Abstract
The CCND1 gene, a key cell-cycle regulator, is often altered in breast cancer, but the mechanisms underlying CCND1 dysregulation and the clinical significance of CCND1 status are unclear. We used real-time quantitative PCR and RT–PCR assays based on fluorescent TaqMan methodology to quantify CCND1 gene amplification and expression in a large series of breast tumours. CCND1 overexpression was observed in 44 (32.8%) of 134 breast tumour RNAs, ranging from 3.3 to 43.7 times the level in normal breast tissues, and correlated significantly with positive oestrogen receptor status (P=0.0003). CCND1 overexpression requires oestrogen receptor integrity and is exacerbated by amplification at 11q13 (the site of the CCND1 gene), owing to an additional gene dosage effect. Our results challenge CCND1 gene as the main 11q13 amplicon selector. The relapse-free survival time of patients with CCND1-amplified tumours was shorter than that of patients without CCND1 alterations, while that of patients with CCND1-unamplified-overexpressed tumours was longer (P=0.011). Only the good prognostic significance of CCND1-unamplified-overexpression status persisted in Cox multivariate regression analysis. This study confirms that CCND1 is an ER-responsive or ER-coactivator gene in breast cancer, and points to the CCND1 gene as a putative molecular marker predictive of hormone responsiveness in breast cancer. Moreover, CCND1 amplification status dichotomizes the CCND1-overexpressing tumors into two groups with opposite outcomes.
British Journal of Cancer (2002) 86, 580–586. DOI: 10.1038/sj/bjc/6600109 www.bjcancer.com
© 2002 Cancer Research UK
Keywords: breast cancer, CCND1 expression, quantitative RT–PCR, real-time PCR detection, prognostic value
Cyclin D1, a protein encoded by the CCND1 gene, has a well-established role in regulating progression through the G1 phase of the cell cycle. Cyclin D1 acts by complexing with the cyclin-dependent kinases CDK4 and CDK6, promoting phosphorylation and inactivation of retinoblastoma protein. CCND1 has been identified as an oncogene, and is rearranged, amplified or overexpressed in a variety of tumours (Motokura and Arnold, 1993). Recent results from several groups suggest that cyclin D1 may also be involved in the activities of transcription factors through CDK-independent mechanisms. Cyclin D1 can bind to and regulate the activity of several proteins, including myb-like transcription factor (DMP1) (Inoue and Sherr, 1998), the myogenic transcription factor MyoD (Skapek et al, 1996), and also the oestrogen receptor, through the recruitment of p300/CBP-associated protein (P/CAF) and steroid receptor coactivator-1 (SRC-1) (Zwijsen et al, 1997, 1998; Neuman et al, 1997; McMahon et al, 1999).
Cyclin D1 aberrations have been strongly linked to human breast cancer. Ectopic expression of cyclin D1 is sufficient to initiate cell cycle progression in the absence of external growth stimuli (Musgrove et al, 1994). Transgenic mice carrying the CCND1 gene driven by the mouse mammary tumour virus terminal repeat show altered mammary cell proliferation and a high incidence of mammary adenocarcinomas (Wang et al, 1994). Clinical studies have found amplification of 11q13 chromosomal region (which contains CCND1) in 10–15% of human primary breast cancers (Ali et al, 1989; Borg et al, 1991; Schuuring et al, 1992; Henry et al, 1993). However, overexpression (at both the mRNA and protein levels) is seen in about 50% of cases, suggesting that mechanisms other than DNA amplification may dysregulate cyclin D1 expression (McIntosh et al, 1995; Gillett et al, 1996; Barbareschi et al, 1997; Jares et al, 1997; Nielsen et al, 1997; Kenny et al, 1999). It is noteworthy that it has been previously described a high correlation between overexpression of CCND1 mRNA and increased presence of Cyclin D1 protein (Bartkova et al, 1994; Gillett et al, 1994).
The regulation of CCND1 gene expression is poorly understood. Experimental data show that cyclin D1 expression can be regulated by several factors which may be dysregulated in breast cancer, including growth factors (Musgrove et al, 1993), p53 through p21WAF1 (Chen et al, 1995) and oestrogen (Musgrove et al, 1994; Altucci et al, 1996). It is noteworthy that most CCND1-overexpressing tumours are oestrogen receptor-positive (Hui et al, 1996; Barbareschi et al, 1997; Jares et al, 1997). Finally, cyclin D1 is frequently overexpressed in ductal carcinoma in situ, and also in some benign breast diseases (Weinstat-Saslow et al, 1995; Alle et al, 1998), pointing to a role in the earliest stages of breast tumour development.
