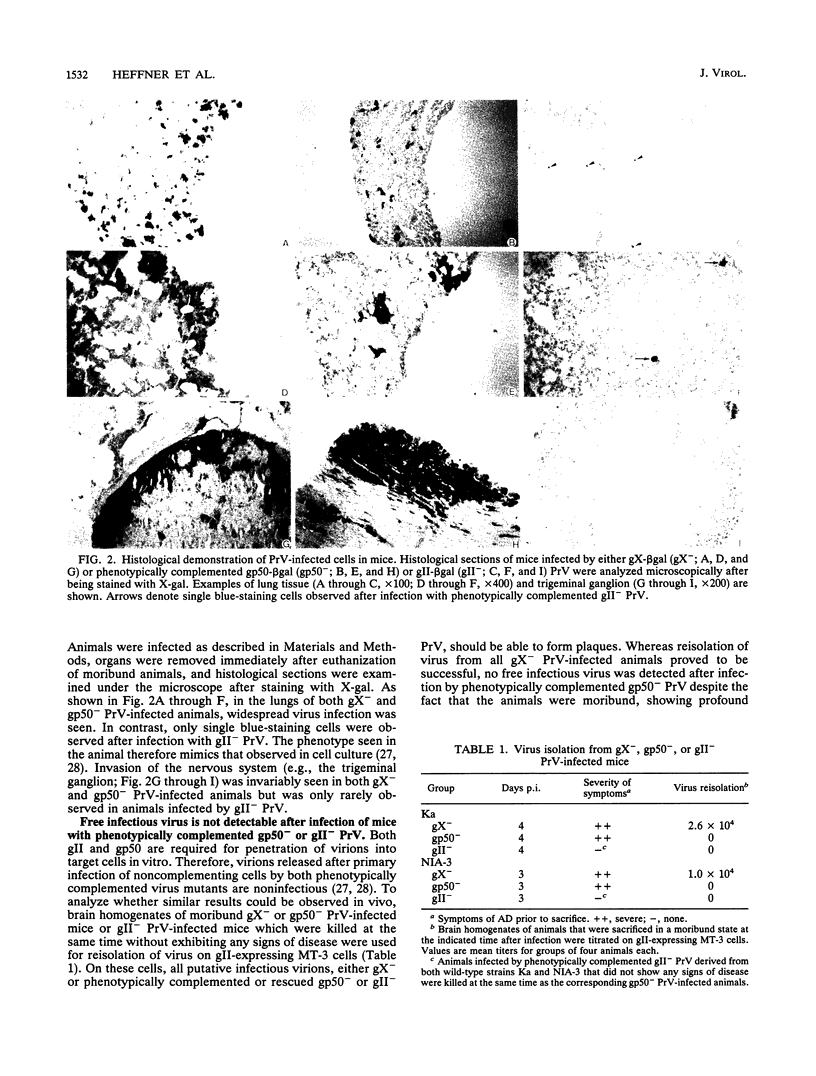
Abstract
Essential herpesvirus glycoproteins are involved in membrane fusion processes during infection, e.g., viral penetration and direct cell-to-cell transmission. We previously showed that the gD-homologous glycoprotein gp50 of pseudorabies virus (PrV) is essential for virus entry into target cells but proved to be dispensable for direct viral cell-to-cell spread in cell culture (I. Rauh and T. C. Mettenleiter, J. Virol. 65:5348-5456, 1991). For gp50-negative (gp50-) viruses, after phenotypic complementation necessary for primary infection, the only means of viral spread is by way of direct cell-to-cell transmission. In contrast, virus mutants lacking the essential gB-homologous glycoprotein gII after phenotypic complementation are only able to infect primary target cells and are blocked in further viral spread. To analyze how these in vitro phenotypes translate into virus replication in the animal, mice were infected intranasally with gp50- or gII- PrV mutants after prior phenotypic complementation by propagation on cell lines providing the essential glycoprotein in trans. Our results show that whereas the gII- mutants did not cause disease or any symptoms, gp50- mutants derived from two different PrV strains were fully virulent, with animals exhibiting severe symptoms ultimately leading to death. However, free infectious virus could not be recovered from either gp50- or gII- PrV-infected animals. We conclude that direct cell-to-cell transmission as the only means of viral spread of the gp50- mutants is sufficient for a full virulent phenotype in mice. After infection of pigs with phenotypically complemented gp50- PrV, only mild symptoms were observed, whereas the gII- mutant was totally avirulent. In both cases, shedding of infectious virus did not occur, in contrast to results with animals infected by gX- PrV that showed severe signs of disease and extensive virus shedding. After challenge infection with the highly virulent NIA-3 strain, the previously gII- PrV-infected animals exhibited severe symptoms, whereas the gp50- PrV-infected pigs showed a significant level of protection. In conclusion, vaccination with a PrV mutant lacking glycoprotein gp50, which is unable to spread between animals because of a lack of formation of free infectious virions, can confer on pigs protection against challenge infection. These results provide the basis for the development of new, nonspreading live herpesvirus vaccines based on gp50- PrV mutants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen L. S., Medveczky I., Strandbygaard B. S., Pejsak Z. Characterization of field isolates of suid herpesvirus 1 (Aujeszky's disease virus) as derivatives of attenuated vaccine strains. Arch Virol. 1992;124(3-4):225–234. doi: 10.1007/BF01309804. [DOI] [PubMed] [Google Scholar]
- Eloit M., Fargeaud D., L'Haridon R., Toma B. Identification of the pseudorabies virus glycoprotein gp50 as a major target of neutralizing antibodies. Arch Virol. 1988;99(1-2):45–56. doi: 10.1007/BF01311022. [DOI] [PubMed] [Google Scholar]
- Fehler F., Herrmann J. M., Saalmüller A., Mettenleiter T. C., Keil G. M. Glycoprotein IV of bovine herpesvirus 1-expressing cell line complements and rescues a conditionally lethal viral mutant. J Virol. 1992 Feb;66(2):831–839. doi: 10.1128/jvi.66.2.831-839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W., Rziha H. J., Lukàcs N., Braunschweiger I., Visser N., Lütticken D., Schreurs C. S., Thiel H. J., Mettenleiter T. C. Pseudorabies virus glycoprotein gI: in vitro and in vivo analysis of immunorelevant epitopes. J Gen Virol. 1990 May;71(Pt 5):1141–1151. doi: 10.1099/0022-1317-71-5-1141. [DOI] [PubMed] [Google Scholar]
