Abstract
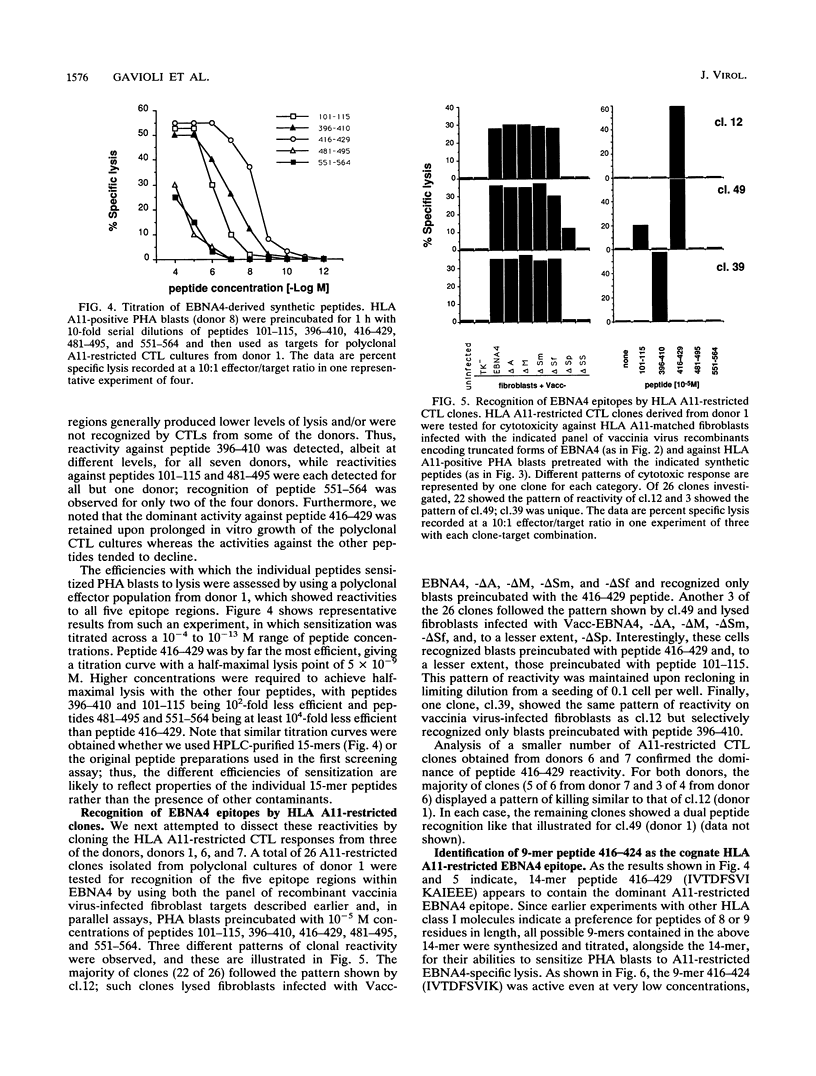
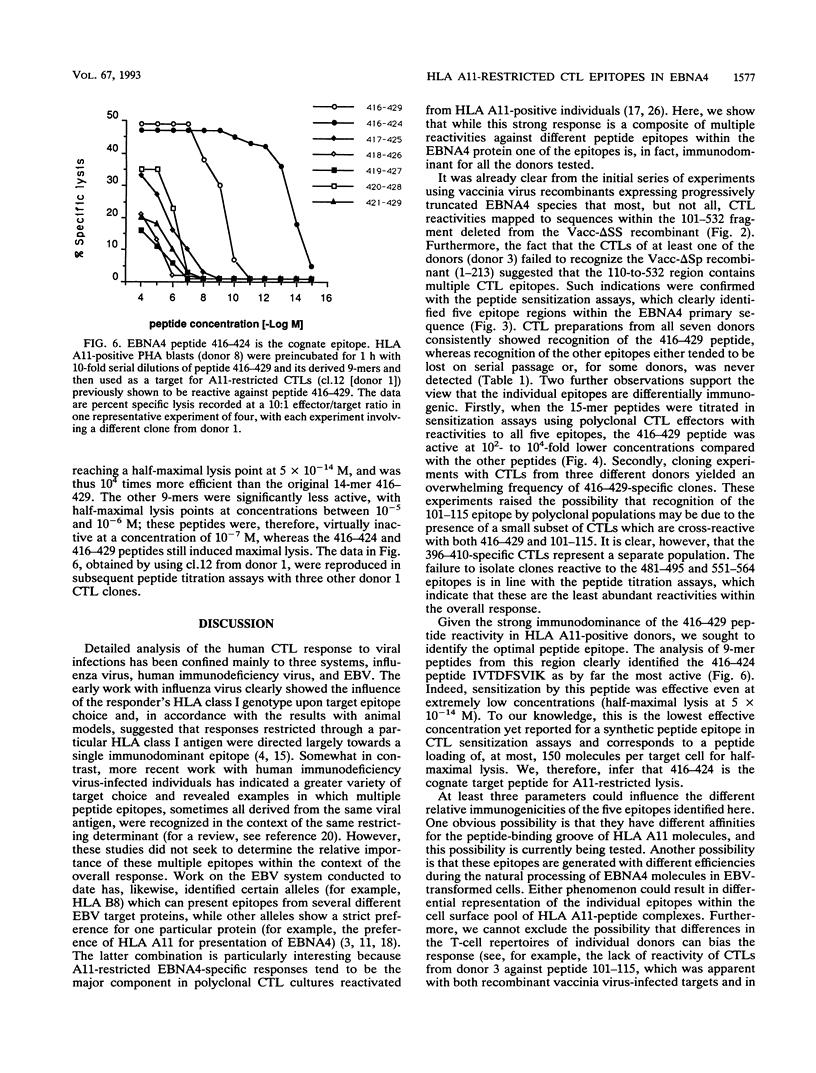
Epstein-Barr virus (EBV), a ubiquitous herpesvirus, induces potent HLA class I-restricted cytotoxic T-lymphocyte (CTL) responses. Analyses of target antigen choice have shown that the very strong CTL responses which are often observed through the HLA A11 allele map are due almost entirely to a single transformation-associated EBV protein, the nuclear antigen EBNA4. Here, we sought to determine the number and relative immunogenicities of HLA A11-restricted epitopes within this 938-amino-acid protein. An initial screening with a series of recombinant vaccinia virus vectors encoding progressively truncated forms of EBNA4 was followed by peptide sensitization experiments using overlapping 14- or 15-mers from the entire sequence. These two approaches allowed the identification of five epitope regions located between residues 101 and 115, 416 and 429, 396 and 410, 481 and 495, and 551 and 564 of the EBNA4 molecule. CTL preparations from all seven HLA A11-positive donors tested had demonstrable reactivities against the 416-to-429 peptide, whereas reactivities against the other epitopes either tended to be lost on serial passage or, for some of the donors, were never detected. The immunodominance of the 416-to-429 epitope was further supported by peptide dilution assays using polyclonal effectors and by CTL cloning experiments. Analysis of the 416-to-429 region identified the nanomer 416-424 (IVTDFSVIK) as the cognate peptide. This peptide was able to sensitize targets to lysis by A11-restricted CTL clones at concentrations as low as 5 x 10(-14) M.