Abstract
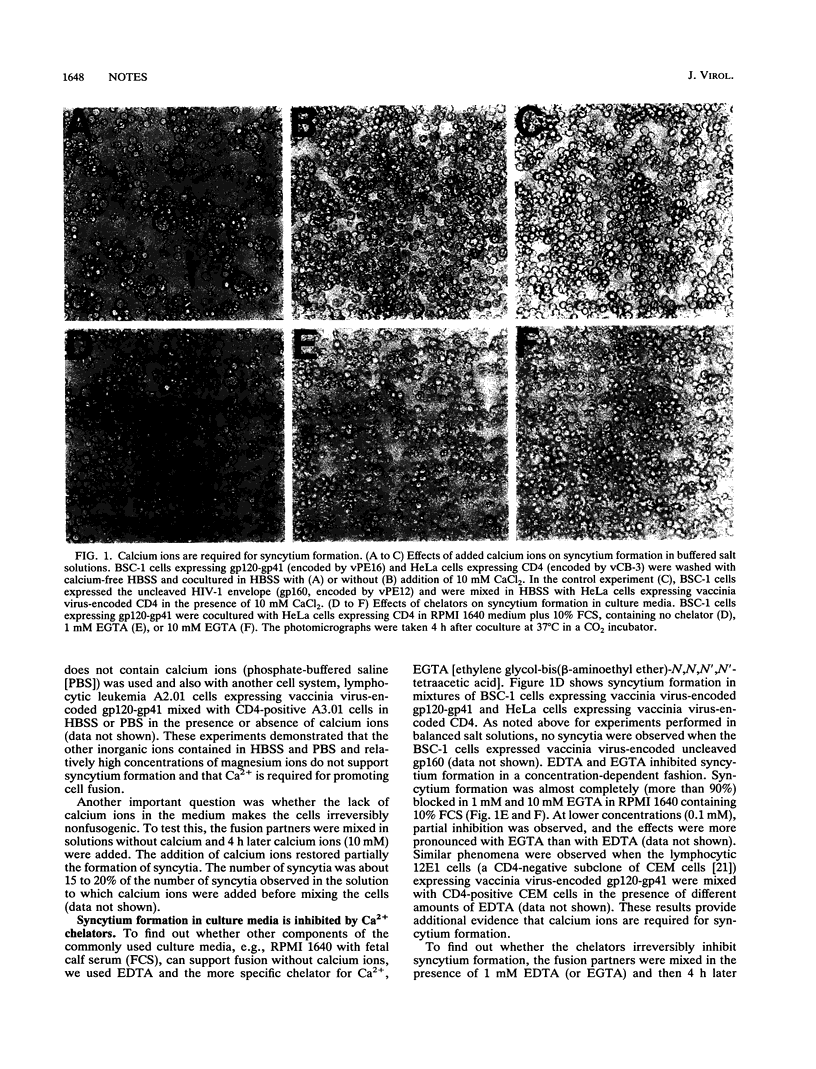
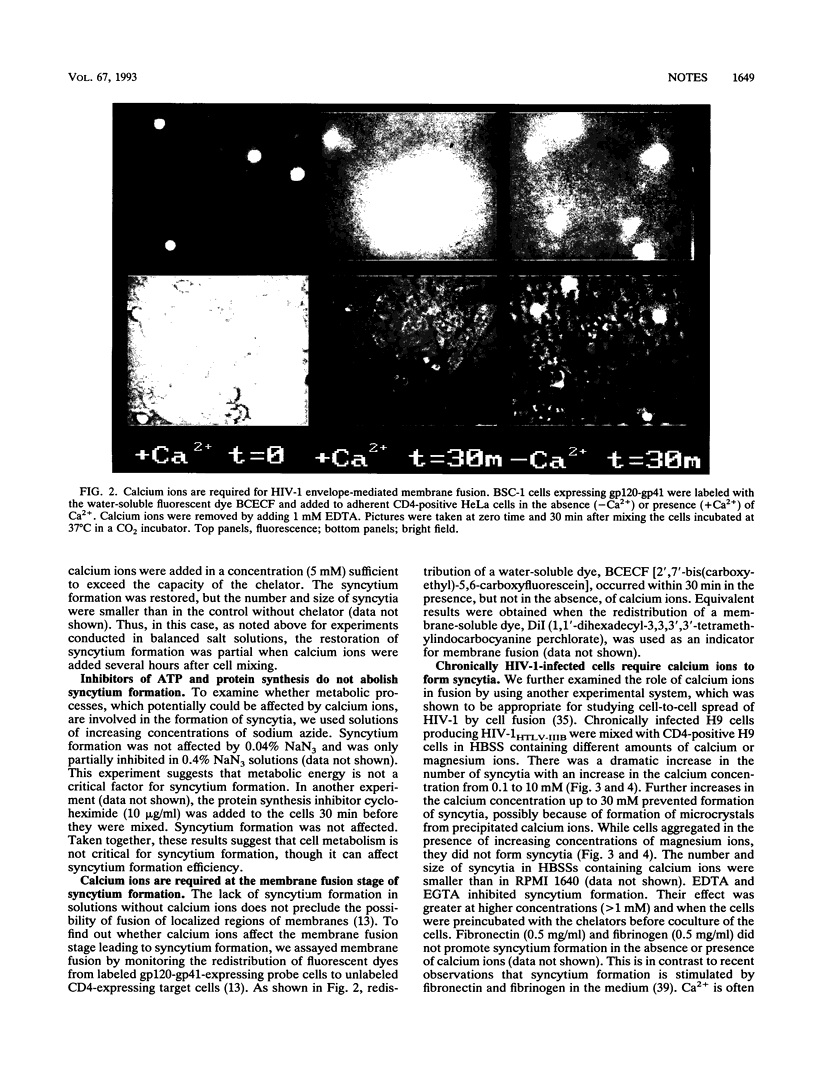
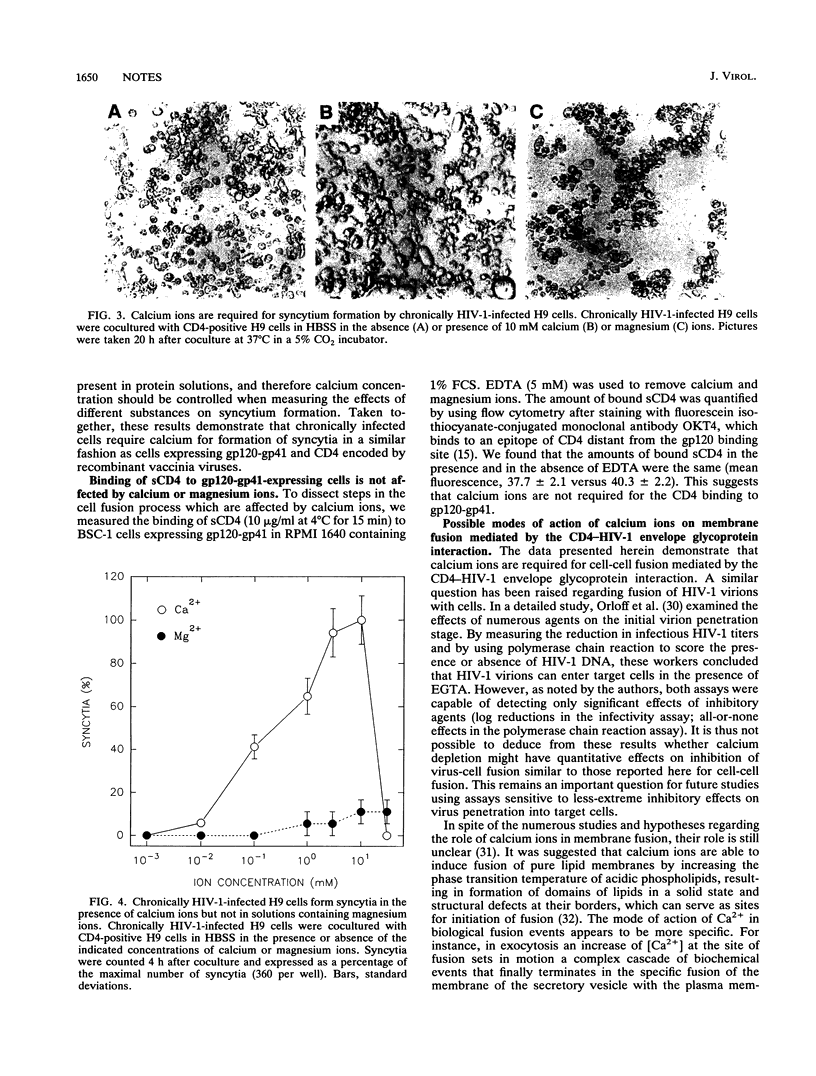
Calcium ions are required for fusion of a wide variety of artificial and biological membranes. To examine the role of calcium ions for cell fusion mediated by interactions between CD4 and the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (gp120-gp41), we used two experimental systems: (i) cells expressing gp120-gp41 and its receptor CD4, both encoded by recombinant vaccinia viruses, and (ii) chronically infected cells producing low levels of HIV-1. Fusion was measured by counting the number of syncytia and by monitoring the redistribution of fluorescence dyes by video microscopy. Syncytia did not form in solutions without calcium ions. Addition of calcium ions partially restored the formation of syncytia. EDTA and EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid] blocked syncytium formation in culture media containing calcium ions. Membrane fusion as monitored by fluorescence dye redistribution also required calcium ions. Cell fusion increased with an increase in calcium ion concentration from 100 microM to 10 mM but was not affected by magnesium ions in the concentration range from 0 to 30 mM. Fibrinogen and fibronectin did not promote fusion in the absence or presence of Ca2+. Binding of soluble CD4 to gp120-gp41-expressing cells was not affected by Ca2+ and Mg2+. We conclude that Ca2+ is involved in postbinding steps in cell fusion mediated by the CD4-HIV-1 envelope glycoprotein interaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashorn P. A., Berger E. A., Moss B. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990 May;64(5):2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Lifson J. D., Eiden L. E. Stimulation of glycoprotein gp120 dissociation from the envelope glycoprotein complex of human immunodeficiency virus type 1 by soluble CD4 and CD4 peptide derivatives: implications for the role of the complementarity-determining region 3-like region in membrane fusion. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8082–8086. doi: 10.1073/pnas.88.18.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Sisler J. R., Earl P. L. Human immunodeficiency virus type 1 envelope glycoprotein molecules containing membrane fusion-impairing mutations in the V3 region efficiently undergo soluble CD4-stimulated gp120 release. J Virol. 