed, and the formation of mono- and oligonucleosomes (23, 24) was studied by Cell Death Detection ELISA following instructions supplied by the manufacturer (Boehringer Mannheim).
Radioimmunoassay of 5-HETE.
Prostate cancer cells (≈106) were plated overnight in 60-mm tissue culture dishes (Falcon) in serum-free RPMI medium 1640. On day 2, experimental (MK886 and/or arachidonic acid) and control agents were added, and the plates were further incubated for various periods of time, as indicated in individual experiments, at 37°C in the CO2 incubator. MK886 was dissolved in DMSO and further diluted with serum-free RPMI medium 1640 to achieve desired concentrations. Arachidonic acid was precomplexed with lipid-free BSA before addition to the culture. Control cells were treated with the plating medium containing 0.02% DMSO and 5 μM lipid-free BSA. At the end of the incubation periods, aliquots of the culture supernatants were taken, and the amounts of 5-HETE measured by radioimmunoassay following the manufacturer’s instructions (PerSeptive Biosystems, Framingham, MA).
RESULTS
Apoptosis Rapidly Follows Inhibition of 5-Lipoxygenase.
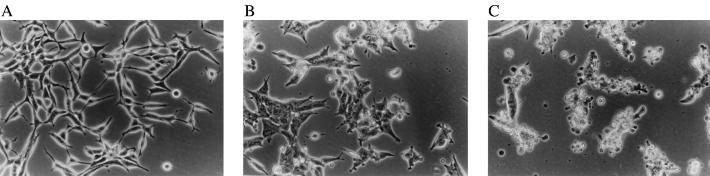
We observed that human prostate cancer cells treated with MK886, a specific inhibitor of arachidonate 5-lipoxygenase (25), showed pronounced alteration of membrane morphology characterized by the formation of membrane blebs, which was noticed as early as 1–2 hr and culminated at 6–8 hr posttreatment (Fig. 1). The morphological alterations were consistent with programmed cell death (apoptosis). Both hormone-responsive (LNCaP) and -nonresponsive (PC3) cells were equally affected by this inhibition. Well spread adherent cells were observed to withdraw their processes, become round, and eventually completely detach from the tissue culture plates. Similar effects were observed with MK591 (26) and AA861 (27), other specific inhibitors of 5-lipoxygenase activity.
Figure 1.
Photomicrographs showing the morphology of LNCaP prostate cancer cells. LNCaP prostate cancer cells (3 × 105) were grown for 48 hr in 60-mm tissue culture plates in RPMI medium 1640 supplemented with 10% FBS. The spent medium was then replaced with fresh serum-free RPMI medium 1640, and the cells were treated with 10 μM MK886 for various periods of time. Control cells were treated with the untreated medium containing 0.02% DMSO. Photographs were taken with a Zeiss inverted microscope at ×20. (A) Control cells. (B and C) Cells 4 and 8 hr, respectively, after treatment with MK886.