- Henderson L. M., Katz J. B., Erickson G. A., Mayfield J. E. In vivo and in vitro genetic recombination between conventional and gene-deleted vaccine strains of pseudorabies virus. Am J Vet Res. 1990 Oct;51(10):1656–1662. [PubMed] [Google Scholar]
- Henderson L. M., Levings R. L., Davis A. J., Sturtz D. R. Recombination of pseudorabies virus vaccine strains in swine. Am J Vet Res. 1991 Jun;52(6):820–825. [PubMed] [Google Scholar]
- Ishii H., Kobayashi Y., Kuroki M., Kodama Y. Protection of mice from lethal infection with Aujeszky's disease virus by immunization with purified gVI. J Gen Virol. 1988 Jun;69(Pt 6):1411–1414. doi: 10.1099/0022-1317-69-6-1411. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S., VATTER A. E. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959 Apr;7(4):394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- Kimman T. G., de Wind N., Oei-Lie N., Pol J. M., Berns A. J., Gielkens A. L. Contribution of single genes within the unique short region of Aujeszky's disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J Gen Virol. 1992 Feb;73(Pt 2):243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- Klupp B. G., Visser N., Mettenleiter T. C. Identification and characterization of pseudorabies virus glycoprotein H. J Virol. 1992 May;66(5):3048–3055. doi: 10.1128/jvi.66.5.3048-3055.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács F., Mettenleiter T. C. Firefly luciferase as a marker for herpesvirus (pseudorabies virus) replication in vitro and in vivo. J Gen Virol. 1991 Dec;72(Pt 12):2999–3008. doi: 10.1099/0022-1317-72-12-2999. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy A. D., Bridgman P. C., Mettenleiter T. C. beta-Galactosidase expressing recombinant pseudorabies virus for light and electron microscopic study of transneuronally labeled CNS neurons. Brain Res. 1991 Aug 2;555(2):346–352. doi: 10.1016/0006-8993(91)90364-2. [DOI] [PubMed] [Google Scholar]
- Lukàcs N., Thiel H. J., Mettenleiter T. C., Rziha H. J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985 Jan;53(1):166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioli C. C., Yancey R. J., Jr, Petrovskis E. A., Timmins J. G., Post L. E. Evaluation of pseudorabies virus glycoprotein gp50 as a vaccine for Aujeszky's disease in mice and swine: expression by vaccinia virus and Chinese hamster ovary cells. J Virol. 1987 Dec;61(12):3977–3982. doi: 10.1128/jvi.61.12.3977-3982.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerran J. B., Dow C. Studies on immunisation of pigs with the Bartha strain of Aujeszky's disease virus. Res Vet Sci. 1975 Jul;19(1):17–22. [PubMed] [Google Scholar]
- Mettenleiter T. C., Kern H., Rauh I. Isolation of a viable herpesvirus (pseudorabies virus) mutant specifically lacking all four known nonessential glycoproteins. Virology. 1990 Nov;179(1):498–503. doi: 10.1016/0042-6822(90)90324-k. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C. Molecular biology of pseudorabies (Aujeszky's disease) virus. Comp Immunol Microbiol Infect Dis. 1991;14(2):151–163. doi: 10.1016/0147-9571(91)90128-z. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Rauh I. A glycoprotein gX-beta-galactosidase fusion gene as insertional marker for rapid identification of pseudorabies virus mutants. J Virol Methods. 