Abstract
Elevated levels of epidermal growth factor receptor in head and neck cancer have been extensively reported, and are correlated with poor prognosis. The combination of cisplatin and 5-fluorouracil is a standard treatment regimen for head and neck cancer, with radiation representing another therapeutic option. Six head and neck cancer cell lines were used to study the cytotoxic effects of combining ZD1839 (‘Iressa’), a new selective epidermal growth factor receptor tyrosine kinase inhibitor, and radiation. Two of the cell lines were also used to study the combination of ZD1839 and cisplatin/5-fluorouracil. Cytotoxic effects were assessed by the MTT test. The results indicated that ZD1839 applied before radiation gave the best effects (P=0.002); an effect that was strongest in those p53-mutated cell lines that express the highest epidermal growth factor receptor levels. The effects of ZD1839 with cisplatin and/or 5-fluorouracil were sequence dependent (P<0.003), with the best results achieved when ZD1839 was applied first. For the triple combinations, ZD1839 applied before cisplatin and 5-fluorouracil resulted in a slight synergistic effect (P=0.03), although the effect was greater when ZD1839 was applied both before and during cytotoxic drug exposure. In conclusion, ZD1839 applied before radiation and before and/or during cisplatin/5-fluorouracil may improve the efficacy of treatment for head and neck cancer.
British Journal of Cancer (2002) 86, 819–827. DOI: 10.1038/sj/bjc/6600103 www.bjcancer.com
© 2002 Cancer Research UK
Keywords: ZD1839, fluorouracil, cisplatin, radiation, head and neck cancer cell lines, combination therapy
Epidermal growth factor receptor (EGFR) has been particularly well studied in head and neck cancer (HNC) because of its association with the cellular mechanisms involved in tumour progression. High levels of EGFR expression have been correlated with poor prognosis (Santini et al, 1991; Dassonville et al, 1993). EGFR signalling has been found to control not only cell growth, but also angiogenesis and DNA repair (Wheeler et al, 1999; Schlessinger, 2000), and has recently been assessed as an innovative target in cancer therapy and particularly in HNC. To date, clinical studies in this area have involved the administration of cetuximab (C225, a chimeric monoclonal antibody), either alone or in combination with chemotherapy or radiotherapy, to patients with advanced head and neck squamous cell carcinoma (Ezekiel et al, 1999; Huang et al, 1999; Mendelsohn et al, 1999; Baselga et al, 2000; Huang and Harari, 2000; Milas et al, 2000). More recently, a wide range of small-molecule tyrosine kinase inhibitors have been developed, and one of the most advanced in this class of compounds is ZD1839 (‘Iressa’), which is a quinazoline derivative. This agent interacts specifically with the highly conserved ATP binding site of the tyrosine kinase domain of EGFR, resulting in inhibition of ligand-induced EGFR activation, and blockade of signal transduction pathways (Ciardiello et al, 2000; Sirotnak et al, 2000).
The cisplatin/5-fluorouracil (5-FU) regimen is considered to be a standard chemotherapy regimen in the treatment of advanced HNC as part of an organ-conserving strategy (Taylor et al, 1997; Brizel et al, 1998; Calais et al, 1999). Another standard treatment is the combination of external beam radiation therapy with surgical extirpation (Bensadoun et al, 1998; Fu et al, 2000; Vokes et al, 2000). Despite the effectiveness of these treatments, the survival rates vary according to the tumour site and stage, and globally, prognosis remains poor (Vokes et al, 1993). Thus, one interesting and promising research direction for altering the natural history of HNC could be a molecular-targeted therapy against EGFR in association with one of the standard therapeutic strategies. Experimental data have indicated that application of EGFR-targeting agents can not only slow down cell proliferation, but also improve apoptotic capacities and decrease DNA repair. These observations suggest that EGFR targeting can lead to chemo- and radiosensitisation and recent experimental results tend to confirm this view (Huang et al, 1999).
The aim of this study was two-fold: firstly, to explore the sequence-dependent cytotoxic effects of combining ZD1839 with radiation using a panel of six human head and neck squamous cell carcinoma cell lines; and secondly, to investigate the sequence-dependent cytotoxic effects of combining ZD1839 with cisplatin and/or 5-FU on two of the cell lines from the panel. This preclinical work was undertaken to serve as a rationale to support ongoing clinical investigations of ZD1839 in HNC patients.
MATERIALS AND METHODS
Chemicals
ZD1839 was kindly provided by AstraZeneca. A 50 mM working solution in dimethysulphoxide (DMSO) was prepared before use. Human recombinant 125I-EGF (ref IM 196, specific activity 4514×1010 Bq mmol−1, 92.5×104 Bq per 250 μl) and unlabelled human recombinant EGF (ref ARN 5100) were obtained from Amersham. Dulbecco's modification of Eagle's medium (DMEM), RPMI 1640 and glutamine were purchased from Whittaker (Verviers, Belgium). Foetal bovine serum (FBS) was obtained from Dutscher (Brumath, France). Penicillin and streptomycin were from Meyrieux (Lyons, France). Transferrin and insulin were purchased from Flow (Irvine, Scotland). Bovine serum albumin (BSA), 3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and DMSO were purchased from Sigma (St Quentin Fallavier, France).
Cell lines
Six HNC cell lines of human origin were used (CAL27, CAL33, CAL60, CAL166, Hep-2, Detroit562) (Table 1). Cells were routinely cultured in DMEM supplemented with 10% FBS, 2 mM glutamine, 600 μg l−1 insulin, 500 μg l−1 transferrin, 50 000 units l−1 penicillin and 80 μM streptomycin in a fully humidified incubator (Sanyo, Japan) at 37°C in an atmosphere containing 8% CO2.