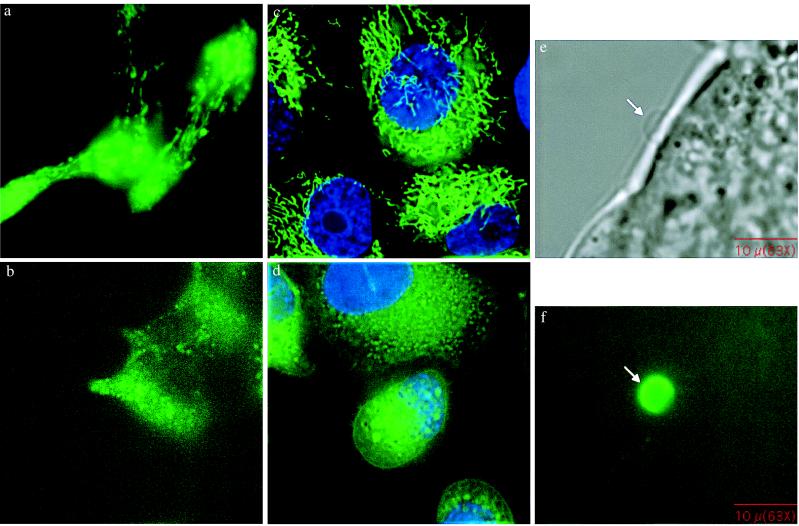
Prostatic epithelial cells synthesize large amounts of citrate that is then secreted into the semen. Perhaps to support citrate synthesis, prostatic epithelial cells are rich in mitochondria. For this reason, we examined the mitochondrial content of both LNCaP and PC3 cells by using Mitotracker Red (Molecular Probes) and found that both cell lines are rich in mitochondria. In some forms of apoptosis, a key element is the loss of mitochondrial membrane permeability followed by leakage of cytochrome C and apoptosis initiating factor into the cytosol, which in turn activates the cell-death proteases (caspases), especially caspase 3. Therefore, we next tested whether loss of mitochondrial membrane potential occurred during MK886-induced apoptosis. In these experiments, the status of mitochondrial membrane potential was assessed by using Rhodamine 123, a dye selectively retained by mitochondria in proportion to their membrane potential. The results (Fig. 2) indicate that the loss of mitochondrial membrane potential occurs between 30 min and 1 hr after MK886 addition. Thus, loss of mitochondrial membrane potential is an early, dramatic event associated with MK886-induced cell death.
Figure 2.
(a–d) Fluorescent microscope images showing MPT. LNCaP and PC3 cells (3 × 105 per well) were plated on cover glasses in 6-well tissue culture plates in RPMI medium 1640 supplemented with 10% FBS and cultured for 48 hr. The cover glasses were then placed in 6-well plates in 1 ml of RPMI medium 1640 and loaded with 5 μM Rhodamine-123 for 30 min. The cells were then treated with 10 μM MK886, incubated for 45 min at 37°C, and observed by fluorescent microscopy. (a and b) LNCaP cells, (c and d) PC3 cells. (a and c) Control. (b) and (d) MK886-treated. (e) and (f) Fluorescent microscope images showing phosphatidylserine externalization. LNCaP prostate cancer cells were plated as described and treated with 10 μM MK886 in serum-free medium for 3 hr. At the end of each incubation period, cells were washed and treated with annexin V-fluorescein isothiocyanate in binding buffer for 15 min at room temperature. The cover glasses were then mounted on glass slides and observed by fluorescent microscopy using fluorescein long pass filter set. e and f represent images of a bleb under phase-contrast and fluorescent microscope, respectively.
The phase-contrast images in Fig. 1 show that bleb formation is characteristic of MK886-induced apoptosis. Bleb formation begins 1–2 hr after drug addition, and thus follows the loss of mitochondrial membrane potential. Apoptosis-associated bleb formation is characterized by cleavage of cortical cytoskeleton followed by externalization of phosphatidylserine at the site of bleb formation. Externalization of phophatidylserine can be assessed by its high-affinity binding with annexin V. As shown in Fig. 2, the blebs that form after addition of MK886 bind selectively to Annexin V–fluorescein isothiocyanate, confirming externalization of phosphatidylserine at those sites.
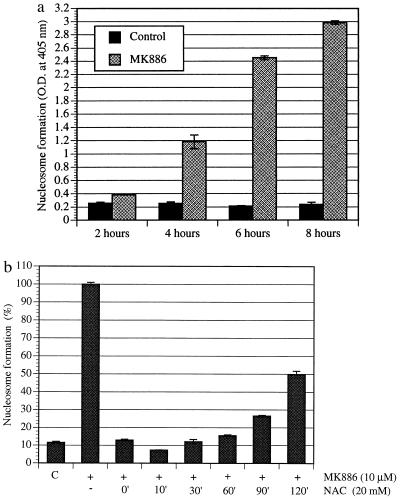
Among other parameters, degradation of DNA to nucleosomal fragments is a well characterized late event of apoptotic cell death. We studied nucleosome formation in the time frame required for the prostate cancer cells to show extensive plasma membrane blebs. Fig. 3A shows MK886-induced nucleosome formation by the androgen-responsive (LNCaP) prostate cancer cells, which began around 2 hr and peaked at 8 hr posttreatment. Similar results were observed with the hormone-resistant cell line PC3 (data not shown).
