Abstract
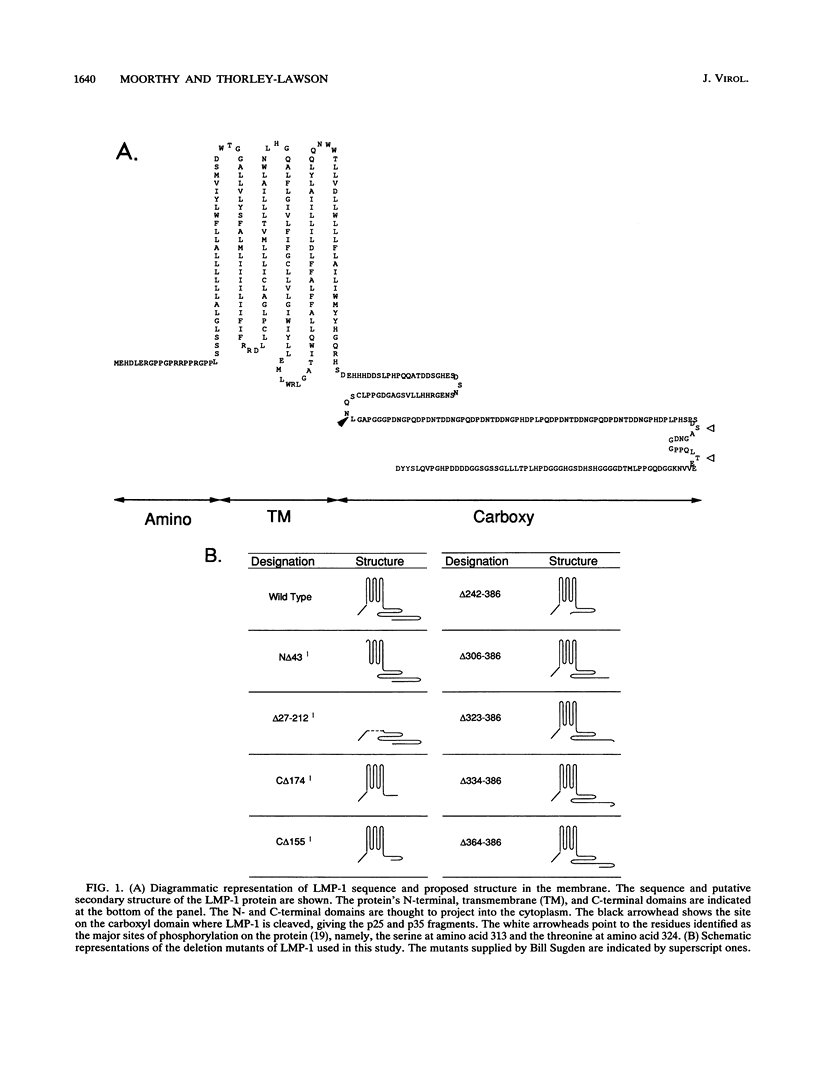
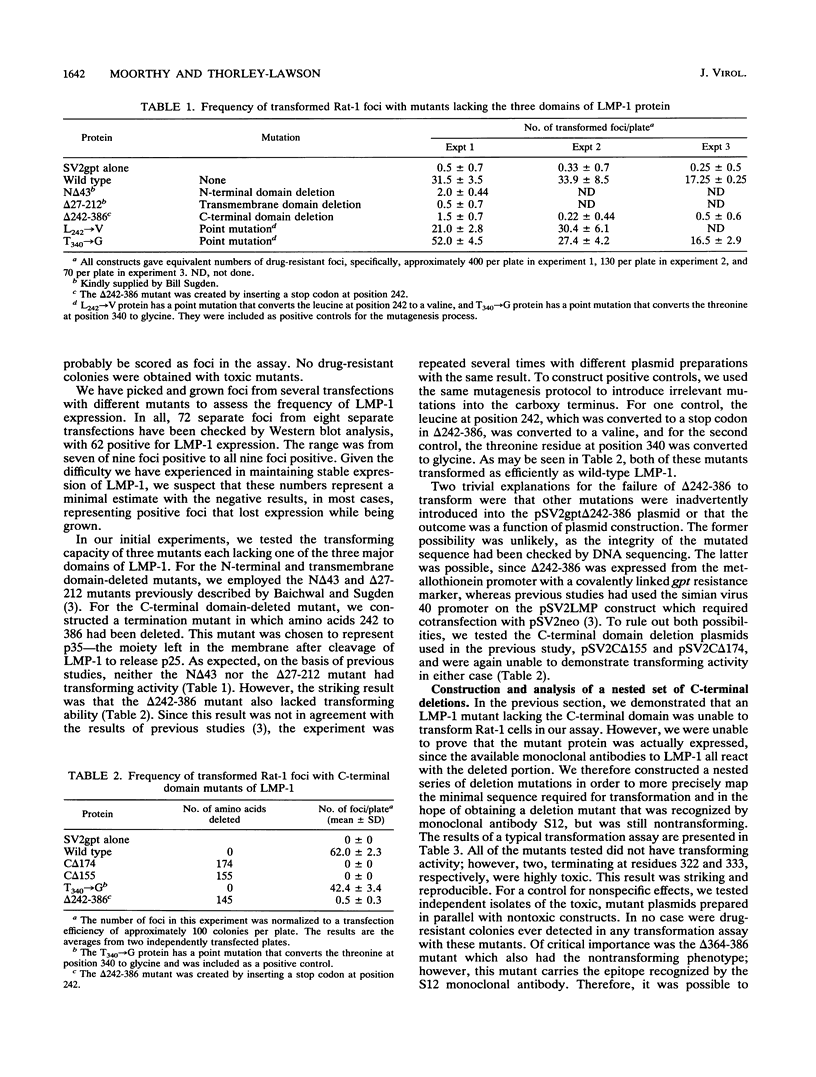
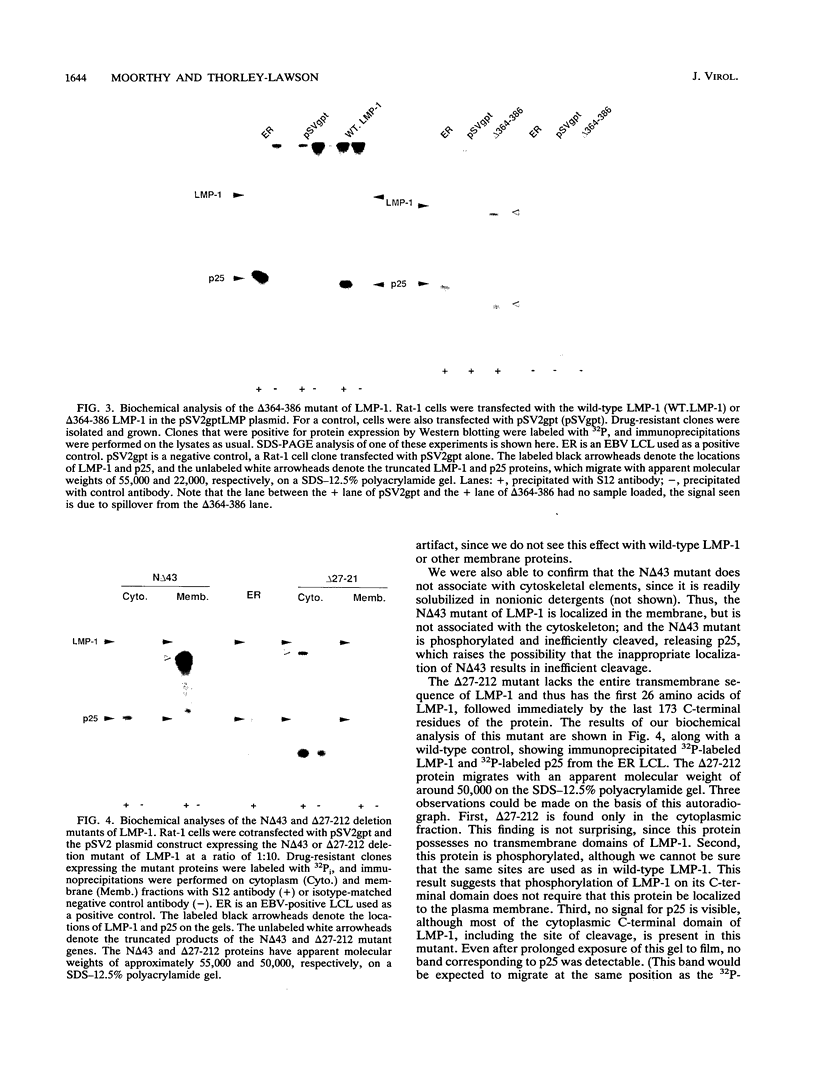
LMP-1, the Epstein-Barr virus latent membrane protein 1, is the only protein encoded by the virus that has been shown to have the properties of a transforming oncogene in rodent fibroblasts such as Rat-1 cells. LMP-1 is phosphorylated and proteolytically cleaved in Rat-1 cells in a manner similar to that seen in human lymphocytes. In this study, we demonstrate that all three major domains of LMP-1 (N-terminal, transmembrane, and C-terminal domains) are required for the ability to transform Rat-1 cells in culture, as assayed by loss of contact inhibition. This study is the first demonstration of a functional role for the C-terminal domain of LMP-1. Our analysis suggests that there are at least three distinct regions of the C terminus involved in signalling. Amino acids 306 to 334, which generate a toxic signal in the absence of amino acids 334 to 364, and the last 23 amino acids, 364 to 386, are essential for transformation. Biochemical analysis of the LMP-1 mutants with the three domains deleted indicate that the mutant N-terminal with the domain deleted is phosphorylated normally but is inefficiently cleaved compared with the wild-type LMP-1. The mutant with the transmembrane domain deleted is also phosphorylated but is not cleaved, showing that phosphorylation of LMP-1 does not require membrane association. The nontransforming mutant with the C-terminal domain deleted that lacks the last 23 amino acids is phosphorylated and cleaved. Therefore, these processing events alone are insufficient to generate a transforming signal.