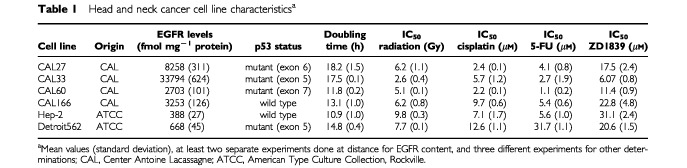
Table 1. Head and neck cancer cell line characteristicsa.
EGFR assay
EGFR expression was assayed by competitive analysis and Scatchard plots as previously described in human cancer cell lines (Olivier et al, 1990). Cells were grown in 24-well plates (105 cells per well) in 10% FBS-DMEM at 37°C. At 80–90% confluence, cells were rinsed three times with 500 μl RPMI 1640 containing 0.1% BSA at 2–4°C (plates placed on a tray with ice). Plates were then incubated for 30 min with the same medium (500 μl per well) at 4°C. Cells were first screened for their capacity to bind EGF specifically. Total binding was measured after incubation with 0.2 nM 125I-EGF (3 h, 4°C, 0.1% BSA–RPMI); non-specific binding was measured in the presence of an excess of unlabelled EGF (20 nM). With no exception, the six tested cell lines exhibited EGF binding and their precise EGFR content was then measured. Cells were incubated in the RPMI medium for 3 h, at 4°C in the presence of various concentrations of 125I-EGF (0.01, 0.02, 0.04, 0.08, 0.12, 0.18, 0.20 nM); for higher EGF concentrations, cells were incubated with 0.2 nM 125I-EGF with increasing concentrations of unlabelled EGF (0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 20, 200 nM). Plates were placed on ice to stop the reaction, and the supernatant was removed from each well. Cells were washed twice with phosphate-buffered saline (PBS) containing 0.1% BSA (4°C, 500 μl per well). After removal of the supernatant, cells were solubilised in 1 M NaOH at 37°C (500 μl per well for 30 min). The radioactivity of each well was determined by gamma counting. Results were expressed in fmol per mg cell protein. Scatchard analysis was used to calculate the number of receptor sites per cell (N) and the dissociation constant (Kd). Each point of every Scatchard plot was performed in quadruplicate. Cells were counted in four wells run in parallel, resuspended in 200 μl PBS at room temperature and counted with a haemocytometer. Experiments were performed in duplicate only, because of the intrinsic reproducibility of the assay (coefficient of variance=7.3%, n=4).
Determination of p53 status
DNA was extracted from all head and neck cell lines of the panel. Exons 4 to 8 were screened for mutations by use of denaturing gradient gel electrophoresis in accordance with the method described by Hamelin et al (1993) for exons 5, 7, and 8, and the method of Gulberg et al (1997) for exons 4 and 6. Exon 9 was screened for mutations by the method described by Cabelguenne et al (2000). Polymerase chain reaction (PCR) amplification products were loaded onto a 6.5% polyacrylamide gel that contained an appropriate gradient of urea and formamide. After electrophoresis, gels were stained with ethidium bromide. Tumours that showed an electrophoresis variant pattern were amplified and sequenced for each variant exon. PCR products were purified with QIAquick PCR Purification Kit (QIAGEN SA, Courtabeuf, France) and sequenced on both strands on an ABI 310 genetic analyser (PE Applied Biosystems, Courtabeuf, France). A Big Dye Terminator sequencing kit (PE Applied Biosystems) was used in accordance to the manufacturer's instructions; this step was followed by ethanol precipitation to remove non-incorporated dyes. Sequences were analysed by Sequence Analysis 3.0 (PE Applied Biosystems).
Evaluation of radiotoxicity
Cells were irradiated with gamma rays during exponential cell growth as monolayers in 96-well microtitration plates using a 60Co unit at a dose rate of 1 Gy min−1. Dose-effect curves were established for all six head and neck cell lines using a total of 10 doses (0.5, 1, 2, 3, 4, 6, 8, 10, 12, 15 Gy). Sequence-dependent cytotoxic effects of binary combinations of ZD1839 (0.2–200 μM) with ionising radiation were then evaluated using eight doses of ionising radiation, ranging from 0.5 to 10 Gy for CAL27 and CAL33, and from 2 to 15 Gy for CAL60, CAL166, Hep-2 and Detroit562 (the radiation doses were selected from the above experiments to encompass more precisely the zone of sensitivity). Cells were maintained in DMEM supplemented with 10% FBS during all radiation exposures, which were performed at room temperature.
Determination of the cytotoxic effects of radiation alone
Cells were seeded in 96-well microtitration plates (100 μl per well) to obtain exponential growth for the whole duration of the experiment: initial cell densities were 2500 (CAL27 and Hep-2), 3000 (CAL33 and Detroit562), 4500 (CAL166) and 5000 (CAL60) cells per well.
The cells were exposed to ionising radiation 48 h later (dose previously described) and the MTT assay was performed 48 h after that. Control cells received no radiation treatment.
Determination of cytotoxicity using ZD1839 in combination with radiation
Cells were seeded in 96-well microtitration plates (100 μl per well) to obtain exponential growth for the whole duration of the experiment (initial cell density is given above). In each plate, one ZD1839 concentration and one radiation dose were tested. Only half of each plate was irradiated in order to measure on the same plate the effects of ZD1839 alone (20 wells), radiation alone (five wells), ZD1839 combined with radiation (20 wells) and a control (no radiation, no ZD1839; five wells).
Forty-eight hours after seeding the cells, three different sequences of radiation and 48-h incubations of ZD1839 were compared: (a) ZD1839 (48 h) followed by radiation, then medium for 48 h; (b) concomitant ZD1839 (48 h) and ionising radiation (radiation delivered in the middle of ZD1839 exposure), followed by medium for 48 h; (c) ionising radiation prior to ZD1839 (48 h), then medium for 48 h.