Figure 3.
(a) Time course of the formation of nucleosomal DNA in prostate cancer cells on treatment with MK886. LNCaP prostate cancer cells (3 × 105) were cultured for 48 hr in 60-mm tissue culture plates in RPMI medium 1640 supplemented with 10% FBS. The medium was then changed to serum-free RPMI medium 1640, and the cells were treated with 10 μM MK886 for various periods of time. Control cells were treated with the untreated medium containing 0.02% DMSO only. At the end of each incubation periods, cells were harvested and lysed, and the formation of nucleosomes was quantitatively measured by Cell Death Detection ELISA taking lysates equivalent to 5,000 cells for each assay. Results are presented as the mean ± standard error (n = 4). (b) Effect of NAC on MK886-induced prostate cancer cell death. LNCaP prostate cancer cells were plated as described in Fig. 3a and treated with 10 μM MK886 in serum-free medium. The thiol antioxidant NAC (20 mM), was added at different time points as indicated (minutes) post-MK886 treatment. Control cells were treated with the plating medium containing the solvent (0.02% DMSO) only. Cells were incubated for 6 hr after the addition of MK886, and the formation of nucleosomes was measured by Cell Death Detection ELISA. Data presented as the mean ± standard error (n = 4).
Role of Oxidative Stress in MK886-Induced Apoptosis.
There are now numerous reports which suggest that oxidative stress can be a common mediator of apoptotic cell death. The antiapoptotic protein Bcl-2 was shown to prevent apoptosis by decreasing the generation of reactive oxygen species (28, 29), and various antioxidants can substitute for Bcl-2 expression in preventing apoptosis (30, 31). Moreover, overexpression of Bcl-2 leads to relocalization of glutathione into the nucleus (32). Reactive oxygen intermediates (ROI), which are byproducts of mitochondrial respiration, can readily react with and damage important cellular structures, such as cell membranes. We wanted to learn whether MK886-induced prostate cancer cell death involves formation of ROI. We observed that treatment of the cells with the thiol antioxidant and glutathione precursor N-acetylcysteine (NAC) rescued these cells from MK886-induced DNA degradation, indicating a role of ROI in this process. Furthermore, NAC protected these cells from DNA degradation when it was added as late as 60–90 min post-MK886 treatment, but after that time, degradation of DNA proceeds even in the presence of NAC (Fig. 3b). NAC does not enhance 5-HETE production in the presence of MK886. These observations suggest that generation of ROI is an obligatory early event in MK886-induced apoptosis of human prostate cancer cells that precedes DNA degradation.
Survival Depends on the Products of Arachidonate 5-Lipoxygenase and Not Other Lipoxygenases.
Whereas MK886 appears to be a specific inhibitor of arachidonate 5-lipoxygenase, arachidonic acid is metabolized to a wide range of eicosanoids that may also have an effect on the survival of prostate cancer cells. Treatment of these cells with inhibitors of other major pathways of arachidonic acid metabolism (cyclooxygenase, cytochrome P450, or 12-lipoxygenase) did not trigger apoptosis under the experimental conditions described above (Table 1).
Table 1.
Degradation of DNA in LNCaP cells by inhibitors of major pathways of arachidonic acid metabolism
| Treatment | % nucleosome formation |
|---|---|
| Control | 10 |
| MK886 (10 μM) | 100 |
| SKF-525A (50 μM) | 15 |
| Ibuprofen (50 μM) | 10 |
| Baicalein (10 μM) | 10 |
Cells were plated as described in Fig. 3a and treated with various inhibitors (MK886 for 5-lipoxygenase, SKF-525A for cytochrome P450, ibuprofen for cyclooxygenase, and baicalein for 12-lipoxygenase) in serum-free medium for 6 hr. At the end of the incubation period, cells were harvested and washed, and the formation of nucleosomes was measured by ELISA. Results are shown as the mean of the amount of DNA degraded to nucleosomes (n = 4).