The action of cyclin D1 in cell cycle control, its role in murine mammary gland development and oncogenesis, its altered expression in half of all human breast tumours and in the earliest stages of breast oncogenesis, as well as its apparent involvement in the action of oestrogen, have led to numerous studies to ascertain whether cyclin D1 may serve as a biological marker in breast cancer. However, clinical studies have produced unexpected results. Indeed, CCND1 amplification has been linked to poor outcome (Ali et al, 1989; Borg et al, 1991; Schuuring et al, 1992; Henry et al, 1993), whereas overexpression of cyclin D1, as determined by immunohistochemical methods, has been linked to good outcome (Gillett et al, 1996). The latter association could be explained by a link between cyclin D1 overexpression and well-differentiated, ER-positive carcinomas, which carry a better prognosis. In this regard, we recently suggested in a small series of breast tumors (n=33) that CCND1 mRNA overexpression is related to oestrogen receptor positively (Spyratos et al, 2000).
CCND1 expression status might also be a useful marker to predict the response to endocrine therapy (Gillett et al, 1996; Sutherland et al, 1997; Wilcken et al, 1997; Barnes and Gillett, 1998).
Finally, CCND1 appears to be an outstanding candidate therapeutic target, and several studies have shown that antisense to CCND1 inhibits the growth and reverses the transformed phenotype of human cancer cells (Zhou et al, 1995; Arber et al, 1997).
These promising clinical perspectives call for a sensitive, accurate and rapid method to screen breast cancer patients for CCND1 amplification/overexpression. We developed a real-time quantitative RT–PCR assay based on TaqMan methodology to quantify CCND1 mRNA in homogeneous total RNA solutions obtained from tumour samples (Gibson et al, 1996). This method has excellent performance, accuracy and sensitivity, together with a wide dynamic range, a high throughput capacity and good interlaboratory agreement. In addition, it eliminates the need for tedious post-PCR processing.
To determine the prognostic value of CCND1 amplification and/or overexpression, we used this real-time PCR method to measure CCND1 gene expression at the mRNA level in a large series of unilateral invasive primary breast tumours (n=134) with known CCND1 gene status (Bièche et al, 1998) and available long-term outcome data.
As several studies have pointed to cooperation between the CCND1 and RB1 genes, and to their joint involvement in the proliferative capacity of tumour cells, we also sought a possible link between CCND1 DNA and/or mRNA status and RB1 mRNA underexpression.
MATERIALS AND METHODS
Patients and samples
We analyzed tissue from excised primary breast tumours of 134 women treated at the Centre René Huguenin from 1977 to 1989. The samples were examined histologically for the presence of tumour cells. A tumour sample was considered suitable for this study if the proportion of tumour cells was more than 60%. Immediately following surgery the tumour samples were stored in liquid nitrogen until RNA extraction.
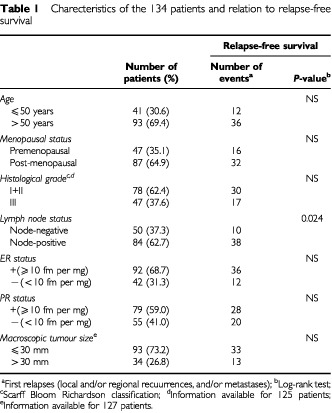
The patients (mean age 58.3 years, range 34–91) met the following criteria: primary unilateral non metastatic breast carcinoma on which complete clinical, histological and biological data were available; and no radiotherapy or chemotherapy before surgery. The main prognostic factors are presented in Table 1 . The median follow-up was 8.8 years (range 1.0–16.2). Forty-eight patients relapsed (the distribution of first relapse events was as follows: 14 local and/or regional recurrences, 30 metastases and four both).
Table 1. Charecteristics of the 134 patients and relation to relapse-free survival.
Specimens of adjacent normal breast tissue from 10 of the breast cancer patients, and normal breast tissue from 10 women undergoing cosmetic breast surgery were used as sources of normal RNA.
Real-time RT–PCR
Theoretical basis
Reactions are characterized by the point during cycling when amplification of the PCR product is first detected, rather than the amount of PCR product accumulated after a fixed number of cycles. The higher the starting quantity of the target molecule, the earlier a significant increase in fluorescence is observed. The parameter Ct (threshold cycle) is defined as the fractional cycle number at which the fluorescence generated by cleavage of the probe passes a fixed threshold above baseline. The CCND1 target message in unknown samples is quantified by measuring Ct and by using a standard curve to determine the starting target message quantity.
The precise amount of total RNA added to each reaction mix (based on optical density) and its quality (i.e. lack of extensive degradation) are both difficult to assess. We therefore also quantified transcripts of the gene coding for the TATA box-binding protein (TBP) (a component of the DNA-binding protein complex TFIID) as the endogeneous RNA control, and each sample was normalized on the basis of its TBP content.
For each experimental sample the amount of the targets and endogeneous reference is determined from the standard curve. Then, the target amount is divided by the endogeneous reference amount to obtain a normalized target value. The relative gene target expression level was also normalized to a normal breast tissue sample (calibrator), or 1×sample. Each of the normalized target values is divided by the calibrator normalized target value to generate the final relative expression levels.