Growth inhibition was assessed 168 h after cell seeding by using the MTT test as described below (Carmichael et al, 1987). Cells were washed with PBS and incubated with MTT; after 2 h of exposure, MTT was released and fixation was revealed by the addition of DMSO (100 μl). Absorbance at 450 nm was measured using a microplate reader (Labsystems, Helsinki, Finland). Results were expressed as the relative percentage of absorbance compared with controls without drug. Cell sensitivity to the tested drugs was expressed by IC50 (concentration leading to 50% cell survival). Triplicate determinations were made in separate experiments.
Evaluation of cisplatin and/or 5-FU cytotoxicity
Cells were seeded in 96-well microtitration plates (100 μl per well) to obtain exponential growth for the duration of the experiments (initial cell density was 2500 and 3000 cells per well for Hep-2 and CAL33, respectively). Forty-eight hours after seeding, cells were exposed to ZD1839 and cisplatin and/or 5-FU in a variety of sequences. In all cases, cells were exposed to ZD1839 for 48 h, and the cisplatin/5-FU sequence was constant (cisplatin applied for 2 h prior to exposure to 5-FU for 48 h). The sequence of ZD1839 varied: (a) ZD1839 (48 h) followed by cisplatin and/or 5-FU; (b) concomitant ZD1839 and cisplatin and/or 5-FU (the exposure to ZD1839 was 50 h); (c) ZD1839 prior to and during cisplatin/5-FU exposure (ZD1839 was given for 48 h before cisplatin/5-FU and then for 50 h concomitant with cisplatin/5-FU, making 98 h in total) followed by medium for 48 h; (d) cisplatin/5-FU prior to ZD1839 followed by medium for 48 h. Eleven concentrations were tested for each drug: ZD1839 0.2–200 μM; cisplatin 0.1–200 μM; 5-FU 0.22–220 μM. The cisplatin/5-FU combination was tested at a constant concentration ratio of the drugs for a given cell line, the ratio being dictated by the drug sensitivity and close to the ratio of the IC50 for each drug.
Thereafter, growth inhibition was assessed 48 h after the end of the experiment (in medium alone) by the MTT test described above. Experimental conditions were tested in sextuplicate (six wells of the 96-well plate per experimental condition) and separate experiments were performed in triplicate. The dose-effect curves were analysed using Prism software (GraphPad Software, San Diego, CA, USA).
Combination index (CI) calculations and determination of the potentiation factor
The cytotoxic effects obtained with the different ZD1839/cisplatin/5-FU combinations were analysed according to the Chou and Talalay (1984) method on Calcusyn software (Biosoft, Cambridge, UK). Interaction between the double combinations (ZD1839 plus either cisplatin or 5-FU), or the triple combinations (ZD1839 plus cisplatin and 5-FU) was assessed by means of an automatically computed combination index (CI). CI was determined at 50 and 75% cell death, and was defined as follows:
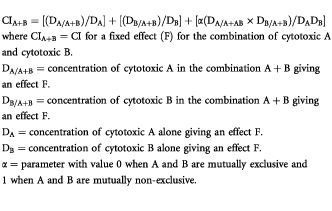 |
The combination index indicated: synergism <0.8; additivity >0.8 and <1.2; antagonism >1.2; slight synergistic and additive cytotoxic activity for values of 0.8 and 1.2, respectively.
The cytotoxic effects obtained with the different ionising radiation/ZD1839 combinations were also analysed according to the Chou and Talalay (1984) method as described above. CIs were determined at 50 and 75% cell death. The application of the Chou and Talalay model recommends the use of cytotoxic agents at a fixed dose ratio (for example, the ratio of their IC50s). However, in the case of ZD1839 and radiation, this strategy would not have been feasible because the dose-effect curves of each cytotoxic agent acting alone are too different (e.g. IC90/IC10=50 for ZD1839 and 2.8 for radiation (CAL33 cell line)). Consequently, ZD1839 and radiation were combined at equitoxic effects (i.e. doses were applied in the combination that would have produced the same cytotoxic effect if given alone); it follows that the ratio between the concentration of ZD1839 and the radiation dose varied from 4×10−7 up to 2×10−5 when progressing from low to high cytotoxic effect. When using non-constant ratios between the doses, the Chou and Talalay Calcusyn programme does not allow direct calculation of the CIs at a fixed final cell death (50 and 75% in the present case). Thus, in order to compare CI at fixed values, i.e. CI50 or CI75, we graphically interpolated between values obtained from the experimental points (11 points generated by the programme between 10 and 90% of final death).
The potentiation factor of ionising radiation by ZD1839 was defined as the ratio of the IC50 of ionising radiation alone to the IC50 of the combination (ZD1839 plus ionising radiation); higher potentiation factors indicate greater cytotoxicity. Potentiation factor I was obtained using sequence I (ZD1839 (48 h) followed by radiation, then medium for 48 h). Potentiation factor II used sequence II (concomitant ZD1839 (48 h) and ionising radiation, radiation delivered in the middle of the ZD1839 exposure), and potentiation factor III used sequence III (ionising radiation prior to ZD1839 (48 h), then medium for 48 h).
Statistical analysis
For the panel of cell lines, the relationship between EGFR expression and response to ionizing radiation was analysed by plotting IC50 values for radiation against the respective EGFR content. The Friedman non-parametric rank test was used to analyse the impact of the sequence ZD1839/cisplatin/5-FU (CI values) or ZD1839/radiation and to compare the different sequences combining ZD1839 and radiation on the basis of the potentiation factors. The correlation coefficients (r) and the P-values were computed using SPSS software (Chicago, IL, USA). A P-value of less than 0.05 was considered to be statistically significant.
RESULTS
The study panel of human squamous cell carcinoma cells showed substantial variability in EGFR expression, ranging from 388 (Hep-2) to 33 794 (CAL33) fmol per mg cell protein. Four of the six cell lines were found to be mutated for p53 gene; CAL166 and Hep-2 were p53 wild type (Table 1).