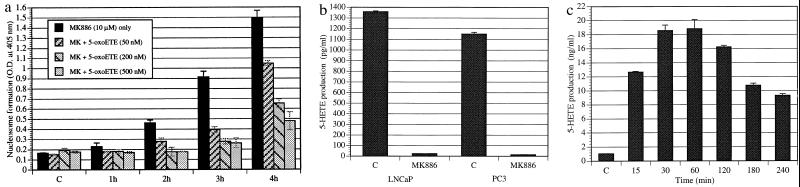
To further confirm that these events result from the specific inhibition of arachidonate 5-lipoxygenase activity, we tested whether MK886-induced degradation of DNA could be prevented by exogenous addition of the metabolic products of 5-lipoxygenase. As shown in Fig. 4a, we observed that simultaneous addition of 5-oxoeicosatetraenoic acid (5-oxoETE), a product of arachidonate 5-lipoxygenase, largely prevented DNA breakdown and nucleosome formation induced by MK886. Similar results were obtained with related 5-lipoxygenase products, 5-HETE and 5-HETE lactone (Table 2). On the other hand, leukotrienes (e.g., LTB4), which are also metabolic products of arachidonate 5-lipoxygenase, failed to protect these cells from MK886-induced apoptosis. Moreover, addition of other lipoxygenase products (12-HETE, 15-HETE, 5,12-diHETE, or 5,15-diHETE) did not protect these cells from MK886-induced apoptosis, indicating the specific requirement for eicosanoids of the 5-HETE series for the survival of human prostate cancer cells.
Figure 4.
(a) Reversal of MK886-induced DNA degradation by 5-oxoETE. Cells in serum-free medium were treated with 10 μM MK886 as described in Fig. 3a in the absence or presence of the 5-lipoxygenase product, 5-oxoETE, and incubated for various periods of time at 37°C. Control cells (C) were treated with serum-free RPMI medium 1640 containing 0.02% DMSO. Formation of nucleosomal DNA was determined by Cell Death Detection ELISA. Results represent the mean ± standard error (n = 4). (b) Constitutive production of 5-HETE by human prostate cancer cells. Cells (106) were plated in serum-free RPMI medium 1640 in 60-mm tissue culture dishes and incubated for 24 hr. Then the cells were treated with 10 μM MK886 or the solvent vehicle (0.02% DMSO) and incubated at 37°C for 2 hr. At the end of the incubation period, aliquots of culture supernatants were taken, and the production of 5-HETE was measured by radioimmunoassay. Each data point represents the mean ± standard error (n = 4). (c) Stimulation of 5-HETE production in LNCaP cells by arachidonic acid. Cells were plated as described in b and treated with 10 μM arachidonic acid precomplexed with lipid-free BSA for various periods of time. Production of 5-HETE was measured by radioimmunoassay using aliquots of culture supernatant. Data is presented as the mean ± standard error (n = 4).
Table 2.
Effect of different lipoxygenase products on apoptosis induced by inhibition of 5-lipoxygenase
| Treatment | % nucleosome formation |
|---|---|
| Control | 10 |
| MK886 | 100 |
| MK886 + 5-HETE | 35 |
| MK886 + 5-HETE lactone | 25 |
| MK886 + 5-oxoETE | 25 |
| MK886 + LTB4 | 100 |
| MK886 + 12-HETE | 100 |
| MK886 + 15-HETE | 100 |
| MK886 + 5,12-diHETE | 100 |
| MK886 + 5,15-diHETE | 100 |
LNCaP prostate cancer cells were plated in 60-mm plates as described in Fig. 3A and treated with 10 μM MK886 with or without the addition of various lipoxygenase products (500 nM). The plates were incubated for 4 hr at 37°C in a CO2 incubator. Control cells were treated with serum-free RPMI medium 1640 containing 0.02% DMSO only. At the end of each incubation period, cells were harvested and lysed, and the formation of nucleosomes was measured by ELISA. Results are reported as the mean of the amount of DNA degraded to nucleosomal fragments (n = 4).