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doumas B. T. Standards for total serum protein assays--a collaborative study. Clin Chem. 1975 Jul;21(8):1159–1166. [PubMed] [Google Scholar]
- Gavioli R., De Campos-Lima P. O., Kurilla M. G., Kieff E., Klein G., Masucci M. G. Recognition of the Epstein-Barr virus-encoded nuclear antigens EBNA-4 and EBNA-6 by HLA-A11-restricted cytotoxic T lymphocytes: implications for down-regulation of HLA-A11 in Burkitt lymphoma. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5862–5866. doi: 10.1073/pnas.89.13.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotch F., Rothbard J., Howland K., Townsend A., McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. 1987 Apr 30-May 6Nature. 326(6116):881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- Gratama J. W., Oosterveer M. A., Zwaan F. E., Lepoutre J., Klein G., Ernberg I. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8693–8696. doi: 10.1073/pnas.85.22.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Zutter M. M., Minarovits J., Oosterveer M. A., Thomas E. D., Klein G., Ernberg I. Expression of Epstein-Barr virus-encoded growth-transformation-associated proteins in lymphoproliferations of bone-marrow transplant recipients. Int J Cancer. 1991 Jan 21;47(2):188–192. doi: 10.1002/ijc.2910470205. [DOI] [PubMed] [Google Scholar]