Hep-2 cells were the most radioresistant (IC50 9.8±0.3 Gy), and CAL33 cells the most radiosensitive (IC50 2.6±0.4 Gy). Figure 1 shows dose-survival curves following irradiation for Hep-2 and CAL33 cells. Over the panel of cell lines, growth inhibition was more pronounced in EGFR-overexpressing cell lines than it was in cell lines expressing low levels of EGFR (Table 1). A statistically significant linear inverse correlation was found between EGFR cellular content and IC50 for radiation (P=0.0091, r2=0.848) (Figure 2). No statistically significant correlation was found between EGFR levels and cell doubling time for the six head and neck cell lines (P=0.093, r2=0.548).
Figure 1.
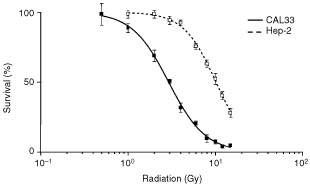
Dose-survival curves following ionising radiation for Hep-2 and CAL33 cells.
Figure 2.
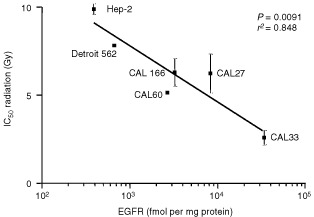
Correlation between cell sensitivity to ionising radiation and EGFR content.
Typical dose-effect curves for the different ZD1839/radiation exposure combinations are shown in Figure 3 for CAL33. In all cell lines, pretreatment with ZD1839 followed by ionising radiation (sequence I) produced a leftward shift of the concentration-response curves, indicating a greater cytotoxic effect. The CI values computed at 50 and 75% cell lethality are given in Table 2. It appears that ZD1839 applied 48 h before radiation (sequence I) led to lower CI values in all head and neck cell lines (P=0.002). More precisely the best synergistic effects were observed in the p53-mutant cell lines that express the highest EGFR levels (CAL27, CAL33, CAL60). Typical examples of CI/fractional effect curves are illustrated in Figure 4 for CAL33.
Figure 3.
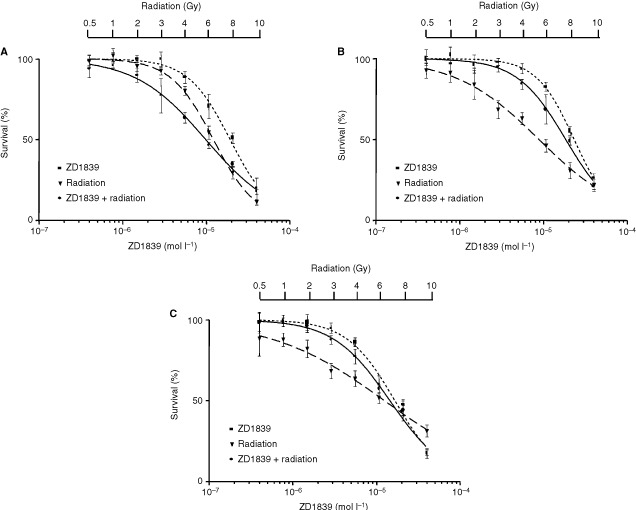
Dose-effect curves for the different ZD1839/radiation exposure combinations for CAL33 cells: (A) sequence I, (B) sequence II, (C) sequence III. For each combination sequence of ZD1839 and radiation, the dose-effect curve of the combination was compared with the dose-effect curves of ZD1839 alone and radiation alone. Radiation dose ranged from 0.5 to 10 Gy.
Table 2. CI valuesa according to the different sequences of ZD1839/radiation.
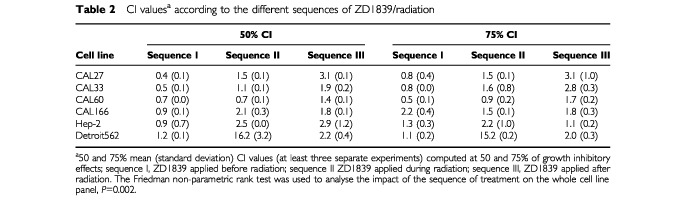
Figure 4.
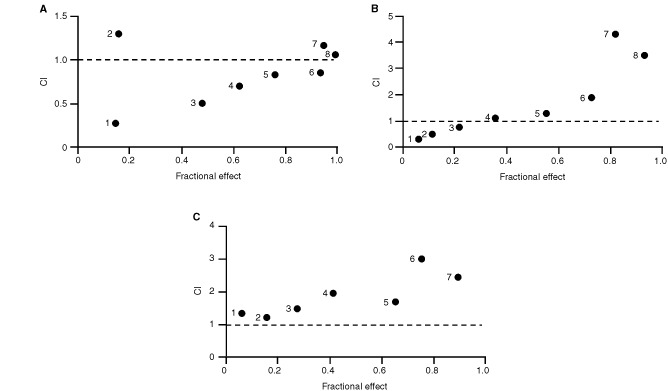
Typical examples of CI/fractional effects curves for CAL33 cells: (a) sequence I, (b) sequence II, (c) sequence III.
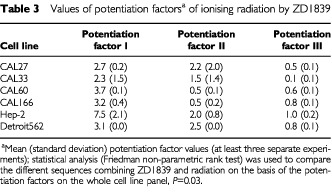
Table 3 gives the potentiation factors for ZD1839 and ionizing radiation. Statistical analyses indicated that the relative contributions of the two treatments are significantly different depending upon the sequence (P=0.001). The greatest contribution to the overall cytotoxicity of the combination came from ZD1839 applied 48 h before irradiation (sequence I; highest relative contribution) and the smallest from ZD1839 following irradiation (sequence III).
Table 3. Values of potentiation factorsa of ionising radiation by ZD1839.