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baichwal V. R., Sugden B. Posttranslational processing of an Epstein-Barr virus-encoded membrane protein expressed in cells transformed by Epstein-Barr virus. J Virol. 1987 Mar;61(3):866–875. doi: 10.1128/jvi.61.3.866-875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. The multiple membrane-spanning segments of the BNLF-1 oncogene from Epstein-Barr virus are required for transformation. Oncogene. 1989 Jan;4(1):67–74. [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988 May;2(5):461–467. [PubMed] [Google Scholar]
- Fennewald S., van Santen V., Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984 Aug;51(2):411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B., Baichwal V. R. The transforming domain alone of the latent membrane protein of Epstein-Barr virus is toxic to cells when expressed at high levels. J Virol. 1989 Jun;63(6):2469–2475. doi: 10.1128/jvi.63.6.2469-2475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Fennewald S., Hummel M., Cole T., Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7207–7211. doi: 10.1073/pnas.81.22.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst H., Dallenbach F., Hummel M., Niedobitek G., Pileri S., Müller-Lantzsch N., Stein H. Epstein-Barr virus latent membrane protein expression in Hodgkin and Reed-Sternberg cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4766–4770. doi: 10.1073/pnas.88.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Adrenergic receptors. Adv Second Messenger Phosphoprotein Res. 1988;21:1–10. [PubMed] [Google Scholar]