- Hanto D. W., Frizzera G., Gajl-Peczalska K. J., Simmons R. L. Epstein-Barr virus, immunodeficiency, and B cell lymphoproliferation. Transplantation. 1985 May;39(5):461–472. doi: 10.1097/00007890-198505000-00001. [DOI] [PubMed] [Google Scholar]
- Herbst H., Niedobitek G., Kneba M., Hummel M., Finn T., Anagnostopoulos I., Bergholz M., Krieger G., Stein H. High incidence of Epstein-Barr virus genomes in Hodgkin's disease. Am J Pathol. 1990 Jul;137(1):13–18. [PMC free article] [PubMed] [Google Scholar]
- Katsoyannis P. G., Schwartz G. P. The synthesis of peptides by homogeneous solution procedures. Methods Enzymol. 1977;47:501–578. doi: 10.1016/0076-6879(77)47049-6. [DOI] [PubMed] [Google Scholar]
- Khanna R., Burrows S. R., Kurilla M. G., Jacob C. A., Misko I. S., Sculley T. B., Kieff E., Moss D. J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992 Jul 1;176(1):169–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Jacob C. A., Burrows S. R., Kurilla M. G., Kieff E., Misko I. S., Sculley T. B., Moss D. J. Expression of Epstein-Barr virus nuclear antigens in anti-IgM-stimulated B cells following recombinant vaccinia infection and their recognition by human cytotoxic T cells. Immunology. 1991 Nov;74(3):504–510. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Santos-Aguado J., Strominger J. L. Effect of mutations and variations of HLA-A2 on recognition of a virus peptide epitope by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9194–9198. doi: 10.1073/pnas.85.