Abstract
The mitochondrial uncoupling proteins-2 and -3 are putative mediators of thermogenesis and energy expenditure. We measured the mRNA levels of uncoupling proteins-2 and -3 in skeletal muscle from 12 gastrointestinal adenocarcinoma patients, of whom six had stable weight and six had lost 2–18 kg, and from six healthy controls undergoing elective surgery. Uncoupling proteins-3 mRNA levels were significantly higher in the muscle of the cancer patients with weight loss (2.2±0.47 arbitrary units) compared both with controls (0.39±0.20) and with cancer patients who had not lost weight (0.47±0.23; P<0.02). Uncoupling proteins-2 mRNA levels did not differ significantly between groups. Elevations in muscle uncoupling proteins-3 activity may enhance energy expenditure and this in turn could contribute to tissue catabolism.
British Journal of Cancer (2002) 86, 372–375. DOI: 10.1038/sj/bjc/6600074 www.bjcancer.com
© 2002 The Cancer Research Campaign
Keywords: uncoupling proteins, cachexia, muscle
Some degree of weight loss occurs in over 80% of cancer patients, and is strongly associated with poor outcome and early death (Puccio and Nathanson, 1997; De Wyes et al, 1980). The aetiology of weight loss in malignancy is multifactorial and may include increases in circulating cytokines, reductions in food intake, and alterations in metabolism (Tisdale et al, 1996). Resting energy expenditure is increased in some animal and human cancers, and is thought to contribute to weight loss (Bosaeus et al, 2001). The sites and mechanisms of excessive heat production in human cancer cachexia are however unknown.
The uncoupling proteins (UCPs) are a family of mitochondrial membrane proteins. UCP-1 is restricted to brown adipose tissue (BAT) where it uncouples oxidative phosphorylation and generates heat instead of ATP (Klaus et al, 1991). Other UCPs include UCP-2 (found in many tissues) and UCP-3 (expressed in BAT and skeletal muscle) (Boss et al, 1997; Fleury et al, 1997). Both have uncoupling activity in vitro and may mediate the increased energy expenditure implicated in energy balance. Transgenic mice over-expressing UCP-3 in muscle are lean despite exhibiting hyperphagia; oxygen consumption in the transgenic animals was increased by 91% indicating an increase in metabolic rate (Clapham et al, 2000). The exact roles of the various UCP isoforms in vivo are still debated and are reviewed elsewhere (Ricquier and Bouillaud, 2000).
We recently found increased UCP-2 and -3 mRNA levels in the muscle of mice with severe cachexia due to the MAC-16 (Bing et al, 2000) This non-metastasizing adenocarcinoma is histologically similar to human gastrointestinal tumours and causes profound weight loss despite only modest reductions in food intake. Here, we aimed to determine whether UCP-2 and -3 expression was similarly up-regulated in human cancer.
METHODS
Patient characteristics
We studied 12 patients with histologically diagnosed adenocarcinoma of the GI tract undergoing elective laparotomy. Subjects were excluded from the study if there was evidence of intercurrent infection at the time of surgery, or mechanical obstruction of the gut. Six had weight loss of 2–18 kg over a period of 4–12 weeks prior to surgery, as documented in medical notes, while the remainder had stable weight documented on at least two occasions over a similar period. Weights were measured on a single set of scales in the surgical outpatient clinic. All patients were operated during a weekly list to minimize the differences in fasting times prior to sample collection. Controls were undergoing laparotomy for a benign disease (Table 1) and had a documented stable weight for at least 8 weeks prior to surgery.
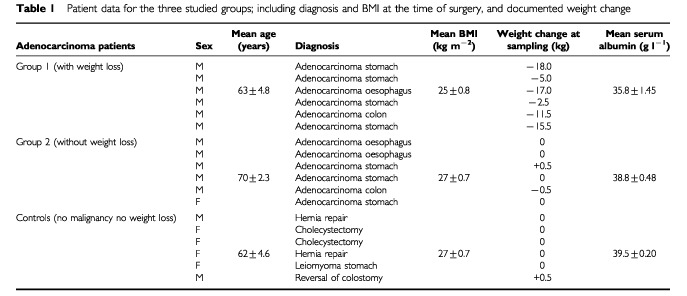
Table 1. Patient data for the three studied groups; including diagnosis and BMI at the time of surgery, and documented weight change.
Fully informed and written consent was obtained in all cases and the study protocol was approved by the Sefton Ethics Committee.
Tissue collection and processing
At laparotomy, samples of rectus abdominus muscle (approximately 10 mg) were collected and immediately placed in storage solution (RNAlater® Ambion Inc., Austin, TX, USA) to stabilize RNA pending transport to the laboratory and storage at −20°C. After collection, all samples were processed at the same time using a commercial spin column kit to isolate total mRNA (Neucleospin® Clontech Labs Inc., CA, USA). The amount and purity of isolated RNA was calculated using spectrometry at 260/280 nM.