1992 Oct;66(10):6208–6212. doi: 10.1128/jvi.66.10.6208-6212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder C. C., Berger E. A. CD4 molecules with a diversity of mutations encompassing the CDR3 region efficiently support human immunodeficiency virus type 1 envelope glycoprotein-mediated cell fusion. J Virol. 1993 Feb;67(2):913–926. doi: 10.1128/jvi.67.2.913-926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Ebenbichler C., Vornhagen R., Schulz T. F., Steindl F., Böck G., Katinger H., Dierich M. P. HIV-1 gp41 contains two sites for interaction with several proteins on the helper T-lymphoid cell line, H9. AIDS. 1992 Jun;6(6):533–539. doi: 10.1097/00002030-199206000-00002. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Schoch C., Blumenthal R. Delay time for influenza virus hemagglutinin-induced membrane fusion depends on hemagglutinin surface density. J Virol. 1991 May;65(5):2402–2407. doi: 10.1128/jvi.65.5.2402-2407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. M., Scharnowske S., Watson A. J. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Golding H., Blumenthal R. Initial stages of HIV-1 envelope glycoprotein-mediated cell fusion monitored by a new assay based on redistribution of fluorescent dyes. AIDS Res Hum Retroviruses. 1991 Oct;7(10):799–805. doi: 10.1089/aid.1991.7.799. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Hillman K., Manischewitz J., Blumenthal R., Golding H. Correlation between kinetics of soluble CD4 interactions with HIV-1-Env-expressing cells and inhibition of syncytia formation: implications for mechanisms of cell fusion and therapy for AIDS. AIDS. 1992 Mar;6(3):249–256. doi: 10.1097/00002030-199203000-00001. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Hillman K., Manischewitz J., Blumenthal R., Golding H. Kinetics of soluble CD4 binding to cells expressing human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1992 Jan;66(1):132–138. doi: 10.1128/jvi.66.1.132-138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T., Charneau P., Clavel F., Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992 Aug;66(8):4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Hügin A. W., Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990 May;64(5):2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Koenig S., Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991 Jan;65(1):31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart T. K., Kirsh R., Ellens H., Sweet R. W., Lambert D. M., Petteway S. R., Jr, Leary J., Bugelski P. J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman K., Shapira-Nahor O., Gruber M. F., Hooley J., Manischewitz J., Seeman R., Vujcic L., Geyer S. J., Golding H. Chemically induced CD4 mutants of a human T cell line. Evidence for dissociation between binding of HIV I envelope and susceptibility to HIV I infection and syncytia formation. J Immunol. 