Final results, expressed as N-fold differences in CCND1 gene expression relative to the TBP gene and the calibrator, termed ‘NCCND1’, was determined as follows:
 |
Primers, probes and PCR consumables
Primers and probes for the TBP and CCND1 genes were chosen with the assistance of the computer programs Oligo 4.0 (National Biosciences, Plymouth, MN, USA) and Primer Express (Perkin-Elmer Applied Biosystems, Foster City, CA, USA). The primer pairs for CCND1 were selected to be unique when compared with the sequences of the closely related CCND2 and CCND3 genes, and CCND2PS and CCND3PS pseudogenes. The nucleotide sequences of the oligonucleotide hybridization probes and primers are shown in Table 2
Table 2. Oligonucleotide primer and probe sequences used.
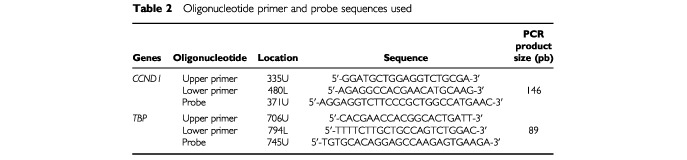
. Primers and Probes are designated by the nucleotide position (relative to TBP GenBank Number X54993 and CCND1 GenBank Number X59798) corresponding to the 5′ position, followed by the letter U for upper (sense strand) or L for lower (antisense strand). To avoid amplification of contaminating genomic DNA, one of the two primers or the probe was placed at the junction between two exons, or in a different exon. For example, the upper primer of TBP (706U) was placed in exon 5, the probe (745U) at the junction between exon 5 and exon 6, and the lower primer (794L) were placed in exon 6.
RNA extraction
Total RNA was extracted from breast specimens by using the acid-phenol guanidium method (Chomczynski and Sacchi, 1987). The quality of the RNA samples was determined by electrophoresis through denaturing agarose gels and staining with ethidium bromide, and the 18S and 28S RNA bands were visualized under ultraviolet light.
Standard curve construction
The relative kinetic method was applied using a standard curve. The latter was constructed with four-fold serial dilutions of total RNA from normal human breast tissues in mouse total RNA (Clontech, Category Number 64042-1). The standard curve used for reverse transcription is composed of five points (1000, 250, 62.5, 15.6 and 3.9 ng of human normal breast total RNA). The series of diluted human total RNAs was aliquoted and stored at −80°C until use.
cDNA synthesis
Reverse transcription of RNA was done in a final volume of 20 μl containing 1× RT–PCR buffer (500 mM each dNTP, 3 mM MgCl2, 75 mM KCl, 50 mM Tris-HCl pH 8.3), 10 units of RNasin™ Ribonuclease inhibitor (Promega, Madison, WI, USA), 10 mM dithiothreitol, 50 units of Superscript II RNase H- reverse transcriptase (Gibco–BRL, Gaithersburg, MD, USA), 1.5 mM random hexamers (Pharmacia, Uppsala, Sweden) and 1 μg of total RNA (standard curve point samples and patients' samples). The samples were incubated at 20°C for 10 min and 42°C for 30 min, and reverse transcriptase was inactivated by heating at 99°C for 5 min and cooling at 5°C for 5 min.
PCR amplification
All PCR reactions were performed using a ABI Prism 7700 Sequence Detection System (Perkin-Elmer Applied Biosystems). For each PCR run a master mix was prepared on ice with 1×TaqMan buffer, 5 mM MgCl2, 200 mM dATP, dCTP and dGTP and 400 mM dUTP, 300 nM each primer, 150 nM probe and 1.25 units of AmpliTaq Gold DNA polymerase (Perkin-Elmer Applied Biosystems). Ten microliters of each appropriate diluted RT sample (standard curve points and patients' samples) was added to 40 μl of the PCR master-mix. The thermal cycling conditions comprised an initial denaturation step at 95°C for 10 min and 50 cycles at 95°C for 15 s and 65°C for 1 min.
Experiments were performed with duplicates for each data point. All the patients' samples with a CV of the number of TBP or CCND1 mRNA copies higher than 10% were retested.
Statistical analysis
Relapse-free survival (RFS) was determined as the interval between diagnosis and detection of the first relapse (local and/or regional recurrences, and/or metastases). Clinical, histological and biological parameters were compared using the chi-square test. Differences between the two populations were judged significant at confidence levels greater than 95% (P<0.05). Survival distributions were estimated by the Kaplan–Meier method (Kaplan and Meier, 1958), and the significance of differences between survival rates was ascertained using the log-rank test. Multivariate analysis using Cox's proportional hazards model (Cox, 1972) was used to assess the independent contribution of each variable to RFS.
RESULTS
Validation of the standard curve and dynamic range of real-time RT–PCR
The dynamic range of the CCND1 real-time RT–PCR assay was wide (at least three orders of magnitude) with samples containing as much as 50 ng or as little as 0.2 ng equivalent total cDNA. A strong linear relationship between the Ct and the log of starting copy number was always demonstrated (R2⩾0.99). The efficiency of the reaction (E), calculated by the formula: E=101/|m|−1, where m is the slope of standard curve line, was ranged from 90 to 100% for the different assays. All breast tissue samples which were analyzed consistently fell within the calibration curve.
