Abstract
Epidemiological findings indicate that hormonal influences may play a role in the etiology of renal cell cancer (RCC). The possible effect of childbearing remains enigmatic; while some investigators have reported a positive association between number of births and renal cell cancer risk, others have not. A case–control study, nested within a nation-wide Fertility Register covering Swedish women born 1925 and later, was undertaken to explore possible associations between parity and age at first birth and the risk of renal cell cancer. Among these women a total of 1465 cases of RCC were identified in the Swedish Cancer Register between 1958 and 1992 and information on the number of live childbirths and age at each birth was obtained by linkage to the Fertility Database. For each case, five age-matched controls were randomly selected from the same register. Compared to nulliparous women, ever-parous women were at a 40% increased risk of RCC (Odds Ratio [OR]=1.42; 95% CI 1.19-1.69). The corresponding OR for women of high parity (five or more live births) was 1.91 (95% CI 1.40–2.62). After controlling for age at first birth among parous women, each additional birth was associated with a 15% increase in risk (OR=1.15; 95% CI 1.08–1.22). The observed positive association between parity and renal cell cancer risk is unlikely to be fully explained by uncontrolled confounding, but warrants further evaluation in large studies, with allowance for body mass index.
British Journal of Cancer (2002) 86, 1425–1429. DOI: 10.1038/sj/bjc/6600263 www.bjcancer.com
© 2002 Cancer Research UK
Keywords: kidney neoplasms, risk factors, parity, pregnancy, case–control studies
Cancer of the renal parenchyma, or renal cell cancer (RCC), represents about 80% of all kidney cancer cases in Sweden and is about twice as common in men than among women. Incidence rates in Sweden have tended to decrease among both men and women (Swedish Cancer Registry, 2000), during the past two decades in contrast to many other areas in the world. Reported international incidence rates vary more than 10-fold, with the highest rates found in northern, western and eastern Europe, Australia and North America (Ferlay et al, 2001). Apart from cigarette smoking and obesity, and possibly weight cycling, hypertension and/or antihypertensive medication, the causes of renal cell cancer are unclear (McLaughlin and Lipworth, 2000).
Experimental findings of oestrogen-induced renal cell carcinogenesis in animals (Li and Li, 1990) have prompted an interest in the possible influence of hormonal factors in humans. Weak positive associations have been reported for use of oral contraceptives (McLaughlin et al, 1992) and replacement oestrogens in some (Asal et al, 1988; McLaughlin et al, 1992), but not all studies (McLaughlin et al, 1984; Adami et al, 1998). A large case–control study, however, found a reduced risk of renal cell cancer following oral contraceptive use (Lindblad et al, 1995), although another recent study found no relationship (Gago-Dominguez et al, 1999). Two studies showed no relation between hysterectomy or oophorectomy and risk of RCC (Wynder et al, 1974; McLaughlin et al, 1984), whereas other investigators have reported an increased risk following hysterectomy (McCredie and Stewart, 1992; Mellemgaard et al, 1994; Gago-Dominguez et al, 1999) and both hysterectomy and oophorectomy (Lindblad et al, 1995). There is also some epidemiological evidence that childbearing may play a role in the development of RCC (Kreiger et al, 1993; Chow et al, 1995; Lindblad et al, 1995).
To further examine this issue, we utilised a large population-based data set to explore possible associations between pregnancy and the risk of renal cell cancer among Swedish women.
MATERIALS AND METHODS
We merged data from two population-based Swedish registers, the Fertility Register and the Cancer Register, both updated through 1992, through a unique national registration number.