The order of association between ZD1839 and combined cytotoxic drugs had a significant impact on their interaction. For both cell lines, application of the Wilcoxon test shows that the association between ZD1839 and cisplatin alone gives supra- additive effects more frequently when ZD1839 is applied first; the same conclusions are true for ZD1839 combined with 5-FU, with mainly synergistic effects when ZD1839 is given before 5-FU (P <0.003) (Table 4). When ZD1839 was applied during or after each of these drugs, effects were usually additive or antagonistic. With the triple combination (ZD1839, cisplatin, 5-FU), the effects were synergistic when ZD1839 was applied first, in both Hep-2 and CAL33 cells (P=0.003) (Table 4). In contrast, when ZD1839 was applied during or after cisplatin/5-FU there were antagonistic or additive effects. Interestingly, the optimal result (lowest CI value) was obtained with sequence III, in which ZD1839 is applied before and during cisplatin/5-FU (Table 4). Typical dose-effect curves comparing sequences I and III are given in Figure 5 for Hep-2 and CAL33.
Table 4. CI results in Hep-2 and CAL33 cell lines corresponding to concentration of drugs leading to 50% cell survival.
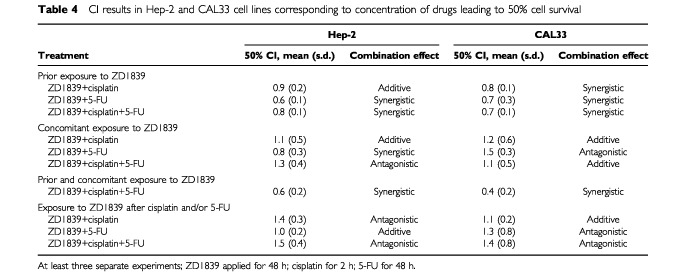
Figure 5.
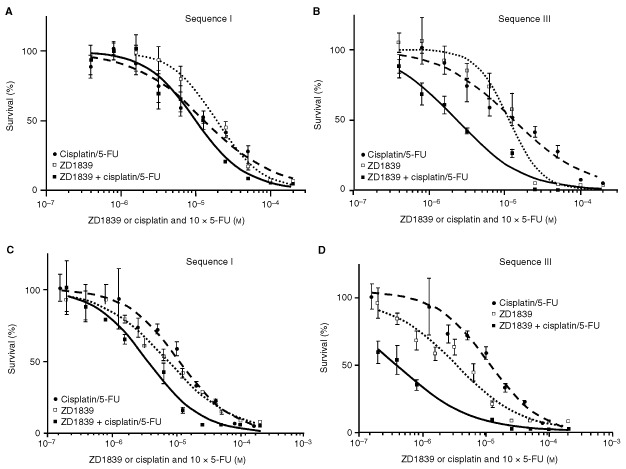
Typical dose-effect curves comparing sequences I and III for (A, B) Hep-2 and (C, D) CAL33. Sequence I consisted of ZD1839, followed by cisplatin/5-FU for 48 h; sequence III was ZD1839 applied first and also during the cisplatin/5-FU exposure, followed by medium for 48 h.
DISCUSSION
Squamous cell carcinomas in the aerodigestive tract remain a significant public health problem (Vokes et al, 1993). Even with multimodal therapy, the long-term remission rate for these cancers is only around 50–60%. External beam radiotherapy represents one of the main therapeutic tools in HNC, producing major antitumour responses when used in conjunction with both cisplatin and 5-FU in either induction or adjuvant treatment settings (Vokes et al, 2000). A recent meta-analysis has shown that a substantial survival benefit can be obtained by chemo-/radiotherapy combination in advanced HNC (Pignon et al, 2000). The challenge in this pathology is to incorporate new agents into established regimens in order to obtain a significant impact on overall outcome. Over the past decade, comprehensive data have been accumulated that strongly support a role for EGFR and its ligands in tumour development and growth. This is particularly true for squamous cell carcinoma of the head and neck, where EGFR is considered to be one of the major prognostic factors (Santini et al, 1991; Dassonville et al, 1993; Salomon et al, 1995; Grandis et al, 1996, 1998; Maurizi et al, 1996). As a result, EGFR has become an attractive target for novel anticancer therapies, particularly in HNC.
Previous studies have shown that stimulation of the EGFR signalling pathway leads to radioresistance both in vitro and in vivo (Wollman et al, 1994; Miyaguchi et al, 1998; Akimoto et al, 1999; Dent et al, 1999). Interestingly, in the present study, we observed a strong positive association between EGFR level and radiosensitivity, which is in agreement with previous results of Bonner et al (1994, 1998, 2000). Among a panel of human tumour cell lines, these authors found that the cell line expressing the lowest EGFR levels demonstrated the greatest radioresistance, and that the greatest growth inhibition was observed for the cell line with the highest EGFR content. In addition, there are some in vitro reports suggesting an EGF-related radiosensitisation. For instance, Kwok and Sutherland (1989, 1991) have shown that EGF-related radiosensitization may be EGFR-density dependent. A link between the presence of transforming growth factor alpha (TGFα) and radiosensitivity was also suggested by the clinical data reported by Sauter et al (1994) and Wen et al (1996). More precisely, Sauter et al (1994) found that low plasma TGFα levels were associated with radioresistance in patients with squamous carcinoma of the tongue. Wen et al (1996) reported that the recurrence rates of early laryngeal cancer treated with radiotherapy correlated with TGFα but not with EGFR. Thus, it appears that not only EGFR but also the tumoural content of its specific ligands may play an important role in radiosensitivity. Cell doubling time and EGFR content were not found to be correlated in the present study. Thus the impact of EGFR on radiosensitivity is not explicable by EGFR-dependent modifications of cell kinetic characteristics, which could enhance the action of irradiation. The classical view of the cellular effects of ionising radiation consists of an initial event involving the induction of DNA damage, in addition to the activation of several intracellular signalling cascades that have commonly been regarded as mitogenic, including the Raf-MEK-Erk kinase cascade (Todd et al, 1999). DNA damage is modulated by metabolic DNA repair processes. Interestingly, Bandyopadhyay et al (1998) have shown that EGFR-mediated signalling may control the activity of DNA-PK, an enzyme directly involved in DNA damage repair; more precisely, from their work, high EGFR expression could maintain a high level of signalling, which would be associated with high basal levels of DNA repair capacity. However, this does not appear to explain the observations reported here. An approach used by Lewis et al (2000), examining the whole pattern of MAP kinase pathway signalling targets, could help to elucidate the molecular origins of the present observation of a radiosensitivity linked to high tumoural EGFR levels.