Autocrine Production of 5-HETE Promotes the Survival of Human Prostate Cancer Cells.
If 5-HETE is an essential factor for the survival of human prostate cancer cells in culture, one would expect that these cancer cells must constitutively produce this eicosanoid. As illustrated in Fig. 4b, both LNCaP and PC3 cells exhibit constitutive production of 5-HETE under serum-free conditions. Furthermore, this constitutive production of 5-HETE is blocked by the addition of MK886. Moreover, addition of exogenous arachidonic acid results in a more than 10-fold increase in the formation of 5-HETE within 1 hr (Fig. 4c). This increased production of 5-HETE is also effectively blocked by the addition of MK886 (not shown). These findings suggest that under serum-free conditions, synthesis of 5-HETE is limited by the availability of arachidonic acid and not by the capacity of these cells to convert arachidonic acid to 5-HETE. Thus, these prostate cancer cells are able to use both endogenous and exogenous arachidonic acid pools to produce a potent survival factor, 5-HETE, and this process is inhibited by MK886.
Androgen Will Not Rescue These Cells from Inhibition of Arachidonate 5-Lipoxygenase.
In prostate cancer cells, androgen has a well characterized antiapoptotic action in addition to its ability to stimulate growth (33–35). In LNCaP cells, although androgen withdrawal does not initiate apoptosis, addition of androgen was observed to diminish drug-induced apoptosis, apparently at least in part through expression of antiapoptotic proteins such as Bcl-2 (34). With this background in mind, we examined whether MK886-induced cell death is prevented by androgen. R1881, a synthetic androgen-receptor agonist, did not protect these cells from MK886-induced apoptosis when used at concentrations of 1–5 nM, sufficient to double their growth in vitro (data not shown).
DISCUSSION
We have established that under serum-free conditions (i) human prostate cancer cells constitutively produce 5-HETE, which is dramatically increased by exogenous addition of arachidonic acid, (ii) inhibition of 5-lipoxygenase activity by MK886 blocks 5-HETE production and induces massive apoptosis in both hormone-responsive and -nonresponsive human prostate cancer cells, and (iii) exogenous addition of 5-HETE and its derivatives protects these cells from apoptotic cell death induced by MK886. Previously, we have shown that arachidonic acid stimulates the proliferation of both PC3 and LNCaP cell lines, that this stimulation results in the formation of 5-HETE, and that MK886 addition inhibits both 5-HETE formation and cell proliferation (10). Moreover, we have shown that the addition of 5-HETE or other 5-HETE derivatives (e.g., 5-HETE lactone, 5-oxoETE, etc.) is sufficient to support proliferation of these cells in the absence of serum stimulation and can reverse the growth-inhibitory effects of MK886 (10). This addition establishes the presence of an autocrine loop involving formation of 5-HETE as the mechanism by which arachidonic acid regulates the growth and survival of human prostate cancer cells. Furthermore, under serum-free conditions, formation of this survival factor is dramatically enhanced by exogenous arachidonic acid.
Thus, in addition to mitogenic effects, the metabolic products of 5-lipoxygenase play an important survival role in human prostate cancer cells. This is of note because serum starvation does not induce apoptosis in LNCaP cells and does so in PC3 cells only after 4 days, consistent with autocrine production of a “survival factor.” We have demonstrated that both of these cell lines produce 5-HETE even under serum-free conditions. Furthermore, 5-HETE and its derivatives act as potent survival factors: concentrations as low as 50 nM can protect against cell death triggered by the addition of MK886. Androgen, a well described and potent survival factor for prostate cancer cells, did not prevent MK886-induced cell death. In contrast, 5-HETE is able to support the survival and growth of the hormone-responsive cell line, LNCaP, in the absence of androgen stimulation. One of the central puzzles of prostate-cancer biology is how these tumor cells survive androgen withdrawal and eventually develop resistance to standard hormonal therapy. The properties of 5-HETE make it a factor potentially capable of promoting survival of prostate cancer cells following androgen withdrawal and supporting tumor growth in the absence of androgen.