The Fertility Register was originally based on women born between 1925 and 1960 who were resident citizens of Sweden in 1960. For these women, reproductive data (nulliparity, number and dates of live births) during the period 1943–1960 were collected retrospectively at the 1960 Census. Later birth cohorts of all Swedish women have been added continuously, with the women's births being recorded annually via vital statistics records. For the present study, the Fertility Register included almost 3.4 million live births recorded from 1943 to 1992 among approximately 2.4 million women born between 1925 and 1972. Based on a nation-wide reporting system of all births to women residing in Sweden, the quality of data concerning number and dates of birth is generally high (Johansson and Finnäs, 1983). In the oldest cohorts, mainly women born 1925–29, fertility may be underestimated since children born before 1943, those not living with their mothers, and those who died before the 1960 Census, were not recorded. There might also be a slight overestimation of fertility between 1943 and 1960 because adopted and foster children were included in the retrospectively collected information. From 1961 and onwards, only biological children were included. Dates of death and emigration among registered women are recorded yearly in the Fertility Register. Because women with 10 or more births are rare in the birth cohorts under study, complete birth dates were only obtained for up to nine children per woman for the purpose of this study. Subjects with 10 or more births were identifiable by a two-digit parity code.
The Swedish Cancer Register started in 1958. All newly diagnosed malignant tumours must be reported to the Register separately by both the clinician and the pathologist or cytologist. Nearly 100% of all diagnosed cancers are recorded (Swedish Cancer Registry, 2000). Among resident Swedish women born between 1925 and 1972, a total of 2086 cases of kidney cancer (International Classification of Diseases (ICD)-7 : 180) were recorded in the Cancer Register from January 1, 1958 to December 31, 1992. Of these, 1856 (88.9 %) were identified in the Fertility Register. Thus, no match was achieved for 230 cases recorded in the Cancer Register, some of which represent mismatches due to aberrant national registration numbers, while the majority are non-Swedish citizens who are recorded only in the Cancer Register. We excluded 14 cases diagnosed in 1958–1960 who were survivors with prevalent cancers at the time of the 1960 Census, two cases with more than nine children, 193 cases with non-renal cell cancer and 368 cases with histopathological-codes other than adenocarcinoma (code: 096). Because of overlap between the excluded groups, the final analysis encompassed 1465 cases of renal cell cancer with concomitant fertility information.
A nested case–control study was undertaken to evaluate possible associations between the recorded reproductive variables and RCC. For every case, five comparison women (controls) matched on year and month of birth were randomly selected among those in the Fertility Register. The controls had to be alive at the date of the diagnosis of the case and be residents of Sweden, without themselves having been diagnosed with kidney cancer. Controls were assigned an index date that was the same as the diagnosis date of their matched case. For both cases and controls, only pregnancies before the date of the diagnosis of the case (the index date for the control) were included in the analysis. Controls with more than nine births were excluded, leaving five case–control sets being matched 1:4.
Statistical analysis
The statistical analyses used matched conditional logistic regression (Breslow and Day, 1980). Associations between the reproductive risk factors and RCC were measured using odds ratios (OR) as estimates of relative risks (Rothman and Greenland, 1998), and their 95% confidence intervals (CI). Age at first birth was analysed both as a categorical variable (nulliparous, <20, 20–24, 25–29, 30+) and as a continuous variable in the analysis for parous women. Risk estimates with time since most recent pregnancy were calculated among uniparous, biparous and triparous women, compared to nulliparous women.
RESULTS
More than half of all renal cell cancers (59.9%) in women were diagnosed at age 50 or over (Table 1), with a mean age at diagnosis of 50.4 years. We found a strong positive association between number of births and risk of RCC (Table 2). Compared to nulliparous women, ever parous women were at a 40% increased risk of RCC (OR=1.42; 95% CI=1.19–1.69). The corresponding OR for women of high parity (five or more live births) was 1.91 (95% CI=1.40–2.62). After controlling for age at first birth among parous women, risk increased monotonically and each additional birth was associated with a 15% increase in risk (OR=1.15; 95% CI=1.08–1.22). Relative to uniparous women, the adjusted odds ratio for women with three births was 1.33 (95% CI=1.10–1.61) and for women with five or more births the OR was 1.62 (95% CI=1.18–2.23) (Table 2). The positive association with parity was similar in women older at diagnosis (age ⩾50 years) and in those younger at diagnosis (<50 years) (data not shown). When age at first birth was analysed as a continuous variable, there was a slight decrease in risk with increasing age at first delivery. After controlling for parity among parous women, the risk decreased by 8% for each 5-year increment of delay of first birth (OR=0.92; 95% CI=0.86–1.00) (Table 2).