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko I. S., Pope J. H., Hütter R., Soszynski T. D., Kane R. G. HLA-DR-antigen-associated restriction of EBV-specific cytotoxic T-cell colonies. Int J Cancer. 1984 Feb 15;33(2):239–243. doi: 10.1002/ijc.2910330212. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Brooks J. M., Thomas W. A., Rowe M., Kieff E., Rickinson A. B. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992 Jul 1;176(1):157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
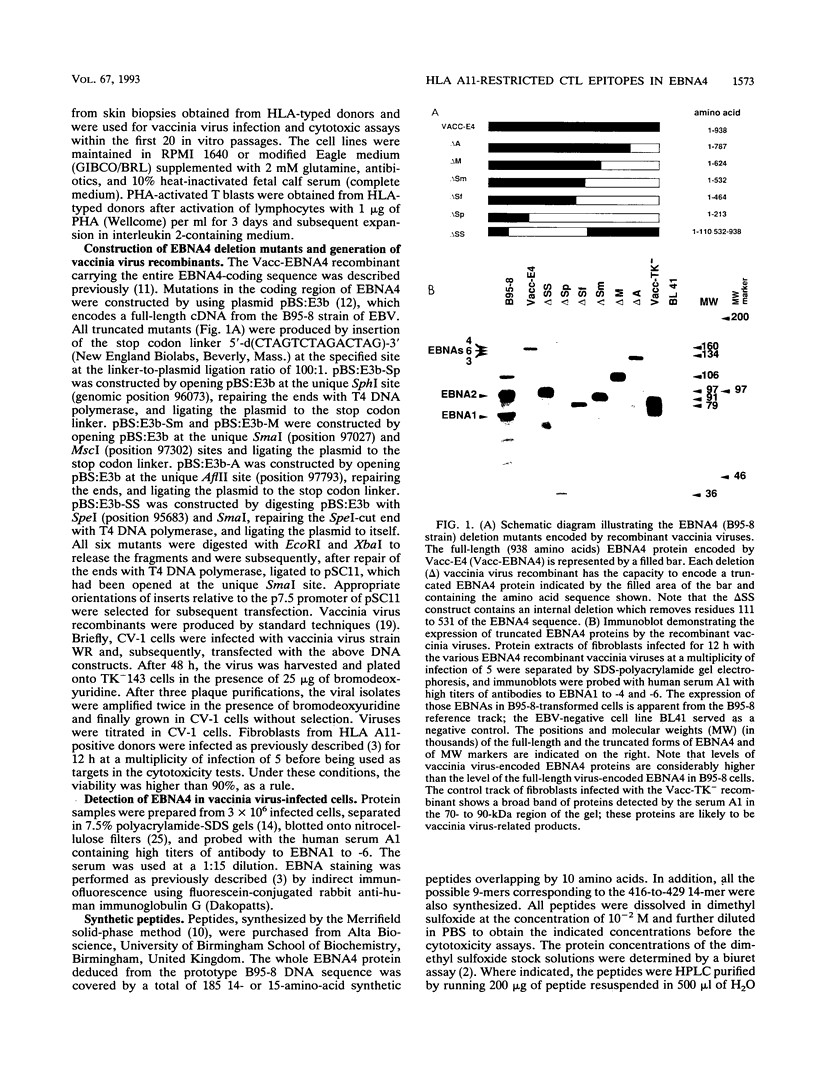
- Murray R. J., Kurilla M. G., Griffin H. M., Brooks J. M., Mackett M., Arrand J. R., Rowe M., Burrows S. R., Moss D. J., Kieff E. Human cytotoxic T-cell responses against Epstein-Barr virus nuclear antigens demonstrated by using recombinant vaccinia viruses. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2906–2910. doi: 10.1073/pnas.87.8.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon D. F., McMichael A. J. Cytotoxic T-cell recognition of HIV proteins and peptides. AIDS. 1991 Sep;5(9):1049–1059. [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Rickinson A. B., Moss D. J., Allen D. J., Wallace L. E., Rowe M., Epstein M. A. Reactivation of Epstein-Barr virus-specific cytotoxic T cells by in vitro stimulation with the autologous lymphoblastoid cell line. Int J Cancer. 1981 May 15;27(5):593–601. doi: 10.1002/ijc.2910270505. [DOI] [PubMed] [Google Scholar]