The mechanism by which 5-lipoxygenase metabolites control the survival of prostate cancer cells is an intriguing problem. Recently, a number of reports suggested MPT and release of cytochrome C and apoptosis initiating factor are the critical molecular events for the initiation of apoptotic cascade (36). Cytochrome C and apoptosis initiating factor then cooperate to activate a cascade of caspases involved in the final execution phase of apoptosis. In prostate cancer cells, we observed that MPT is an early event (occurring within 1 hr) in MK886-induced apoptosis, indicating that MPT is an integral event in prostate cancer cell death induced by 5-lipoxygenase inhibition. Moreover, the speed and uniformity with which all of the tumor cells undergo MPT make this one of the most dramatic examples of mitochondrial apoptosis yet described.
Recent observations have linked phosphorylation of the proapoptotic protein BAD (Bcl-2 Associated Death promoter) to the regulation of MPT by Bcl-2 and related proteins (37–39). BAD is a direct substrate of PKB/cAKT, which is regulated by phosphoinositide 3-kinase. In a separate set of experiments, we observed that 5-oxoETE fails to prevent MK886-induced apoptosis in prostate cancer cells in the presence of wortmannin, a specific inhibitor of phosphoinositide 3-kinase (unpublished observation), suggesting involvement of phosphoinositide 3-kinase-mediated signaling as a downstream event in the prevention of apoptosis by 5-lipoxygenase metabolites. Moreover, direct stimulation of phosphoinositide 3-kinase activity by 5-oxoETE has been recently reported in other cell types (40).
These findings represent an insight into events regulating apoptosis in human prostate cancer cells. Although various eicosanoids have been implicated in the regulation of cell proliferation and cell death in other cell types, this report substantiates that eicosanoids of the 5-HETE series act as critical survival factors for human prostate cancer cells. This antiapoptotic action is quite dramatic in that once the synthesis of this eicosanoid is blocked, onset of massive apoptosis is apparent within 30–60 min. These cell cultures are not synchronized, and both LNCaP and PC3 cell lines have cell-cycle times of 24 hr or longer. However, after 5-HETE synthesis is blocked, essentially all of the cells, in both cell lines, enter apoptosis within 1 hr and die within 8 hr. This cell death indicates that 5-lipoxygenase inhibition-initiated apoptosis in human prostate cancer cells is largely independent of cell-cycle progression.
Although 5-HETE eicosanoids thus appear to play an important role in the regulation of cell death in these prostate-cancer cell lines, there are a number of observations that suggest this is not a general phenomenon. Mice in which the 5-lipoxygenase gene has been knocked out mature normally and are fertile (41, 42). The most notable aspect of the phenotype is that these knockout mice are resistant to shock induced by platelet activating factor (43). Moreover, the knockout mice showed substantially reduced collagen-induced arthritis (44). Additionally, drugs that inhibit 5-lipoxygenase have been extensively studied as treatments for asthma and appear to be well tolerated in both man and a range of experimental animals (45). Finally, we have found that the hormone-independent breast cancer cell line, MDA-MB-231, is completely refractory to MK886 (data not shown). These results suggest that agents inhibiting the formation and function of 5-HETE eicosanoids may make attractive new tools for the treatment of prostate cancer.
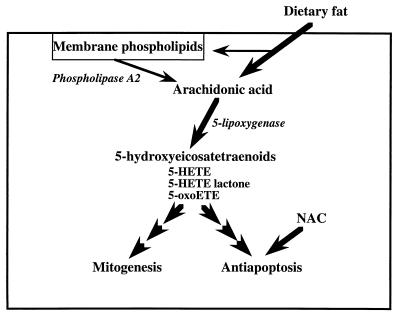
In conclusion, our experimental observations indicate that the production of 5-HETE is a critical requirement for the survival of both hormone-responsive and -nonresponsive human prostate cancer cells. Furthermore, the ability of arachidonic acid to stimulate proliferation of these cells appears also to depend on its metabolic conversion to 5-HETE. Finally, our findings provide a molecular mechanism by which high intake of dietary fat may foster the progression of prostate cancer by supporting the production of 5-HETE eicosanoids (Fig. 5).