Table 1. Distribution of 1465 case women with renal cell cancer, by year of birth and age at diagnosis.

Table 2. Distribution of 1465 women with renal cell cancer and 7320 controls by number of live births and age at first birth. Odds ratios (OR) and 95% confidence intervals (CI) by parity and age at first birth.
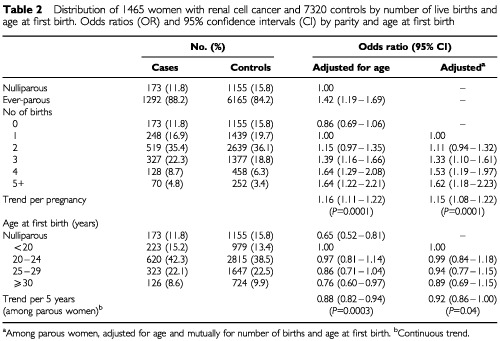
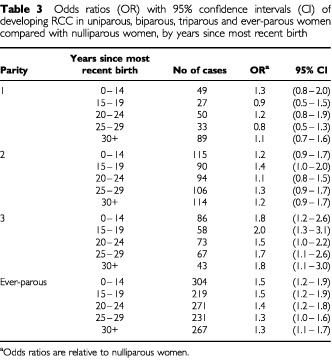
Table 3 shows the risk of developing RCC by years since most recent birth among uniparous, biparous, triparous and ever-parous women relative to nulliparous women. Except for women with one live birth, risk estimates were consistently and often significantly elevated in all time periods since delivery.
Table 3. Odds ratios (OR) with 95% confidence intervals (CI) of developing RCC in uniparous, biparous, triparous and ever-parous women compared with nulliparous women, by years since most recent birth.
DISCUSSION
The influence of number of births on the risk of RCC remains unclear with a majority of studies finding no clear relation (Wynder et al, 1974; McLaughlin et al, 1984, 1992; Cantor et al, 1993; La Vecchia et al, 1993; Mellemgaard et al, 1994; Gago-Dominguez et al, 1999), while some investigators have reported positive associations (Kreiger et al, 1993; Chow et al, 1995; Lindblad et al, 1995).
Our results indicate that women with a history of high parity are at an increased risk of renal cell cancer. Compared to nulliparous women, the risk was nearly two times higher among women with five or more live births. This finding is in agreement with that of a large international multi-centre case–control study that found an 80% increased risk among high parity women. However, in that study there was no regular trend with increasing parity. In the same study, age at first birth was not associated with RCC risk after adjustment for age, study centre, body mass index and number of births (Lindblad et al, 1995). Compared to women with one or two births, multiparous women showed a more than two-fold risk increase, an association that was stronger among hypertensive or overweight women (Chow et al, 1995). In another study, a risk increase of similar magnitude was reported in multiparous women compared to nulliparous women, which was strictly limited to women with a previous history of foetal loss; among women with no foetal loss, the number of live births was unrelated to the risk of RCC (Kreiger et al, 1993). The latter study, as in our own, showed evidence of an inverse association between age at first birth and the risk of RCC.
The main strengths of the present study were its large size and the population-based design that minimised selection and information biases. By using nation-wide registry information, we were able to ascertain virtually all incident cases of RCC. The primary weakness was the absence of information on potential confounders such as Body Mass Index (BMI), weight cycling, hypertension, and smoking habits. Furthermore, in our assessment, pregnancy histories on complications or outcomes such as spontaneous and induced abortions were not available.