A strong positive interaction between irradiation and ZD1839, when the ZD1839 was applied before the radiation, was demonstrated in the present study (Tables 2 and 3). This could be explained by an intrinsic activity of ZD1839 as radiosensitiser, as has previously been shown for cetuximab, another EGFR-targeting drug (Ezekiel et al, 1999; Huang et al, 1999; Mendelsohn et al, 1999; Baselga et al, 2000; Huang and Harari, 2000; Milas et al, 2000), and also for trastuzumab (Herceptin™), a HER-2-targeting drug (Pegram et al, 1998; Pietras et al, 1999; Pegram and Slamon, 2000). Trastuzumab increased the radiosensitivity of HER-2-overexpressing MCF7 breast cancer cells as measured by in vitro colony-forming assays, and the combination of trastuzumab and radiation showed synergistic tumour reduction in nude mice (Pietras et al, 1999). According to Pietras et al (1999) the mechanism of radiosensitization appears to involve both cell cycle regulation and DNA repair. Interestingly, the application of ZD1839 in our study was found to increase the percentage of cells in the G1 phase (results not shown), which are particularly sensitive to radiation (Teyssier et al, 1999). Thus, there are several potential mechanisms of radiosensitisation by inhibitors of the EGFR family; they may include favourable cell cycle reorganisation and/or abrogation or attenuation of signals required for survival during cell cycle arrest. Marked antagonistic effects were observed when exposing cells to ZD1839 after irradiation (sequence III). This observation may be related to the fact that irradiation is able to upregulate EGFR phosphorylation in a similar manner to EGF (Wan et al, 2001) and thus create the circonstances of opposite effects to ZD1839.
Two cell lines of the initial panel of six were considered for analysis of the association between ZD1839 and cytotoxic drugs. We have recently demonstrated an inverse correlation between p53 content (representative of p53 mutations) and EGFR levels in a group of HNC patients (Etienne et al, 1999). Thus, the two cell lines were selected because they are representative of human HNC characteristics on the basis of their p53 status and EGFR status: CAL33 is p53 mutant and has high EGFR expression, and Hep-2 is p53 wild type and has low EGFR expression. We observed synergistic effects when ZD1839 was applied before cisplatin and/or 5-FU in both cell lines (Table 4). The impact of ZD1839 on the effects of the combined drugs was sequence dependent. The highest synergistic effects were obtained when ZD1839 was applied before and during exposure to cytotoxic drugs (Table 4). Previous in vivo and in vitro studies have shown that the anti-EGFR receptor monoclonal antibody C225 can potentiate the effects of a number of chemotherapeutic agents, including doxorubicin, paclitaxel and cisplatin (Ezekiel et al, 1999; Mendelsohn et al, 1999; Huang et al, 1999; Baselga et al, 2000; Huang and Harari, 2000; Milas et al, 2000). The mechanisms by which receptor blockade by C225 augments the cytotoxic effect of anti-neoplastic agents was attributed by these authors to cell cycle effects and an enhanced capacity for apoptosis by C225. The antiproliferative activity of ZD1839 combined with a number of cytotoxic drugs (cisplatin, carboplatin, oxaliplatin, paclitaxel, docetaxel, doxorubibin, etoposide, topotecan and raltitrexed) has been recently assessed in a variety of human cancer cell lines (Ciardiello et al, 2000; Sirotnak et al, 2000). The co-administration of ZD1839 was found to enhance the growth-inhibitory effects of all cytotoxic drugs tested. The present data confirm this interesting property of ZD1839 as a strong radio- and chemosensitizer. In addition, the present study brings particular focus to the importance of the combination sequence: it is shown that synergy is not a rule and that some antagonistic effects may result from inappropriate sequences, e.g. where ZD1839 is applied after the cytotoxic drugs (Table 4). In conclusion, the present investigations into the combinations of ZD1839 and radiation, and ZD1839 and cisplatin/5-FU, led to similar findings of sequence-dependent synergy. Although an unavoidable gap does exist between the bench and the bed side, these data may be useful for the design of future clinical trials combining ZD1839 and cytotoxic agents.
Acknowledgments
Ligue Régionale de lutte contre le Cancer and AstraZeneca group for financial support. Results presented in part during the 2001 meeting of the American Association for Cancer Research (AACR). ‘Iressa’ is a trade mark of the AstraZeneca group of companies.