Measurement of UCP-2 and -3 mRNA
Semi-quantitative RT–PCR was performed to calculate amounts of UCP-2 and -3 mRNA, relative to the housekeeping gene, hypoxanthine phosphoribosyl transferase (HPRT). One ng of total RNA was added to 50 μl of PCR mixture containing UCP-2 or -3 specific primers (0.2 μM each of forward and reverse), reverse transcriptase, DNA polymerase, MgCl2 (1.5 mM) (OneStep® Abgene, Epsom, Surrey, UK). A further 1 ng sample was added to an identical PCR mixture containing HPRT-specific primers. Preliminary experiments identified RNA template concentrations, which yielded a product within a linear range for both HPRT and the UCPs, under the standard conditions defined below, and PCR products were sequenced to ensure specificity.
cDNA synthesis and PCR were completed in a single reaction: 30 min at 47°C for first-strand generation, inactivation for 2 min at 95°C, followed by 30 cycles (20 s at 94°C, 30 s at 56°C, 1 min at 72°C) and a final extension step at 72°C for 5 min. After completion, a 5 μl aliquot of the HPRT reaction was mixed with 10 μl of the UCP-2 or -3 reaction and the resulting mixture subjected to electrophoresis on a 1% agarose gel, stained with ethidium bromide. Bands were visualized and quantitated using a digital image analysis system (Kodak 1-D Eastman Kodak Co, MA, USA). Optical density was calculated for the two products and the results expressed as a ratio of UCP-2 product to HPRT product. A negative control (without reverse transcriptase) was run with each experiment to ensure the absence of DNA contamination.
RESULTS
There were no significant differences in age between the three groups (Table 1). Most of the cancer group were male, while three of the control group were female. The origins of the adenocarcinomas were similarly distributed in the cancer patients with or without weight loss. In the cancer with documented weight loss group, this ranged from 2–18 kg. BMI and serum albumin were significantly lower in the cancer patients with weight loss than in both other groups.
Table 2 shows the levels of UCP-2 and -3 mRNA, expressed as ratios to HPRT mRNA; these data are expressed graphically in Figure 1. UCP-2 mRNA levels were similar among all three groups. Mean UCP-3 levels, however, were over five times higher in the cancer patients with weight loss group than in controls. In the cancer without weight loss group, mean UCP-3 levels were similar to controls.
Table 2. UCP-2 and -3 mRNA expression for each of the studied groups. Data is expressed as a ratio: optical density (OD) UCP-2 or -3 to OD of HPRT.
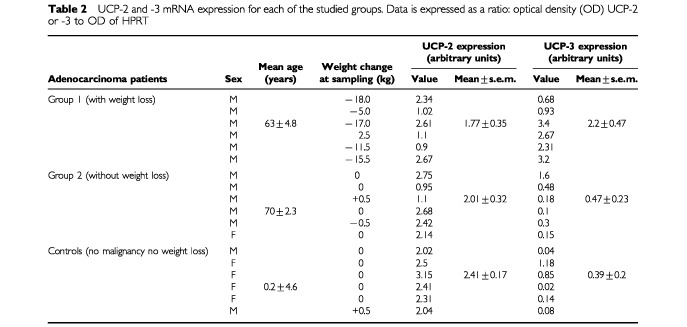
Figure 1.
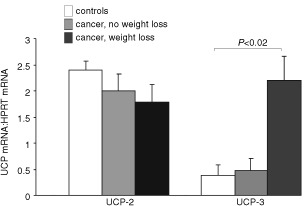
Mean UCP-2 and UCP-3 levels for the studied groups. Data is expressed as a ratio: optical density (OD) UCP-2 or -3 to OD of HPRT ±s.e.m.
DISCUSSION
This is the first demonstration of up-regulation of UCPs in human cancer associated weight loss. We have shown that UCP-3 mRNA levels are increased in muscle only when weight loss is associated with cancer. UCP-2 mRNA levels in muscle seems unaffected by cancer either with or without weight loss. This finding correlates with that previously demonstrated in the MAC-16 model of cancer cachexia (although in this model UCP-2 was also increased in skeletal muscle compared with non tumour-bearing controls).
Our data suggests that UCP-3 in skeletal muscle might either contribute to weight loss, or be up-regulated as a consequence of metabolic changes brought about by tissue loss. It is well recognized that energy expenditure is increased in cancer often despite reduced energy intake, and that it seems directly related to body cell mass (Simons et al, 1999). That this increase in energy expenditure is directly related to the tumour effect is evidenced by the fact that removal of tumour ensures prompt restoration of normal metabolism (Luketich et al, 1990). UCP-3, when overexpressed in the skeletal muscle of transgenic mice results in a lean phenotype, with increased energy expenditure compared to controls (Clapham et al, 2000). Over expression of UCP-3 in the skeletal muscle of human cancer patients might contribute to increase energy expenditure and thus to weight loss.