Of particular concern was the influence on our risk estimates by uncontrolled confounding by BMI. Childbearing is associated with a considerable increase in weight, usually followed by some degree of permanent weight-retention after delivery and lactation (Rössner, 1997). Results from a meta-analysis of 14 studies on obesity and the risk of RCC in women, indicate that the risk increases by 7% for each unit increase in BMI (Bergstrom et al, 2001).
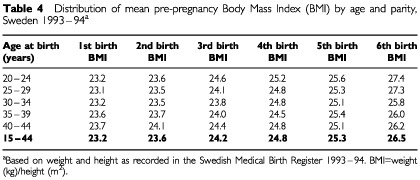
Thus, pregnancy-associated weight gain may have influenced the observed relation to parity. To indirectly assess the relative importance of parity and BMI, we obtained data from the Swedish Medical Birth Register, a population-based database that since 1992 records pre-pregnancy weight among expecting mothers. These data showed that pre-pregnancy BMI increased by an average of 3.3 units between a first and sixth pregnancy (Table 4), producing an expected risk increase for RCC equalling 25.0% (1.073.3). In the present study, we found an estimated risk increase of 62% for women of parity 5+, relative to uniparous women. Taken together, these data provide evidence of an independent risk-modifying effect of parity on the risk of developing RCC, over and beyond the influence of BMI. This assumption is supported by the findings of the collaborative international study on renal cell cancer, where neither adjustment for smoking nor body mass index altered the estimates for parity (Lindblad et al, 1995). Chow et al (1995) reported an interaction between parity, obesity and the risk of RCC. In that study, the positive association with number of births was present in women with normal weight, but was stronger among obese subjects.
Table 4. Distribution of mean pre-pregnancy Body Mass Index (BMI) by age and parity, Sweden 1993–94a.
We can only speculate about the biological mechanisms whereby childbearing could affect renal carcinogenesis. Pregnancy-associated hormonal changes, particularly high oestrogen levels, may act as promotors of malignant change by stimulating renal cell proliferation either directly or via paracrine growth factors (Concolino et al, 1993). Both oestrogen and progesterone receptors are present in normal and malignant renal cells (Ronchi et al, 1984). High doses of potent oestrogens have been shown to induce renal cell tumours in laboratory rodents (Li and Li, 1990). The proliferative effect of oestrogen on kidney tissue seen experimentally may be mediated through the up-regulation of IGF-I receptors (Chen et al, 1996). Also, both insulin and IGF-I might contribute to the growth and proliferation of renal cell cancer. Experimentally evidence indicates that such growth factors and their receptors play a role in several renal diseases, including renal tumours (Zumkeller and Schofield, 1992; Stadler and Vogelzang, 1993; Kellerer et al, 1995). The consistent finding that obesity is a risk factor for human renal cell cancer, and the knowledge that obesity may lead to a hyperestrogenic environment and increased levels of insulin and insulin-like growth factor (IGF-1) (Azziz, 1989; Frystyk et al, 1995), provides indirect support that hormonal factors may play a role in human renal carcinogenesis. Also, epidemiological studies indicate that patients with diabetes have an increased risk of renal cell cancer (Schlehofer et al, 1996; Lindblad et al, 1999). In addition, a normal pregnancy is associated with hyperfiltration (Krutzen et al, 1992; Sturgiss et al, 1996). Animal studies suggest that glomerular hyperfiltration could play a role in the development of glomerulosclerosis, (Neuringer and Brenner, 1992; Westenend et al, 1992), which makes the nephrons more vulnerable for exposure to carcinogens.
The results of the present study are interesting because of both the highly regular trend between increasing parity and the risk of RCC, and the strength of the associations. Our observations appear unlikely to be fully explained by uncontrolled confounding and warrant further evaluation in large studies where detailed information on other risk factors for RCC and possible confounders also can be considered.