Figure 5.
A model illustrating regulation by dietary fat of prostate cancer cell growth and survival.
Acknowledgments
We are indebted to the Reynolds and Lynch Foundations for their financial support. This work was also partially supported by the CaPCure Foundation and by an National Cancer Institute Development Grant in Prostate Cancer (R21-CA 69848).
ABBREVIATIONS
- 5-HETE
5-hydroxyeicosatetraenoic acid
- 5-oxoETE
5-oxoeicosatetraenoic acid
- PGE2
prostaglandin E2
- FBS
fetal bovine serum
- DMSO
dimethyl sulfoxide
- ROI
reactive oxygen intermediates
- NAC
N-acetylcysteine
- MPT
mitochondrial permeability transition
References
- 1. Parker S L, Tong T, Bolden S, Wingo P A. Ca Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Crawford E D, Eisenberger M A, McLeod D G, Spaulding J T, Benson R, Dorr A, Blumenstein B A, Davis M A, Goodman P J. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 3.Gann P H, Hennekens C H, Sacks F M, Grodstein F, Giovannucci E L, Stampfer M J. J Natl Cancer Inst. 1994;86:281–286. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 4.West D W, Slattery M L, Robison L M, French T K, Mahoney A W. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Rimm E B, Colditz G A, Stampfer M J, Ascherio A, Chute C C, Willett W C. J Natl Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 6.Brawley O W, Thompson I M. Urology. 1994;43:594–599. doi: 10.1016/0090-4295(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Corr J G, Thaler H T, Tao Y, Fair W R, Heston W D. J Natl Cancer Inst. 1995;87:1456–1462. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 8.Chaudry A A, Wahle K W, McClinton S, Moffat L E. Int J Cancer. 1994;57:176–180. doi: 10.1002/ijc.2910570208. [DOI] [PubMed] [Google Scholar]
- 9.Rose D P, Connolly J M. Prostate. 1991;18:243–254. doi: 10.1002/pros.2990180306. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh J, Myers C E. Biochem Biophys Res Commun. 1997;235:418–423. doi: 10.1006/bbrc.1997.6799. [DOI] [PubMed] [Google Scholar]
- 11.Needleman P, Turk J, Jakschik B A, Morrison A R, Lefkowith J B. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 12.Ara G, Teicher B A. Prostaglandins Leukotrienes Essent Fatty Acids. 1996;54:3–16. doi: 10.1016/s0952-3278(96)90075-7. [DOI] [PubMed] [Google Scholar]
- 13.Tang D G, Renaud C, Stojakovic S, Diglio C A, Porter A, Honn K V. Biochem Biophys Res Commun. 1995;211:462–468. doi: 10.1006/bbrc.1995.1836. [DOI] [PubMed] [Google Scholar]
- 14.Young M R. Cancer Metastasis Rev. 1994;13:337–348. doi: 10.1007/BF00666103. [DOI] [PubMed] [Google Scholar]
- 15.Peppelenbosch M P, Qiu R G, de Vries-Smits A M, Tertoolen L G, de Laat S W, McCormick F, Hall A, Symons M H, Bos J L. Cell. 1995;81:849–856. doi: 10.1016/0092-8674(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 16.Peppelenbosch M P, Tertoolen L G, den Hertog J, de Laat S W. Cell. 1992;69:295–303. doi: 10.1016/0092-8674(92)90410-e. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Wartman M, Lin A Y, Knopf J L, Seth A, Davis R J. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 18.Currie S, Smith G L, Crichton C A, Jackson C G, Hallam C, Wakelam M J. J Biol Chem. 1992;267:6056–6062. [PubMed] [Google Scholar]
- 19.Lim H, Paria B C, Das S K, Dinchuk J E, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 20.Goetzl E J, An S, Zeng L. J Immunol. 1995;154:1041–1047. [PubMed] [Google Scholar]