- Liebowitz D., Kopan R., Fuchs E., Sample J., Kieff E. An Epstein-Barr virus transforming protein associates with vimentin in lymphocytes. Mol Cell Biol. 1987 Jul;7(7):2299–2308. doi: 10.1128/mcb.7.7.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz D., Mannick J., Takada K., Kieff E. Phenotypes of Epstein-Barr virus LMP1 deletion mutants indicate transmembrane and amino-terminal cytoplasmic domains necessary for effects in B-lymphoma cells. J Virol. 1992 Jul;66(7):4612–4616. doi: 10.1128/jvi.66.7.4612-4616.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz D., Wang D., Kieff E. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J Virol. 1986 Apr;58(1):233–237. doi: 10.1128/jvi.58.1.233-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Thorley-Lawson D. Posttranslational processing of the Epstein-Barr virus-encoded p63/LMP protein. J Virol. 1987 Jul;61(7):2100–2108. doi: 10.1128/jvi.61.7.2100-2108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Sugden B. Transformation by the oncogenic latent membrane protein correlates with its rapid turnover, membrane localization, and cytoskeletal association. J Virol. 1991 Jun;65(6):3246–3258. doi: 10.1128/jvi.65.6.3246-3258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy R., Thorley-Lawson D. A. Processing of the Epstein-Barr virus-encoded latent membrane protein p63/LMP. J Virol. 1990 Feb;64(2):829–837. doi: 10.1128/jvi.64.2.829-837.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. The truncated form of the Epstein-Barr virus latent-infection membrane protein expressed in virus replication does not transform rodent fibroblasts. J Virol. 1988 Jul;62(7):2337–2346. doi: 10.1128/jvi.62.7.2337-2346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]