CCND1 mRNA level in normal breast tissues
To determine the cut-off point for altered CCND1 gene expression at the RNA level in breast cancer tissue, the NCCND1 value, calculated as described in Materials and methods, was determined for 20 normal breast tissue RNAs. As this value consistently fell between 0.6 and 1.8 (mean 1.03±0.37 standard deviation), values of three (mean+5 s.d.) or more were considered to reflect overexpression of the CCND1 gene in tumour RNA samples.
CCND1 mRNA level in breast tumour tissues
Among the 134 breast tumour RNA samples tested, 44 (32.8%) showed CCND1 overexpression. Major differences in the amount of CCND1 mRNA were observed (NCCND1 from 3.3 to 43.7); 19 tumours (14.2%) had an expression level three to five times higher than that of normal breast tissue, while 15 tumours (11.2%) contained amounts five to 10 times higher, six tumours (4.5%) 10 to 20 times higher, and four tumours (3.0%) more than 20 times higher. Among the 10 patients in whom both the primary breast tumour and matched normal breast tissue were investigated, CCND1 expression was far higher in three tumours than in the normal tissue (NCCND1=8.1, 4.8 and 3.6, compared to 0.9, 1.2 and 0.2, respectively).
Relationship between the CCND1 RNA levels and CCND1 amplification levels
All 134 tumours studied for CCND1 expression at the RNA level had previously been tested for CCND1 amplification by Southern blot analysis (unpublished data), and 94 had also been tested (when DNA was still available) with a real-time quantitative PCR assay based on TaqMan technology (Bièche et al, 1998). As the TaqMan technology was more sensitive than Southern blotting (Bièche et al, 1998), we increased the cut-off for gene amplification in the real-time PCR assay from two to 2.5 to have a total correlation between this latter method and Southern blot analysis. With the new cut-off, 15 (11.2%) of the 134 tumours tested here showed CCND1 amplification. CCND1 overexpression was found in all but three of the tumours that showed 11q13 amplification ( Table 3 ). The CCND1 mRNA and DNA status of these three tumours was confirmed by conducting a second RNA and DNA extraction, by additional real-time quantitative PCR and RT–PCR analyses (use of new primer pairs for the CCND1 and TBP genes, and an additional endogeneous RNA control; the RPLP0 gene (also known as 36B4) encoding human acidic ribosomal phosphoprotein P0) and by Northern and Southern analysis.
Table 3. NCCND1 value and ER status in the 15 CCND1-amplified tumours.

Interestingly, the CCND1-amplified-overexpressed tumours contained larger amounts of CCND1 mRNA (12 tumours; mean NCCND1 17.9, range 7.6 to 43.7) than did the CCND1-unamplified-overexpressed tumours (32 tumours; mean NCCND1 5.6; range 3.3 to 8.6).
Correlation between CCND1 mRNA and DNA status and clinical, pathological and biological parameters
We sought links between CCND1 mRNA and DNA status (alteration versus normal) and standard clinical, pathological and biological factors in breast cancer ( Table 4 ). Patients with CCND1-altered tumours (n=47) were subdivided into those with CCND1-amplified tumours (n=15) and those with CCND1-unamplified-overexpressed tumours (n=32).
Table 4. Relationship between CCND1 mRNA and DNA status and standard clinicopathological and biological factors.
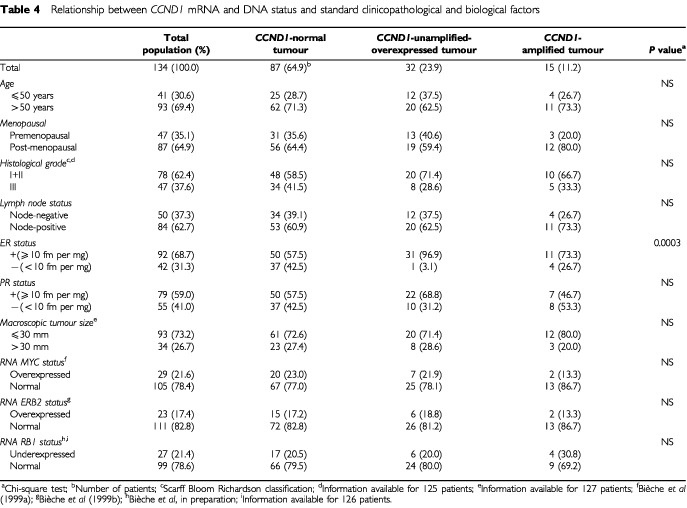
The only statistically significant link was between CCND1-unamplified-overexpressed tumours and oestrogen receptor positivity (P=0.0003). Only one (3.1%) of the 32 patients with CCND1-unamplified-overexpressed tumours was oestrogen receptor-negative, compared with 41 (40.2%) of the other 102 patients. It should be noted that this isolated tumour had a very low level of CCND1 overexpression (NCCND1=3.3).