1990 Oct;30(1):55–65. doi: 10.1016/0166-0934(90)90043-f. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987 Sep;61(9):2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Zuckermann F., Sugg N., Kern H., Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990 Jan;64(1):278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters B., de Wind N., Broer R., Gielkens A., Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992 Jun;66(6):3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters B., de Wind N., Hooisma M., Wagenaar F., Gielkens A., Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992 Feb;66(2):894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh I., Mettenleiter T. C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991 Oct;65(10):5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh I., Weiland F., Fehler F., Keil G. M., Mettenleiter T. C. Pseudorabies virus mutants lacking the essential glycoprotein gII can be complemented by glycoprotein gI of bovine herpesvirus 1. J Virol. 1991 Feb;65(2):621–631. doi: 10.1128/jvi.65.2.621-631.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere M., Tartaglia J., Perkus M. E., Norton E. K., Bongermino C. M., Lacoste F., Duret C., Desmettre P., Paoletti E. Protection of mice and swine from pseudorabies virus conferred by vaccinia virus-based recombinants. J Virol. 1992 Jun;66(6):3424–3434. doi: 10.1128/jvi.66.6.3424-3434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedegah M., Chiang C. H., Weiss W. R., Mellouk S., Cochran M. D., Houghten R. A., Beaudoin R. L., Smith D., Hoffman S. L. Recombinant pseudorabies virus carrying a plasmodium gene: herpesvirus as a new live viral vector for inducing T- and B-cell immunity. Vaccine. 1992;10(9):578–584. doi: 10.1016/0264-410x(92)90436-n. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Marchioli C. C., Yancey R. J., Jr, Post L. E. Replication and virulence of pseudorabies virus mutants lacking glycoprotein gX. J Virol. 1987 Jan;61(1):229–232. doi: 10.1128/jvi.61.1.229-232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Marotti K. R., Palermo D. P., Post L. E. Pseudorabies virus as a live virus vector for expression of foreign genes. Gene. 1987;57(2-3):261–265. doi: 10.1016/0378-1119(87)90130-2. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Isolation, characterization, and physical mapping of a pseudorabies virus mutant containing antigenically altered gp50. J Virol. 1984 Jul;51(1):57–62. doi: 10.1128/jvi.51.1.57-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G. Spread and control of Aujeszky's disease (AD). Comp Immunol Microbiol Infect Dis. 1991;14(2):165–173. doi: 10.1016/0147-9571(91)90129-2. [DOI] [PubMed] [Google Scholar]
- Zsak L., Mettenleiter T. C., Sugg N., Ben-Porat T. Release of pseudorabies virus from infected cells is controlled by several viral functions and is modulated by cellular components. J Virol. 1989 Dec;63(12):5475–5477. doi: 10.1128/jvi.63.12.5475-5477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992 Apr;66(4):2316–2325. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F. A., Mettenleiter T. C., Schreurs C., Sugg N., Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988 Dec;62(12):4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl M., Wensvoort G., de Kluyver E., Hulst M., van der Gulden H., Gielkens A., Berns A., Moormann R. Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol. 1991 May;65(5):2761–2765. doi: 10.1128/jvi.65.5.2761-2765.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]