References
- AkimotoTHunterNRBuchmillerLMasonKAngKKMilasL1999Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas Clin Cancer Res 528842890 [PubMed] [Google Scholar]
- BandyopadhyayDMandalMAdamLMendelsohnJKumarR1998Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells J Biol Chem 27315681573 [DOI] [PubMed] [Google Scholar]
- BaselgaJPfisterDCooperMRCohenRBurtnessBBosMD'AndreaGSeidmanANortonLGunnettKFalceyJAndersonVWaksalHMendelsohnJ2000Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin J Clin Oncol 18904914 [DOI] [PubMed] [Google Scholar]
- BensadounRJEtienneMCDassonvilleOChauvelPPivotXMarcyPYPrevostBCoche-DequeantBBourdinSVallicioniJPoissonnetGCourdiATeissierELagrangeJLThyssASantiniJDemardFSchneiderMMilanoG1998Concomitant B.I.D. radiotherapy and chemotherapy with cisplatin and 5-fluorouracil in unresectable squamous-cell carcinoma of the pharynx: clinical and pharmacological data of a French multicenter phase II study Int J Radiat Oncol Biol Phys 42237245 [DOI] [PubMed] [Google Scholar]
- BonnerJAMaihleNJFolvenBRChristiansonTJHSpainK1994The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivity Int J Radiat Oncol Biol Phys 29243247 [DOI] [PubMed] [Google Scholar]
- BonnerJAVromanBTChristiansonTHJKarnitzLM1998Ionizing radiation-induced MEK and ERK activation does not enhance survival of irradiated human squamous carcinoma cells Int J Radiat Oncol Biol Phys 42921925 [DOI] [PubMed] [Google Scholar]
- BonnerJARaishKPTrummellHQRobertFMeredithRFSpencerSABushbaumDJSalehMNStackhouseMALoBugliaAFPetersGECarollWRWaksalHW2000Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers J Clin Oncol 184753 [PubMed] [Google Scholar]
- BrizelDMAlbersMEFisherSRScherRLRichtsmeierWJHarsVGeorgeSLHuangATProsnitzLR1998Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer N Engl J Med 33817981804 [DOI] [PubMed] [Google Scholar]
- CabelguenneABlonsHde WaziersICarnotFHoullierAMSoussiTBrasnuDBeaunePLaccourreyeOLaurent-PuigP2000P53 alterations predict tumor response to neoadjuvant chemotherapy in head and neck squamous cell carcinoma: a prospective series J Clin Oncol 1814651473 [DOI] [PubMed] [Google Scholar]
- CalaisGAlfonsiMBardetESireCGermainTBergerotPRheinBTortochauxJOudinotPBertrandP1999Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma J Nat Cancer Inst 9120812086 [DOI] [PubMed] [Google Scholar]
- CarmichaelJDe GraffWGGazdarAFMinnaJDMitchellJB1987Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing Cancer Res 47936940 [PubMed] [Google Scholar]
- ChouTTalalayP1984Quantitative analysis of dose-effects relationships: the combined effects of multiple drugs or enzyme inhibitors Adv Enzyme Regul 222755 [DOI] [PubMed] [Google Scholar]
- CiardielloFCaputoRBiancoRDamianoVPomaticoGDe PlacidoSBiancoARTortoraG2000Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa™), an epidermal growth factor receptor-selective tyrosine kinase inhibitor Clin Cancer Res 620532063 [PubMed] [Google Scholar]
- DassonvilleOFormentoJLFrancoualMRamaioliASantiniJSchneiderMDemardFMilanoG1993Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer J Clin Oncol 1118731878 [DOI] [PubMed] [Google Scholar]
- DentPReardonDBParkJSBowersGLogsdonCValerieKSchmidt-UlrichR1999Radiation-induced release of transforming growth factor α activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death Mol Biol Cell 1024932506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EtienneMCPivotXFormentoJLBensadounRJFormentoPDassonvilleOFrancoualMPoissonnetGFontanaXSchneiderMDemardFMilanoG1999A multifactorial approach including tumoural epidermal growth factor receptor, p53, thymidylate synthase and dihydropyrimidine dehydrogenase to predict treatment outcome in head and neck cancer patients receiving 5-fluorouracil Br J Cancer 7918641869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EzekielMPBonnerJARobertFMeredithRFSpencerSALoBuglioAFWaksalHW1999Phase I trial of chimerized anti-epidermal growth factor receptor (anti-EGFR) antibody in combination with either once-daily or twice-daily irradiation for locally advanced head and neck malignancies Proc Am Soc Clin Oncol 18388a(abstract 1501) [Google Scholar]
- FuKKPajakTFTrottiAJonesCUSpencerSAPhillipsTLGardenASRidgeJACooperJSAngKK2000A radiation therapy oncology group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003 Int J Radiat Oncol 48716 [DOI] [PubMed] [Google Scholar]
- GrandisJRMelhemMFBarnesEL1996Quantitative immunohistochemical analysis growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck Cancer 7812841292 [DOI] [PubMed] [Google Scholar]
- GrandisJRMelhemMFGoodingWEDayRHolstVAWagenerMMDreningSDTweardyDJ1998Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival J Natl Cancer Inst 90824832 [DOI] [PubMed] [Google Scholar]
- GulbergPNedergaardTNielsenHJOlsenACAhenkielVZeuthenJ1997Single-step DGGE-based mutation scanning of the p53 gene: application to genetic diagnosis of colorectal cancer Hum Mutat 9348355 [DOI] [PubMed] [Google Scholar]
- HamelinRJegoNLaurent-PuigPVidaudMThomasG1993Efficient screening of p53 mutations by denaturing gradient gel electrophoresis in colorectal tumors Oncogene 822132220 [PubMed] [Google Scholar]
- HuangSMBockJMHarariPM1999Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck Cancer Res 5919351940 [PubMed] [Google Scholar]
- HuangSMHarariPM2000Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis Clin Cancer Res 621662174 [PubMed] [Google Scholar]