- 21.Robinson B S, Hii C S, Poulos A, Ferrante A. J Lipid Res. 1996;37:1234–1245. [PubMed] [Google Scholar]
- 22.Herrmann J L, Menter D G, Beham A, Eschenbach A, McDonnell T J. Exp Cell Res. 1997;234:442–451. doi: 10.1006/excr.1997.3653. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton R F, Li L, Iyer R, Holian A. Am J Physiol. 1996;271:L813–819. doi: 10.1152/ajplung.1996.271.5.L813. [DOI] [PubMed] [Google Scholar]
- 24.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann P G, Wendel A. Am J Pathol. 1995;146:1220–1234. [PMC free article] [PubMed] [Google Scholar]
- 25.Ford-Hutchinson A W, Gresser M, Young R N. Annu Rev Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- 26.Brideau C, Chan C, Charleson S, Denis D, Evans J F, Ford-Hutchinson A W, Fortin R, Gillard J W, Guay J. Can J Physiol Pharmacol. 1992;70:799–807. doi: 10.1139/y92-107. [DOI] [PubMed] [Google Scholar]
- 27.Ancill R J, Takahashi Y, Kibune Y, Campbell R, Smith J R. J Intern Med Res. 1990;18:75–88. doi: 10.1177/030006059001800202. [DOI] [PubMed] [Google Scholar]
- 28.Hockenberry D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Cell. 1994;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 29.Kane D J, Sarafian T A, Anton R, Hahn H, Gralla E B, Valentine J S, Ord T, Bredesen D E. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 30.Buttke T M, Sandstrom P A. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 31.Fesus L, Szondy Z, Uray I. J Cell Biochem. 1995;22:151–161. doi: 10.1002/jcb.240590820. [DOI] [PubMed] [Google Scholar]
- 32.Voehringer D W, McConkey D J, McDonnell T J, Brisbay S, Meyn R E. Proc Natl Acad Sci USA. 1998;95:2956–2960. doi: 10.1073/pnas.95.6.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limonta P, Dondi D, Marelli M M, Moretti R M, Negri-Cesi P, Motta M. J Steroid Biochem Mol Biol. 1995;53:401–405. doi: 10.1016/0960-0760(95)00086-f. [DOI] [PubMed] [Google Scholar]
- 34.Berchem G H, Bosseler M, Sugars L Y, Voeller H J, Zeitlin S, Gelmann E P. Cancer Res. 1995;55:735–738. [PubMed] [Google Scholar]
- 35.Isaacs J T, Lundmo P I, Berges R, Martikainen P, Kyprianou N, English H F. J Androl. 1992;13:457–463. [PubMed] [Google Scholar]
- 36.Kroemer G, Zamzami N, Susin S A. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 37.Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 38.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 40.Norgauer J, Barbisch M, Czech W, Pareigis J, Schwenk U, Schroder J. Eur J Biochem. 1996;236:1003–1009. doi: 10.1111/j.1432-1033.1996.01003.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Sheller J, Johnson E, Funk C D. Nature (London) 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 42.Funk C D, Kurre U, Griffis G. Ann NY Acad Sci. 1994;714:252–258. doi: 10.1111/j.1749-6632.1994.tb12051.x. [DOI] [PubMed] [Google Scholar]
- 43.Byrum R S, Goulet J L, Griffiths R J, Koller B H. J Exp Med. 1997;185:1065–1075. doi: 10.1084/jem.185.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths R J, Smith M A, Roach M L, Stock J L, Stam E J, Milici A J, Scampoli D N, Eskra J D, Byrum R S, Koller B H, et al. J Exp Med. 1997;185:1123–1129. doi: 10.1084/jem.185.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hay D W, Torphy T J, Undem B J. Trends Pharmacol Sci. 1995;16:304–309. doi: 10.1016/s0165-6147(00)89059-8. [DOI] [PubMed] [Google Scholar]