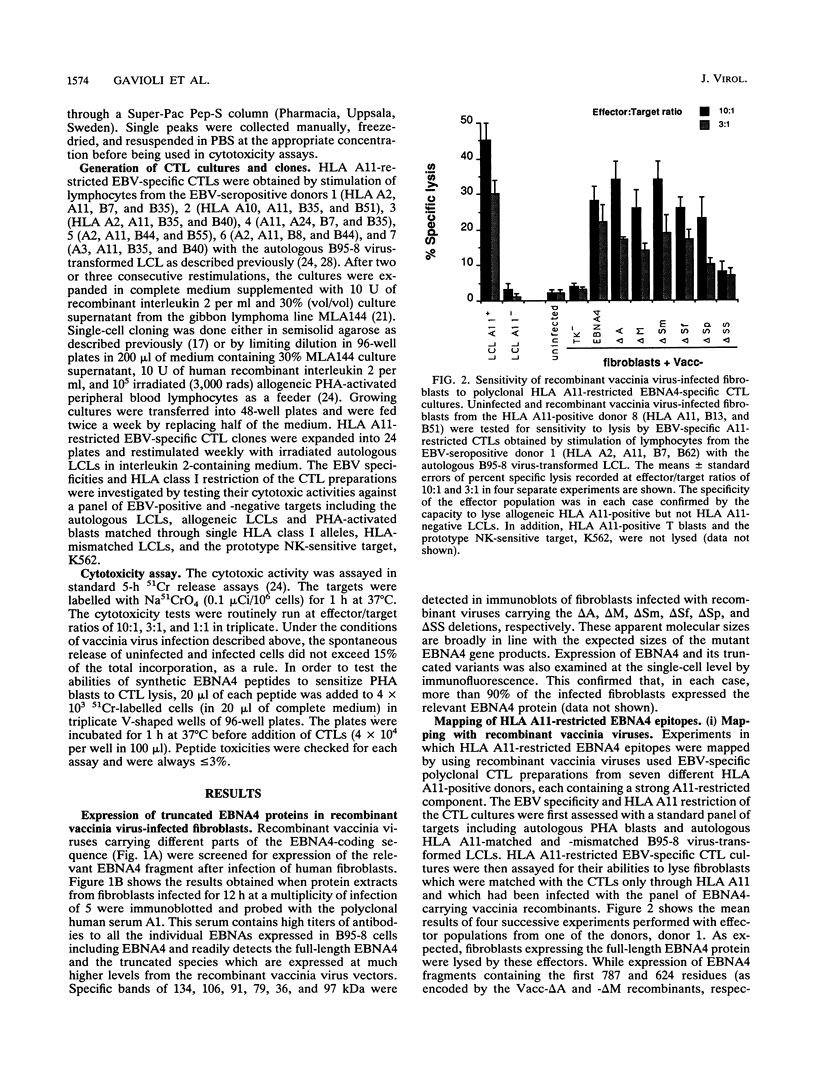
- Schmidt C., Burrows S. R., Sculley T. B., Moss D. J., Misko I. S. Nonresponsiveness to an immunodominant Epstein-Barr virus-encoded cytotoxic T-lymphocyte epitope in nuclear antigen 3A: implications for vaccine strategies. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9478–9482. doi: 10.1073/pnas.88.21.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsteinsdottir S., Masucci M. G., Ehlin-Henriksson B., Brautbar C., Ben Bassat H., Klein G., Klein E. Differentiation-dependent sensitivity of human B-cell-derived lines to major histocompatibility complex-restricted T-cell cytotoxicity. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5620–5624. doi: 10.1073/pnas.83.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
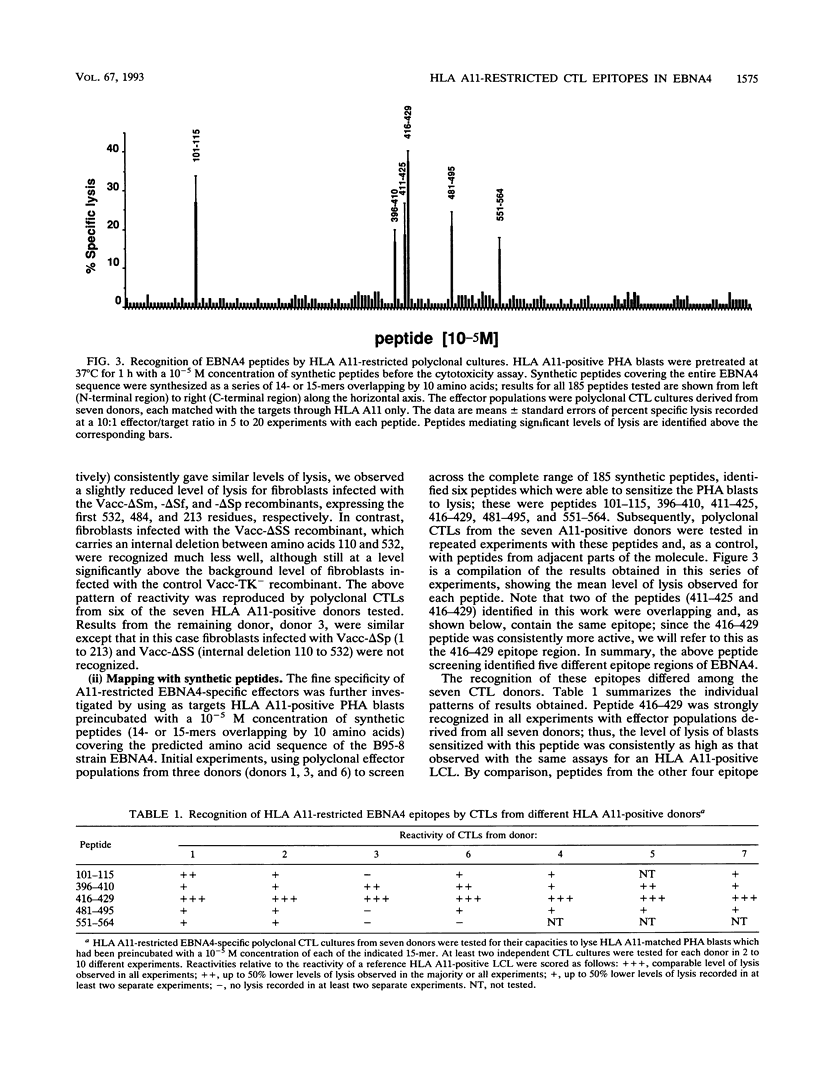
- Wallace L. E., Rickinson A. B., Rowe M., Epstein M. A. Epstein-Barr virus-specific cytotoxic T-cell clones restricted through a single HLA antigen. Nature. 1982 Jun 3;297(5865):413–415. doi: 10.1038/297413a0. [DOI] [PubMed] [Google Scholar]
- Wallace L. E., Rowe M., Gaston J. S., Rickinson A. B., Epstein M. A. Cytotoxic T cell recognition of Epstein-Barr virus-infected B cells. III. Establishment of HLA-restricted cytotoxic T cell lines using interleukin 2. Eur J Immunol. 1982 Dec;12(12):1012–1018. doi: 10.1002/eji.1830121206. [DOI] [PubMed] [Google Scholar]