Neither CCND1 amplification nor CCND1 overexpression without amplification was significantly linked to menopausal status or standard prognostic factors such as macroscopic tumour size, histopathological grade and lymph-node or progesterone receptor status.
Univariate analysis (log-rank test) showed that relapse-free survival (RFS) was linked to CCND1 status (P=0.011; Figure 1 ). The RFS of the 15 patients with CCND1-amplified tumours (5-year RFS 63.0% (36.6–89.4); RR=0.37 (0.15–0.87)) and of the 32 patients with CCND1-unamplified-overexpressed tumours (5-year RFS 93.8% (85.4–100); RR=1.8 (0.7–4.0)) were respectively shorter and longer than the RFS of the 87 patients without CCND1 alterations (5-year RFS 80.7% (72.2–89.2); RR=1). The prognostic significance of CCND1 mRNA and DNA status persisted for lymph-node-negative (P=0.022) but not for lymph-node-positive patients (P=0.13). Using a Cox proportional hazards model, we also assessed the prognostic value, for RFS, of parameters that were significant in univariate analysis, i.e. lymph-node status (Table 1) and CCND1-unamplified-overexpression and CCND1-amplification status (Figure 1). The prognostic significance of lymph-node and CCND1-unamplified-overexpression status only persisted in Cox multivariate regression analysis ( Table 5 ). The adjusted relative risk associated with these two parameters, taking into account menopausal status, macroscopic tumour size, histological grade and steroid receptor status, did not change their prognostic significance for RFS (data not shown).
Figure 1.

RFS curves for patients with CCND1-unamplified-over expressed, CCND1-amplified and CCND1-normal tumours.
Table 5. Multivariate analysis of relapse-free survival.

Relationship between CCND1 mRNA and DNA status and MYC, ERBB2 and RB1 expression status
Because alterations in any component in the cell cycle regulatory p16/CCND1/RB1 pathway may have similar oncogenic effects, we studied the relationship between abnormalities of the CCND1 gene and altered expression of the RB1 gene, which had already been tested at the mRNA level (Bièche and Lidereau, 2000). We observed no correlation (or a negative correlation) between CCND1 mRNA and/or DNA alterations and RB1 underexpression (Table 4).
We also observed no link between CCND1 gene abnormalities and altered mRNA expression of the MYC and ERBB2 genes (Table 4).
DISCUSSION
The aim of this study was to assess the prognostic significance of CCND1 status at both the RNA level (to identify overexpression) and the DNA level (to identify gene amplification) in 134 unilateral invasive primary breast tumours with a known long-term outcome. The frequencies of CCND1 amplification (11.2%) and overexpression (33.6%) are in agreement with those previously reported in the literature (Ali et al, 1989; Borg et al, 1991; Schuuring et al, 1992; Henry et al, 1993; McIntosh et al, 1995; Gillett et al, 1996; Barbareschi et al, 1997; Jares et al, 1997; Nielsen et al, 1997; Kenny et al, 1999). Joint analysis of the CCND1 gene at both the mRNA and DNA levels showed that patients with a good outcome had CCND1-unamplified-overexpressed tumours while those with a poor outcome had CCND1-amplified tumours. Our results confirm the poor outcome associated with CCND1 amplification (Ali et al, 1989; Borg et al, 1991; Schuuring et al, 1992; Henry et al, 1993). More interestingly, they suggest that CCND1 amplification status should be taken into account when studying the prognostic significance of CCND1 overexpression. Indeed, the good outcome of patients with CCND1-overexpressing tumours was reverted by CCND1 amplification. This may explain why some authors have linked CCND1 overexpression to good outcome (Gillett et al, 1996; Nielsen et al, 1997), while others to poor outcome (McIntosh et al, 1995; Kenny et al, 1999).
These data on the CCND1 gene status obtained at both the RNA and DNA levels shed light on several important questions.