- KwokTTSutherlandRM1989Enhancement of sensitivity of human squamous carcinoma cells to radiation by epidermal growth factor J Natl Cancer Inst 8110201024 [DOI] [PubMed] [Google Scholar]
- KwokTTSutherlandRM1991Differences in EGF-related radiosensitization of human squamous carcinoma cells with high and low numbers of EGF receptors Br J Cancer 64251254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LewisTSHuntJBAvelineLDJonscherKRLouieDFYehJMNaheiniTSResingKAAhnNG2000Identification of novel MAP kinase pathway signaling targets by functional proteomics and mass spectrometry Mol Cell 613431354 [DOI] [PubMed] [Google Scholar]
- MauriziMAlmadoriGFerrandinaGDistefanoMRomaniniMECadoniGBenedetti-PaciniPPaludettiGScambiaGMancusoS1996Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma Br J Cancer 7412531257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MendelsohnJShinDMDonatoNKhuriFRadinskyRGlissonBSShinHJMetzEPfisterDPerez-SolerRLawhornKMatsumotoTGunnetKFalceyJWaksalHHongWK1999Phase I study of chimerized anti-epidermal growth factor receptor (EGFR) monoclonal antibody, C225, in combination with cisplatin (CDDP) in patients (PTS) with recurrent head and neck and squamous cell carcinoma (SCC) Proc Am Soc Clin Oncol 18389a(abstract 1502) [Google Scholar]
- MilasLMasonKHunterNPetersenSYamakawaMAngKMendelsohnJFanZ2000In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody Clin Cancer Res 6701708 [PubMed] [Google Scholar]
- MiyaguchiMTakeuchiTMorimotoKKuboT1998Correlation of epidermal growth factor receptor and radiosensitivity in human maxillery carcinoma cell lines Acta Otolaryngol 118428431 [DOI] [PubMed] [Google Scholar]
- OlivierSFormentoPFischelJLEtienneMCMilanoG1990Epidermal growth factor receptor expression and suramin cytotoxicity in vitro Eur J Cancer 26867871 [DOI] [PubMed] [Google Scholar]
- PegramMDLiptonAHayesDFWeberBLBaselgaJMTripathyDBalyDBaughmanSATwaddellTGlaspyJASlamonDJ1998Phase II study receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/ neu monoclonal antibody plus cisplatin in patients with HER2/ neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment J Clin Oncol 1626592671 [DOI] [PubMed] [Google Scholar]
- PegramMSlamonD2000Biological rationale for HER2/neu (c-erbB2) as a target for monoclonal antibody therapy Semin Oncol 271319 [PubMed] [Google Scholar]
- PietrasRJPoenJCGallardoDWongvipatPNLeeHJSlamonDJ1999Monoclonal antibody to HER-2/neu receptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene Cancer Res 5913471355 [PubMed] [Google Scholar]
- PignonJPBourhisJDomengeCDesignéL2000Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data Lancet 355949955 [PubMed] [Google Scholar]
- SalomonDSBradtRCiardelloFNormannoN1995Epidermal growth factor-related peptides and their receptors in human malignancies Crit Rev Oncol Hematol 19183232 [DOI] [PubMed] [Google Scholar]
- SantiniJFormentoJLFrancoualMMilanoGSchneiderMDassonvilleODemardF1991Characterization, quantification and potential clinical value of the epidermal growth factor receptor in head and neck squamous cell carcinomas Head Neck 13132139 [DOI] [PubMed] [Google Scholar]
- SauterERCoiaLREisenbergBLRidgeJA1994Radiation treatment decreases transforming growth factor alpha expression in squamous carcinoma of the tongue Cancer Lett 78159162 [DOI] [PubMed] [Google Scholar]
- SchlessingerJ2000Cell signaling by receptor tyrosine kinases Cell 103211225 [DOI] [PubMed] [Google Scholar]
- SirotnakFMZakowskiMFMillerVAScherHIKrisMG2000Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase Clin Cancer Res 648854892 [PubMed] [Google Scholar]
- TaylorSGMurthyAKGriemKLRecineDCKielKBlendowskiCHurstPBShowelJTHutchinsonJCCampanellaRSChenSCaldarelliDD1997Concomitant cisplatin/5-FU infusion and radiotherapy in advanced head and neck cancer: 8-year analysis of results Head Neck 19684691 [DOI] [PubMed] [Google Scholar]
- TeyssierFBayJODionetCVerelleP1999Cell cycle regulation after exposure to ionizing radiation Bull Cancer 86345357 [PubMed] [Google Scholar]
- ToddDGMikkelsenRBRorrerWKValerieKSchmidt-UlrichRK1999Ionizing radiation stimulates existing signal transduction pathways involving the activation of epidermal growth factor receptor and erbB-3, and changes of intracellular calcium in A431 human squamous carcinoma cells J Recept Signal Transduct Res 19885908 [DOI] [PubMed] [Google Scholar]
- VokesEEWeichselbaumRRLipmanSMHongWK1993Head and neck cancer N Engl J Med 328184194 [DOI] [PubMed] [Google Scholar]
- VokesEEKiesMSHarafDJStensonKListMHumerickhouseRDolanMEPelzerHSulzenLWittMEHsiehYCMittalBBWeichselbaumRR2000Concomitant chemoradiotherapy as primary therapy for locoregionally advanced head and neck cancer J Clin Oncol 1816521661 [DOI] [PubMed] [Google Scholar]
- WanYSWangZQVoorheesJFisherG2001EGF receptor crosstalks with cytokine receptors leading to the activation of c-Jun kinase in response to UV irradiation in human keratinocytes Cell Signal 13139144 [DOI] [PubMed] [Google Scholar]
- WenQHMiwaTYoshikaziTNagayamaMNishijimaH1996Prognostic value of EGFR and TGF-alpha in early laryngeal cancer treated with radiotherapy Laryngoscope 106884888 [DOI] [PubMed] [Google Scholar]
- WheelerRHSpencerSBuchsbaumDRobertF1999Monoclonal antibodies as potentiators of radiotherapy and chemotherapy in the management of head and neck cancer Curr Opin Oncol 11187190 [DOI] [PubMed] [Google Scholar]
- WollmanRYahalomJMaxyRPintoJFuksZ1994Effect of epidermal growth factor on the growth and radiation sensitivity of human breast cancer cells in vitro Int J Radiat Oncol Biol Phys 309198 [DOI] [PubMed] [Google Scholar]