Of course the fact that a protein associated with an increase in energy expenditure is over expressed in a situation where energy conservation would seem more appropriate, appears at first to be counterintuitive. However one of the putative roles of UCP-3 in vivo is the facilitation of lipid substrate utilization. Certainly lipid infusions result in increased skeletal muscle UCP-3 levels both in vivo and in vitro (Hwang and Lane, 1999; Samec et al, 1999). Lipid dysregulation occurs in cancer associated weight loss, with increases in lipolysis (caused by cytokines and tumour derived lipolytic factors) off-set by increases in lipid utilization so that plasma free fatty acid levels may remain normal or only slightly elevated (Younes et al, 1990). The role of UCP-3 may be to assist in the utilization of excess FFA as fuel in the muscle or to prevent possible harmful effects of the intermediates of FFA metabolism. Unfortunately in this study we were unable to obtain fasting FFA levels in enough of the subjects studied to enable a statistically meaningful correlation with UCP-3 levels. In our animal model, however UCP-3 mRNA levels correlated well with plasma FFA levels and there is no evidence to suggest that this would not be the case in humans.
If the up-regulation of UCP-3 in skeletal muscle in human cancers does result in increased energy expenditure and result in weight loss, this offers potential new targets for therapeutic intervention. Indeed, this observation may not be confined to weight loss associated with malignancy, but may also occur in other wasting conditions such as, HIV disease, rheumatoid and cardiac failure. Unfortunately due to the difficulty in obtaining laparotomy specimens from these groups we were unable to address this specific issue in the current study, but aim to investigate it at a future date. If mechanisms could be found to block the up-regulation of UCP-3 in malignancy and this resulted in a reduction in metabolic rate, weight stabilization may occur. Cachexia is one of the most important contributors to mortality in cancer, and its arrest is likely to lead to meaningful increases in survival time.
Acknowledgments
The authors would like to thank Dr Robin Walker, Gastroenterology Unit, University Hospital Aintree, who provided financial support for Dr Collins.
References
- BingCBrownMKingPCollinsPTisdaleMJWilliamsG2000Increased gene expression of brown fat uncoupling protein (UCP)1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia Cancer Res 6024052410 [PubMed] [Google Scholar]
- BosaeusIDanerydPSvanbergELundholmK2001Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients Int J Cancer 93380383 [DOI] [PubMed] [Google Scholar]
- BossOSamecSPaoloni-GiacobinoARossierCDullooASeydouxJMuzzinPGiacobinoJP1997Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue specific expression FEBS Lett 4083942 [DOI] [PubMed] [Google Scholar]
- ClaphamJArchJRSChapmanHHaynesAListerCMooreGBTPiercyVCarterSALehnerISmithSABeeleyLJGoddenRJHerrityNSkehelMChanganiKHockingsPDReidDGSquiresSMHatcherJTrailBLatchamJRastanSHarperAJCadenesSBuckinghamJABrandMAbuninA2000Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean Nature 406415418 [DOI] [PubMed] [Google Scholar]
- De WyesWDBeggDLavinPTBandPRBennetJMBertinoJRCohenMHDouglassHOEngstromPFEzdinliEZHortonJJhonsonGJMoertelCGOakenMMPerliaCRowenbaumCSilversteinMNSkeelRTSpnzoRWTormeyDC1980Prognostic effect of weight loss prior to chemotherapy in cancer patients Am J Med 69491497 [DOI] [PubMed] [Google Scholar]
- FleuryCNeverovaMCollinsSRaimbaultSChampignyOLevi-meyrueisCBouillardFSeldinMFSurwitRSRicquierDWardenmCH1997Nature Genet 152692729054939
- HwangCLaneD1999Up-regulation of uncoupling protein-3 by fatty acid in C2C12 myotubes Biochem Biophys Res Commun 25464469 [DOI] [PubMed] [Google Scholar]
- KlausSCasteillaLBouillaudFRicquierD1991The uncoupling protein UCP: a membranous mitochondrial ion carrier exclusively expressed in brown adipose tissue Int J Biochem 23791801 [DOI] [PubMed] [Google Scholar]
- LuketichJDMullenJLFeurerIDSternliebJFriedRC1990Ablation of abnormal energy expenditure by curative tumour resection Arch Surg 125337341 [DOI] [PubMed] [Google Scholar]
- PuccioMNathansonL1997The cancer cachexia syndrome Semin Onc 24277287 [PubMed] [Google Scholar]
- RicquierDBouillaudF2000The uncoupling protein homologues UCP1, UCP2, UCP3, StUCP and AtUCP Biochem J 345161179 [PMC free article] [PubMed] [Google Scholar]
- SamecSSeydouxJDullooAG1999Skeletal muscle UCP3 and UCP2 gene expression in response to inhibition of free fatty acid flux through mitochondrial β-oxidation Eur J Physiol 438452457 [DOI] [PubMed] [Google Scholar]
- SimonsJPScholsAMBuurmanWAWoutersEF1999Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute-phase response resting energy expenditure and catabolic and anabolic hormones Clin Sci 97215223 [PubMed] [Google Scholar]
- TisdaleMJMcDevittTMTodorovPTCariukP1996Catabolic factors in cancer cachexia In vivo 10131136 [PubMed] [Google Scholar]
- YounesRNVydelingumNANoguchiYBrennanMF1990Lipid kinetic alterations in tumour bearing rats: reversal by tumour excision J Surg Res 48324328 [DOI] [PubMed] [Google Scholar]