Acknowledgments
We thank Dr Petra Otterblad-Olausson for kindly providing data from the Swedish Medical Birth Register. Supported by grants from the Swedish Cancer Society and the Swedish Society of Medicine. Rachel Remler was supported in part by National Institutes of Health, National Research Service Award 5 T32 ES07262 from the National Institute of Environmental Health Sciences.
References
- AdamiHOSignorelloLBTrichopoulosD1998Towards an understanding of breast cancer etiology Semin Cancer Biol 8255262 [DOI] [PubMed] [Google Scholar]
- AsalNRGeyerJRRisserDRLeeETKadamaniSCherngN1988Risk factors in renal cell carcinoma. II. Medical history, occupation, multivariate analysis, and conclusions Cancer Detect Prev 13263279 [PubMed] [Google Scholar]
- AzzizR1989Reproductive endocrinologic alterations in female asymptomatic obesity Fertil Steril 52703725 [DOI] [PubMed] [Google Scholar]
- BergstromAHsiehCCLindbladPLuCMCookNRWolkA2001Obesity and renal cell cancer – a quantitative review Br J Cancer 85984990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BreslowNEDayNE1980Statistical Methods in Cancer Research. Vol 1 - The analysis of case-control studies Vol. 32Lyon: IARC [PubMed] [Google Scholar]
- CantorKPLynchCFJohnsonD1993Reproductive factors and risk of brain, colon, and other malignancies in Iowa (United States) Cancer Causes Control 4505511 [DOI] [PubMed] [Google Scholar]
- ChenCWOberleyTDRoyD1996Inhibition of stilbene estrogen-induced cell proliferation of renal epithelial cells through the modulation of insulin-like growth factor-I receptor expression Cancer Lett 1055159 [DOI] [PubMed] [Google Scholar]
- ChowWHMcLaughlinJKMandelJSBlotWJNiwaSFraumeniJrJF1995Reproductive factors and the risk of renal cell cancer among women Int J Cancer 60321324 [DOI] [PubMed] [Google Scholar]
- ConcolinoGLubranoCOmbresMSantonatiAFlammiaGPDi SilverioF1993Acquired cystic kidney disease: the hormonal hypothesis Urology 41170175 [DOI] [PubMed] [Google Scholar]
- FerlayJBrayFPisaniPParkinDM2001GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0IARC CancerBase No. 5Lyon: IARC Press [Google Scholar]
- FrystykJVestboESkjaerbaekCMogensenCEOrskovH1995Free insulin-like growth factors in human obesity Metabolism 443744 [DOI] [PubMed] [Google Scholar]
- Gago-DominguezMCastelaoJEYuanJMRossRKYuMC1999Increased risk of renal cell carcinoma subsequent to hysterectomy Cancer Epidemiol Biomarkers Prev 89991003 [PubMed] [Google Scholar]
- JohanssonLFinnäsF1983Fertility of Swedish women born 1927–1960Stockholm: Statistics Sweden [Google Scholar]
- KellererMvon Eye CorletaHMuhlhoferACappEMosthafLBockSPetridesPEHaringHU1995Insulin- and insulin-like growth-factor-I receptor tyrosine-kinase activities in human renal carcinoma Int J Cancer 62501507 [DOI] [PubMed] [Google Scholar]
- KreigerNMarrettLDDoddsLHilditchSDarlingtonGA1993Risk factors for renal cell carcinoma: results of a population-based case-control study Cancer Causes Control 4101110 [DOI] [PubMed] [Google Scholar]
- KrutzenEOlofssonPBackSENilsson-EhleP1992Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes Scand J Clin Lab Invest 52387392 [DOI] [PubMed] [Google Scholar]
- La VecchiaCNegriEFranceschiSParazziniF1993Long-term impact of reproductive factors on cancer risk Int J Cancer 53215219 [DOI] [PubMed] [Google Scholar]
