Abstract
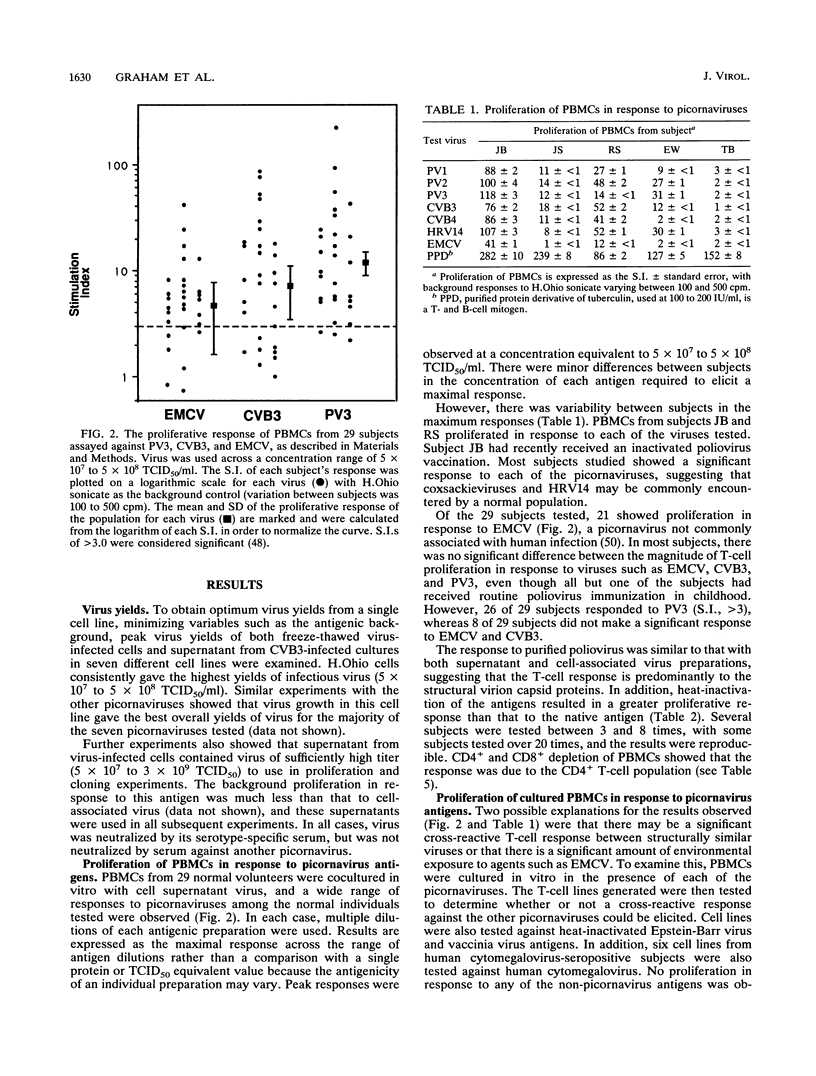
Little is known about the nature and specificity of T-cell-mediated responses to picornaviruses in humans. In this study, the nature of the T-cell response to seven picornaviruses, including polioviruses, coxsackieviruses B3 and B4, human rhinovirus 14, and encephalomyocarditis virus, was determined. Twenty-nine individuals responded to poliovirus type 3, coxsackievirus B3, and encephalomyocarditis virus by proliferation of T cells, and from such cultures, 130 virus-specific T-cell lines were established. T-cell lines generated in response to encephalomyocarditis virus were exclusively strain specific. However, the majority of T-cell lines established in response to viruses, other than encephalomyocarditis virus, were cross-reactive to each other. Their cross-reactivity was confirmed in 2 of the 30 picornavirus-specific clonally derived T-cell lines from two subjects, but the majority of these lines were serotype specific. T-cell epitopes adjacent to each of the B-cell antigenic sites in VP1 of poliovirus type 3 were identified. The response to the region adjacent to B-cell antigenic site 1 (residues 97 to 114) was dominant between individuals. The localization of this major CD4 T-cell epitope may permit the construction of chimeric viruses utilizing the natural picornavirus T-cell response to augment production of antibody specific for inserted sequences.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alp N. J., Sissons J. G., Borysiewicz L. K. Automation of limiting dilution cytotoxicity assays. J Immunol Methods. 1990 May 25;129(2):269–276. doi: 10.1016/0022-1759(90)90447-4. [DOI] [PubMed] [Google Scholar]
- Barnett B. C., Graham C. M., Burt D. S., Skehel J. J., Thomas D. B. The immune response of BALB/c mice to influenza hemagglutinin: commonality of the B cell and T cell repertoires and their relevance to antigenic drift. Eur J Immunol. 1989 Mar;19(3):515–521. doi: 10.1002/eji.1830190316. [DOI] [PubMed] [Google Scholar]
- Beck M. A., Tracy S. M. Evidence for a group-specific enteroviral antigen(s) recognized by human T cells. J Clin Microbiol. 1990 Aug;28(8):1822–1827. doi: 10.1128/jcm.28.8.1822-1827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M. A., Tracy S. M. Murine cell-mediated immune response recognizes an enterovirus group-specific antigen(s). J Virol. 1989 Oct;63(10):4148–4156. doi: 10.1128/jvi.63.10.4148-4156.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke K. L., Dunn G., Ferguson M., Minor P. D., Almond J. W. Antigen chimaeras of poliovirus as potential new vaccines. Nature. 1988 Mar 3;332(6159):81–82. doi: 10.1038/332081a0. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Collen T., Dimarchi R., Doel T. R. A T cell epitope in VP1 of foot-and-mouth disease virus is immunodominant for vaccinated cattle. J Immunol. 1991 Jan 15;146(2):749–755. [PubMed] [Google Scholar]
- Collen T., Doel T. R. Heterotypic recognition of foot-and-mouth disease virus by cattle lymphocytes. J Gen Virol. 1990 Feb;71(Pt 2):309–315. doi: 10.1099/0022-1317-71-2-309. [DOI] [PubMed] [Google Scholar]
- Collen T., Pullen L., Doel T. R. T cell-dependent induction of antibody against foot-and-mouth disease virus in a mouse model. J Gen Virol. 1989 Feb;70(Pt 2):395–403. doi: 10.1099/0022-1317-70-2-395. [DOI] [PubMed] [Google Scholar]