1990 Mar 15;144(6):2131–2139. [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Portner A., Schwartz A. L. An alternative route of infection for viruses: entry by means of the asialoglycoprotein receptor of a Sendai virus mutant lacking its attachment protein. Proc Natl Acad Sci U S A. 1985 Feb;82(4):978–982. doi: 10.1073/pnas.82.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Orloff G. M., Orloff S. L., Kennedy M. S., Maddon P. J., McDougal J. S. Penetration of CD4 T cells by HIV-1. The CD4 receptor does not internalize with HIV, and CD4-related signal transduction events are not required for entry. J Immunol. 1991 Apr 15;146(8):2578–2587. [PubMed] [Google Scholar]
- Papahadjopoulos D., Nir S., Düzgünes N. Molecular mechanisms of calcium-induced membrane fusion. J Bioenerg Biomembr. 1990 Apr;22(2):157–179. doi: 10.1007/BF00762944. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Newton C., Nir S., Jacobson K., Poste G., Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim Biophys Acta. 1977 Mar 17;465(3):579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Qureshi N. M., Coy D. H., Garry R. F., Henderson L. A. Characterization of a putative cellular receptor for HIV-1 transmembrane glycoprotein using synthetic peptides. AIDS. 1990 Jun;4(6):553–558. doi: 10.1097/00002030-199006000-00009. [DOI] [PubMed] [Google Scholar]
- Sato H., Orenstein J., Dimitrov D., Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992 Feb;186(2):712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Ushijima H., Unten S., Honma H., Tsuchie H., Kitamura T., Weiler B. E., Müller W. E. Effect of serum components on syncytium formation and virus production by cells infected with human immunodeficiency virus in vitro. AIDS Res Hum Retroviruses. 1992 Apr;8(4):513–520. doi: 10.1089/aid.1992.8.513. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Loyter A. Role of Ca++ in virus-induced membrane fusion. Ca++ accumulation and ultrastructural changes induced by Sendai virus in chicken erythrocytes. J Cell Biol. 1978 Aug;78(2):465–479. doi: 10.1083/jcb.78.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]