- LiJJLiSA1990Estrogen carcinogenesis in hamster tissues: a critical review Endocr Rev 11524531 [DOI] [PubMed] [Google Scholar]
- LindbladPChowWHChanJBergstromAWolkAGridleyGMcLaughlinJKNyrenOAdamiHO1999The role of diabetes mellitus in the aetiology of renal cell cancer Diabetologia 42107112 [DOI] [PubMed] [Google Scholar]
- LindbladPMellemgaardASchlehoferBAdamiHOMcCredieMMcLaughlinJKMandelJS1995International renal-cell cancer study. V. Reproductive factors, gynecologic operations and exogenous hormones Int J Cancer 61192198 [DOI] [PubMed] [Google Scholar]
- McCredieMStewartJH1992Risk factors for kidney cancer in New South Wales, Australia. II. Urologic disease, hypertension, obesity, and hormonal factors Cancer Causes Control 3323331 [DOI] [PubMed] [Google Scholar]
- McLaughlinJKGaoYTGaoRNZhengWJiBTBlotWJFraumeniJrJF1992Risk factors for renal-cell cancer in Shanghai, China Int J Cancer 52562565 [DOI] [PubMed] [Google Scholar]
- McLaughlinJKLipworthL2000Epidemiologic aspects of renal cell cancer Semin Oncol 27115123 [PubMed] [Google Scholar]
- McLaughlinJKMandelJSBlotWJSchumanLMMehlESFraumeniJrJF1984A population–based case–control study of renal cell carcinoma J Natl Cancer Inst 72275284 [PubMed] [Google Scholar]
- MellemgaardAEngholmGMcLaughlinJKOlsenJH1994Risk factors for renal-cell carcinoma in Denmark. III. Role of weight, physical activity and reproductive factors Int J Cancer 566671 [DOI] [PubMed] [Google Scholar]
- NeuringerJRBrennerBM1992Glomerular hypertension: cause and consequence of renal injury J Hypertens Suppl 10S91S97 [PubMed] [Google Scholar]
- RonchiEPizzocaroGMiodiniPPivaLSalvioniRDi FronzoG1984Steroid hormone receptors in normal and malignant human renal tissue: relationship with progestin therapy J Steroid Biochem 21329335 [DOI] [PubMed] [Google Scholar]
- RothmanKJGreenlandS1998Case–control studiesInModern epidemiologypp 95Philadelphia: Lippincott - Raven [Google Scholar]
- RössnerS1997Weight gain in pregnancy Hum Reprod 12Suppl 1110115 [DOI] [PubMed] [Google Scholar]
- SchlehoferBPommerWMellemgaardAStewartJHMcCredieMNiwaSLindbladPMandelJSMcLaughlinJKWahrendorfJ1996International renal-cell-cancer study. VI. the role of medical and family history Int J Cancer 66723726 [DOI] [PubMed] [Google Scholar]
- StadlerWVogelzangNJ1993Human renal cancer carcinogenesis: a review of recent advances Ann Oncol 4451462 [DOI] [PubMed] [Google Scholar]
- SturgissSNWilkinsonRDavisonJM1996Renal reserve during human pregnancy Am J Physiol 271F16F20 [DOI] [PubMed] [Google Scholar]
- Swedish Cancer Registry2000Cancer Incidence in Sweden 1998Stockholm: Socialstyrelsen [Google Scholar]
- WestenendPJNooyenYAvan der KrogtJAvan BrummelenPWeeningJJ1992Functional and structural determinants of glomerulosclerosis in the fawn-hooded rat Eur J Clin Invest 22391395 [DOI] [PubMed] [Google Scholar]
- WynderELMabuchiKWhitmoreJrWF1974Epidemiology of adenocarcinoma of the kidney J Natl Cancer Inst 5316191634 [PubMed] [Google Scholar]
- ZumkellerWSchofieldPN1992The role of insulin-like growth factors and IGF-binding proteins in the physiological and pathological processes of the kidney Virchows Arch B Cell Pathol Incl Mol Pathol 62207220 [DOI] [PubMed] [Google Scholar]