The mechanisms underlying CCND1 overexpression in unamplified tumours
These data confirm our previous report from a small series of breast tumours (Spyratos et al, 2000) where CCND1 overexpression is strongly linked to oestrogen receptor positivity. However, is CCND1 overexpression a cause or a consequence of transcriptional activation of oestrogen receptors in breast tumour cells? Previously reported in vitro data suggest that CCND1 overexpression is dependent on the presence of oestrogen and oestrogen receptors, and that anti-oestrogens inhibit cyclin D1 expression in breast cancer cells (Sutherland et al, 1997). However, no oestrogen response element (ERE) has been identified in the CCND1 promoter. Sabbah et al (1999) recently suggested a mechanism by which ER regulates CCND1 gene transcription through a cyclic AMP response element (CRE). Alternatively, there is increasing evidence that cyclin D1 forms a direct complex with the oestrogen receptor and can regulate this transcriptional activity without the need for oestrogen (Neuman et al, 1997; Zwijsen et al, 1997; McMahon et al, 1999). Zwijsen et al (1997) observed direct physical binding of cyclin D1 to the hormone-binding domain of the oestrogen receptor, resulting in increased binding of the receptor to oestrogen response-element sequences and upregulating oestrogen receptor-mediated transcription. Activation of the oestrogen receptor by cyclin D1 is independent of complex formation to a CDK partner but necessitates the recruitment of p300/CREB-binding protein-associated protein (P/CAF) and steroid receptor coactivator-1 (SRC-1), and is not inhibited by anti-oestrogens. Wilcken et al (1997) showed that tamoxifen inhibition of cell progression was overcome in both T-47D and MCF7 cells when cyclin D1 expression was ectopically induced. The influence of cyclin D1 status on the response to oestrogen and anti-oestrogens such as the tamoxifen warrants further study.
The good outcome of patients with CCND1 overexpressing tumours
One simple explanation is that CCND1 overexpression is associated with well-differentiated, oestrogen receptor-positive tumours (which are known to have a more favourable prognosis and a respond better to anti-oestrogen therapy. Alternatively, it may be due to more rapid cell proliferation and, thus, greater chemosensitivity. The possible relation between CCND1 overexpression and the response to chemotherapy could not be studied in this retrospective series of unselected patients because the treatments used after surgery were highly variable. To test this hypothesis, it will be necessary to conduct a prospective randomized clinical study to show that CCND1 overexpression do influence outcome only in patients who received chemotherapy as compared to untreated patients.
The possible involvement of the CCND1 gene in 11q13 amplicon selection and the poorer outcome of patients with 11q13-amplified tumours
The more plausible explanation is that CCND1 has a true role of oncogene and it is a more important gene as a driving force for 11q13 amplification. Amplification at 11q13 leads to higher CCND1 expression, resulting in more rapid proliferation of epithelial breast tumours and, thus, in poorer outcome.
However, three of the 15 11q13-amplified tumours were examined had not CCND1 overexpression (Table 3). Similar breast tumours have been observed by several other authors (Gillett et al, 1994; Barbareschi et al, 1997). Moreover, we previously showed that CCND1 mRNA overexpression is not related to a proliferative marker, the S-phase fraction, measured by flow cytometry (Spyratos et al, 2000).
Conversely, the amplification unit on chromosome 11q13 may encompass other gene(s) that could be the major 11q13 amplicon selector and whose overexpression might contribute to poor clinical outcome. One candidate is the oncogene EMS1, which is located approximately 800 kb telomeric to CCND1 and encodes an cytoskeletal actin-binding protein. It is amplified and overexpressed independently of CCND1 and oestrogen receptor expression, and, in contrast to cyclin D1, is not regulated by oestrogen (Hui et al, 1998). Thus, CCND1 overexpression could be due exclusively to the presence of oestrogen receptors, and the higher CCND1 overexpression observed in amplified tumours than in overexpressed-unamplified tumours could be due to a simple gene dosage effect. In agreement with this hypothesis, the three 11q13-amplified tumours with CCND1 normal expression were oestrogen receptor-negative (Table 3). However, it should be noted that we also observed one oestrogen receptor-negative tumour with CCND1 overexpression and amplification (Tumour CCND1209; Table 3).
Finally, 11q13 amplification may simply reflect genomic instability in breast tumours. Coquelle et al (1998) showed a key role of hypoxia in inducing breaks at fragile sites and initiating intrachromosomal amplification. Such fragile sites (FRA11A and FRA11F) are located on each side (centromeric and telomeric) of the 11q13 amplicon.
In conclusion, we observed a major link between CCND1 mRNA status and ER status, confirming a role for the CCND1 gene as an ER-responsive gene or ER-coactivator gene in breast cancer. CCND1 amplification might simply be an additional mechanism contributing to high levels of CCND1 overexpression observed in oestrogen receptor-positive tumours, through a simple gene dosage effect. These findings, together with the observation of several oestrogen receptor-negative tumours with 11q13 amplification but no CCDN1 overexpression, challenge CCND1 gene as the main 11q13 amplicon selector.
CCND1 may serve as a molecular marker for predicting hormone responsiveness in breast cancer, as hypothesis currently being tested in a large and homogeneous clinical study.
Acknowledgments
This work was supported by the Association pour la Recherche sur le Cancer and the Ministère de l'Enseignement Supérieur de la Recherche. We thank the Centre René Huguenin staff for assistance in specimen collection and patient care.