- DiMarchi R., Brooke G., Gale C., Cracknell V., Doel T., Mowat N. Protection of cattle against foot-and-mouth disease by a synthetic peptide. Science. 1986 May 2;232(4750):639–641. doi: 10.1126/science.3008333. [DOI] [PubMed] [Google Scholar]
- Evans D. J., McKeating J., Meredith J. M., Burke K. L., Katrak K., John A., Ferguson M., Minor P. D., Weiss R. A., Almond J. W. An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature. 1989 Jun 1;339(6223):385-8, 340. doi: 10.1038/339385a0. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Non-specific propagation of human antigen-dependent T lymphocyte clones. J Immunol Methods. 1988 May 9;109(2):215–219. doi: 10.1016/0022-1759(88)90245-1. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Hastings G. Z., Syred A. D., McGinn B., Brown F., Rowlands D. J. Non-responsiveness to a foot-and-mouth disease virus peptide overcome by addition of foreign helper T-cell determinants. Nature. 1987 Nov 12;330(6144):168–170. doi: 10.1038/330168a0. [DOI] [PubMed] [Google Scholar]
- Graham C. M., Barnett B. C., Hartlmayr I., Burt D. S., Faulkes R., Skehel J. J., Thomas D. B. The structural requirements for class II (I-Ad)-restricted T cell recognition of influenza hemagglutinin: B cell epitopes define T cell epitopes. Eur J Immunol. 1989 Mar;19(3):523–528. doi: 10.1002/eji.1830190317. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Valentine S., Dietzschold B., Burns-Purzycki C. Overlapping T cell antigenic sites on a synthetic peptide fragment from herpes simplex virus glycoprotein D, the degenerate MHC restriction elicited, and functional evidence for antigen-Ia interaction. J Exp Med. 1988 Feb 1;167(2):275–287. doi: 10.1084/jem.167.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Heintz N., Tracy R. Coxsackievirus B-3-induced myocarditis. Virus and actinomycin D treatment of myocytes induces novel antigens recognized by cytolytic T lymphocytes. J Immunol. 1988 Nov 1;141(9):3214–3219. [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. Lysis of infected myofibers by coxsackievirus B-3-immune T lymphocytes. Am J Pathol. 1980 Mar;98(3):681–694. [PMC free article] [PubMed] [Google Scholar]
- Jenkins O., Booth J. D., Minor P. D., Almond J. W. The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the Picornaviridae. J Gen Virol. 1987 Jul;68(Pt 7):1835–1848. doi: 10.1099/0022-1317-68-7-1835. [DOI] [PubMed] [Google Scholar]
- Jenkins O., Cason J., Burke K. L., Lunney D., Gillen A., Patel D., McCance D. J., Almond J. W. An antigen chimera of poliovirus induces antibodies against human papillomavirus type 16. J Virol. 1990 Mar;64(3):1201–1206. doi: 10.1128/jvi.64.3.1201-1206.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrak K., Mahon B. P., Minor P. D., Mills K. H. Cellular and humoral immune responses to poliovirus in mice: a role for helper T cells in heterotypic immunity to poliovirus. J Gen Virol. 1991 May;72(Pt 5):1093–1098. doi: 10.1099/0022-1317-72-5-1093. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Deriaud E., Mimic V., van der Werf S. Identification of a T-cell epitope adjacent to neutralization antigenic site 1 of poliovirus type 1. J Virol. 1991 Feb;65(2):711–718. doi: 10.1128/jvi.65.2.711-718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Walker P., Smerdon R., Childerstone A., Bergmeier L. A., Haron J. Identification of T- and B-cell epitopes in synthetic peptides derived from a Streptococcus mutans protein and characterization of their antigenicity and immunogenicity. Arch Oral Biol. 1990;35 (Suppl):39S–45S. doi: 10.1016/0003-9969(90)90129-x. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L., CLARKE N. A., KRAFT L. M. Immunological reactions of the Coxsackie viruses. III. Cross-protection tests in infant mice born of vaccinated mothers; transfer of immunity through the milk. J Exp Med. 1950 Nov 1;92(5):499–505. doi: 10.1084/jem.92.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J. H., Allan J. E., Roehrig J. T., Brubaker J. R., Uren M. F., Hunt A. R. T-helper cell and associated antibody response to synthetic peptides of the E glycoprotein of Murray Valley encephalitis virus. J Virol. 1991 Oct;65(10):5141–5148. doi: 10.1128/jvi.65.10.5141-5148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Thornton G. B. Nonoverlapping T and B cell determinants on an hepatitis B surface antigen pre-S(2) region synthetic peptide. J Exp Med. 1986 Aug 1;164(2):532–547. doi: 10.1084/jem.164.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Evans D. M., Ferguson M., Schild G. C., Westrop G., Almond J. W. Principal and subsidiary antigenic sites of VP1 involved in the neutralization of poliovirus type 3. J Gen Virol. 1985 May;66(Pt 5):1159–1165. doi: 10.1099/0022-1317-66-5-1159. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Ferguson M., Evans D. M., Almond J. W., Icenogle J. P. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol. 1986 Jul;67(Pt 7):1283–1291. doi: 10.1099/0022-1317-67-7-1283. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Ferguson M., Katrak K., Wood D., John A., Howlett J., Dunn G., Burke K., Almond J. W. Antigenic structure of chimeras of type 1 and type 3 poliovirus involving antigenic site 1. J Gen Virol. 1990 Nov;71(Pt 11):2543–2551. doi: 10.1099/0022-1317-71-11-2543. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Schild G. C., Bootman J., Evans D. M., Ferguson M., Reeve P., Spitz M., Stanway G., Cann A. J., Hauptmann R. Location and primary structure of a major antigenic site for poliovirus neutralization. Nature. 1983 Feb 24;301(5902):674–679. doi: 10.1038/301674a0. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. The role of antibody and host cells in the resistance of mice against infection by coxsackie B-3 virus. J Gen Virol. 1973 Jun;19(3):329–338. doi: 10.1099/0022-1317-19-3-329. [DOI] [PubMed] [Google Scholar]
- Stanway G., Cann A. J., Hauptmann R., Hughes P., Clarke L. D., Mountford R. C., Minor P. D., Schild G. C., Almond J. W. The nucleotide sequence of poliovirus type 3 leon 12 a1b: comparison with poliovirus type 1. Nucleic Acids Res. 1983 Aug 25;11(16):5629–5643. doi: 10.1093/nar/11.16.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G. Structure, function and evolution of picornaviruses. J Gen Virol. 1990 Nov;71(Pt 11):2483–2501. doi: 10.1099/0022-1317-71-11-2483. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Vallbracht A., Maier K., Stierhof Y. D., Wiedmann K. H., Flehmig B., Fleischer B. Liver-derived cytotoxic T cells in hepatitis A virus infection. J Infect Dis. 1989 Aug;160(2):209–217. doi: 10.1093/infdis/160.2.209. [DOI] [PubMed] [Google Scholar]
- Wahren B., Robèrt K. H., Nordlund S. Conditions for cytomegalovirus stimulation of lymphocytes. Scand J Immunol. 1981;13(6):581–586. doi: 10.1111/j.1365-3083.1981.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Borysiewicz L. K. Viruses and autoimmunity. Autoimmunity. 1990;5(4):277–292. doi: 10.3109/08916939009014712. [DOI] [PubMed] [Google Scholar]