References
- AliIUMerloGCallahanRLidereauR1989The amplification unit on chromosome 11q13 in aggressive primary human breast tumors entails the bcl-1, int-2 and hst loci Oncogene 48992 [PubMed] [Google Scholar]
- AlleKMHenshallSMFieldASSutherlandRL1998Cyclin D1 protein is overexpressed in hyperplasia and intraductal carcinoma of the breast Clin Cancer Res 4847854 [PubMed] [Google Scholar]
- AltucciLAddeoRCicatielloLDauvoisSParkerMGTrussMBeatoMSicaMVBrescianiFWeiszA199617 beta-estradiol induces cyclin-D-1 gene transcription, p36(D1)-p34(cdk4) complex activation and p105(Rb) phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer Oncogene 1223152324 [PubMed] [Google Scholar]
- ArberNDokiYHanEKSgambatoAZhouPKimNHDeloheryTKleinMGHoltPRWeinsteinIB1997Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells Cancer Res 5715691574 [PubMed] [Google Scholar]
- BarbareschiMPelosioPCaffoOButittaFPellegriniSBarbazzaRDalla PalmaPBevilacquaGMarchettiA1997Cyclin-D1-gene amplification and expression in breast carcinoma: relation with clinicopathological characteristics and with retinoblastoma gene product, p53 and p21WAF1 immunohistochemical expression Int J Cancer (Pred Oncol) 74171174 [DOI] [PubMed] [Google Scholar]
- BarnesDMGillettCE1998Cyclin D1 in breast cancer Breast Cancer Res Treat 52115 [DOI] [PubMed] [Google Scholar]
- BartkovaMLucasJMullerHLutzhoftDStraussMBartekJ1994Cyclin D1 protein expression and function in human breast cancer Int J Cancer 57353361 [DOI] [PubMed] [Google Scholar]
- BiècheIOliviMChampemeMHVidaudDLidereauRVidaudM1998Novel approach to quantitative polymerase chain reaction using real-time detection: application to the detection of gene amplification in breast cancer Int J Cancer 78661666 [DOI] [PubMed] [Google Scholar]
- BiècheILaurendeauITozluSOliviMVidaudDLidereauRVidaudM1999aQuantitation of myc gene expression in sporadic breast tumors with a real-time reverse transcription-PCR assay Cancer Res 5927592765 [PubMed] [Google Scholar]
- BiècheIOnodyPLaurendeauIOliviMVidaudDLidereauRVidaudM1999bReal-time reverse transcription PCR assay for future management of ERBB2-based clinical applications Clin Chem 4511481156 [PubMed] [Google Scholar]
- BiècheILidereauR2000Loss of heterozygosity at 13q14 correlates with RB1 gene underexpression in human breast cancer Mol Carcinog 29151158 [PubMed] [Google Scholar]
- BorgASigurdssonHClarkGMFernoMFuquaSAOlssonHKillanderDMcGurieWL1991Association of INT2/HST1 complification in primary breast cancer with hormone-dependent phenotype and poor prognosis Br J Cancer 63136142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChenXBargonettiJPrivesC1995P53, through p21 (WAF1-CIP1), induces cyclin-D1 synthesis Cancer Res 5542574263 [PubMed] [Google Scholar]
- ChomczynskiPSacchiN1987Single-step method of RNA isolation by acid guanidium thlocyanate-phenol-chloroform extraction Anal Biochem 162156159 [DOI] [PubMed] [Google Scholar]
- CoquelleAToledoFSternSBiethADebatisseM1998A new role for hypoxia in tumor progression: induction of fragile site triggering genomic rearrangements and formation of complex DMs and HSRs Mol Cell 2259265 [DOI] [PubMed] [Google Scholar]
- CoxDR1972Regression models and life-tables J R Stat Soc (B) 34187200 [Google Scholar]
- GibsonUEMHeidCAWilliamsPM1996A novel method for real time quantitative RT–PCR Genome Res 69951001 [DOI] [PubMed] [Google Scholar]
- GillettCFantlVFisherCBartekJDicksonCBarnesDPetersG1994Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining Cancer Res 5418121817 [PubMed] [Google Scholar]
- GillettCSmithPGregoryWRichardsMMillisRPetersGBarnesD1996Cyclin D1 and prognosis in human breast cancer Int J Cancer (Pred Oncol) 699299 [DOI] [PubMed] [Google Scholar]
- HenryJAHennessyCLevettDLLennardTWWestleyBRMayFE1993Int-2 amplification in breast cancer: association with decreased survival and relationship to amplification of c-erbB-2 and c-myc Int J Cancer 53774780 [DOI] [PubMed] [Google Scholar]
- HuiRCornishALMcClellandRARobertsonJFRBlameyRWMusgroveEANicholsonRISutherlandRL1996Cyclin D1 and estrogen receptor messenger RNA levels are positively correlated in primary breast cancer Clin Cancer Res 2923928 [PubMed] [Google Scholar]
- HuiRBallJRMacMillanRDKennyFSPrallOWJCampbellDHCornishALMcClellandRADalyRJForbesJFBlameyRWMusgroveEARobertsonJFNicholsonRISutherlandRL1998EMS1 gene expression in primary breast cancer: relationship to cyclin D1 and oestrogen receptor expression and patient survival Oncogene 1710531059 [DOI] [PubMed] [Google Scholar]
- InoueKSherrCJ1998Gene expression and cell cycle arrest mediated by transcription factor DMP1 is antagonized by D-type cyclins through a cyclin-dependent-kinase-independent mechanism Mol Cell Biol 1815901600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JaresPReyMJFernandezPLCampoENadalAMunozMMallofréCMuntanéJNayachILEstapéJCardesaA1997Cyclin D1 and retinoblastoma gene expression in human breast carcinoma: correlation with tumour proliferation and oestrogen receptor status J Pathol 182160166 [DOI] [PubMed] [Google Scholar]
- KaplanELMeierP1958Nonparametric estimation from incomplete observations J Am Stat Assoc 53457481 [Google Scholar]
- KennyFSHuiRMusgroveEAGeeJMWBlameyRWNicholsonRISutherlandRLRobertsonJFR1999Overexpression of Cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer Clin Cancer Res 520692076 [PubMed] [Google Scholar]
- McIntoshGGAndersonJJMiltonIStewaedMParrAHThomasMDHenryJAAngusALennardTWJHorneCHW1995Determination of the prognostic value of cyclin D1 overexpression in breast cancer Oncogene 11885891 [PubMed] [Google Scholar]
- McMahonCSuthiphongchaiTDiRenzoJEwenME1999P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor Proc Natl Acad Sci USA 9653825387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MotokuraTArnoldA1993Cyclins and oncogenesis Biochim Biophys Acta 11556378 [DOI] [PubMed] [Google Scholar]
- MusgroveEAHamiltonJALeeCSSweeneyKJWattsCKSutherlandRL1993Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression Mol Cell Biol 1335773587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MusgroveEALeeCSBuckleyMFSutherlandRL1994Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle Proc Natl Acad Sci USA 9180228026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NeumanELadhaMHLinNUptonTMMillerSJDiRenzoJPestellRGHindsPWDowdySFBrownMEwenME1997Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4 Mol Cell Biol 1753385347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NielsenNHEmdinSOCajanderJLandbergG1997Deregulation of cyclin E and D1 in breast cancer is associated with inactivation of the retinoblastoma protein Oncogene 14295304 [DOI] [PubMed] [Google Scholar]
- SabbahMCourilleauDMesterJRedeuilhG1999Estrogen induction of the cyclin D1 promoter: Involvement of a cAMP response-like element Proc Natl Acad Sci USA 961121711222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchuuringEVerhoevenEVan TinterenHPeterseJLNunninkBThunnissenFBDevileePCornelisseCJVan de VijverMJMooiWJMichalidesRJAM1992Amplification of genes within the chromosome 11q13 region is indicative of poor prognosis in patients with operable breast cancer Cancer Res 5252295234 [PubMed] [Google Scholar]
- SkapekSXRheeJKimPSNovitchBGLassarAB1996Cyclin-mediated inhibition of muscle gene expression via a mechanism that is independent of pRB hyperphosphorylation Mol Cell Biol 1670437053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SpyratosFAndrieuCVidaudDBriffodMVidaudMLidereauRBiècheI2000CCND1 mRNA overexpression is highly related to estrogen receptor positively but not to proliferative markers in primary breast cancer. Int J Biol Markers 15210214 [PubMed] [Google Scholar]
- SutherlandRLPrallOWJAlleKMWilckenNRCHuiRBallJRSarcevicBHenshallSMMusgroveEAWattsCKW1997Cyclin-dependent kinases as down-stream targets of oestrogen action: potential prognostic indicators and therapeutic targets Endocrine Related Cancer 4357370 [Google Scholar]
- WangTCCardiffRDZukerbergLLeesEArnoldASchmidtEV1994Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice Nature 369669671 [DOI] [PubMed] [Google Scholar]
- Weinstat-SaslowDMerinoMJManrowRELawrenceJABluthRFWittenbelKDSimpsonJFPageDLSteegPS1995Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions Nature Med 112571260 [DOI] [PubMed] [Google Scholar]
- WilckenNRPrallOWMusgroveEASutherlandRL1997Inductible overexpression of cyclin D1 in breast cancer cells reverses the growth-inhibitory effects of antiestrogenes Clin Cancer Res 3849854 [PubMed] [Google Scholar]
- ZhouPJiangWZhangYJKahnSMSchierenISantellaRMWeinsteinIB1995Antisense to cyclin D1 inhibits growth and reverses the transformed phenotype of human esophageal cancer cells Oncogene 11571580 [PubMed] [Google Scholar]
- ZwijsenRMWientjensEKlompmakerRVan der SmanJBernardsRMichalidesRJ1997CDK-independent activation of estrogen receptor by cyclin D1 Cell 88405415 [DOI] [PubMed] [Google Scholar]
- ZwijsenRMBuckleRSHijmansEMLoomansCJBernardsR1998Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1 Genes Dev 1234883498 [DOI] [PMC free article] [PubMed] [Google Scholar]


