Abstract
Benzodiazepines (BDZs) are known to increase the amplitude and duration of IPSCs. Moreover, at low [GABA], BDZs strongly enhance GABAergic currents suggesting the up-regulation of agonist binding while their action on gating remains a matter of debate. In the present study we have examined the impact of flurazepam and zolpidem on mIPSCs by investigating their effects on GABAAR binding and gating and by considering dynamic conditions of synaptic receptor activation. Flurazepam and zolpidem enhanced the amplitude and prolonged decay of mIPSCs. Both compounds strongly enhanced responses to low [GABA] but, surprisingly, decreased the currents evoked by saturating or half-saturating [GABA]. Analysis of current responses to ultrafast GABA applications indicated that these compounds enhanced binding and desensitization of GABAA receptors. Flurazepam and zolpidem markedly prolonged deactivation of responses to low [GABA] but had almost no effect on deactivation at saturating or half-saturating [GABA]. Moreover, at low [GABA], flurazepam enhanced desensitization–deactivation coupling but zolpidem did not. Recordings of responses to half-saturating [GABA] applications revealed that appropriate timing of agonist exposure was sufficient to reproduce either a decrease or enhancement of currents by flurazepam or zolpidem. Recordings of currents mediated by recombinant (‘synaptic’) α1β2γ2 receptors reproduced all major findings observed for neuronal GABAARs. We conclude that an extremely brief agonist transient renders IPSCs particularly sensitive to the up-regulation of agonist binding by BDZs.
GABA (γ-aminobutyric acid) is the major inhibitory neurotransmitter in the adult mammalian central nervous system. To date, as many as 20 subunits of GABAA receptors (γ-aminobutyric acid receptor type A) (α1–6, β1–4, γ1–3, δ, ρ1–3, ɛ, π and θ) have been cloned (Cherubini & Conti, 2001; Fritschy & Brunig, 2003) suggesting an overwhelming heterogeneity. However, most common GABAARs consist of two α, two β and one γ or δ subunit (Whiting, 2003; Wafford, 2005).
Benzodiazepine (BDZ) receptor agonists are known as positive modulators of specific GABAA receptors (GABAARs) (Rudolph & Mohler, 2004; Wafford, 2005; Rudolph & Mohler, 2006). BDZs were commonly found to enhance the amplitude and to prolong GABAergic IPSCs (inhibitory postsynaptic currents) (Frerking et al. 1995; Nusser et al. 1997; Perrais & Ropert, 1999; Hajos et al. 2000; Perrais & Ropert, 2000). Several lines of evidence indicate that BDZs up-regulate the binding affinity of GABAARs. A compelling indication for this mechanism is a strong BDZ-induced enhancement of amplitude and onset rate of currents evoked by non-saturating [GABA] but these effects tend to disappear at saturating [GABA] (Lavoie & Twyman, 1996; Krampfl et al. 1998). BDZs do not clearly affect the GABAAR single channel lifetimes (Twyman et al. 1989; Rogers et al. 1994). These findings, taken altogether, suggest that BDZs enhance binding affinity while their effect on GABAAR gating appears minor. However, Rusch & Forman (2005) as well as Downing et al. (2005) have proposed a novel mechanism for GABAAR modulation by BDZs. They considered a spontaneously active GABAAR mutant and reported that BDZs might effectively modulate the GABAAR gating. However, it was not clear to what extent these observations applied to the native GABAARs in which coupling between binding and gating has not been altered by mutations. More recently, Campo-Soria et al. (2006) have reported that in oocytes expressing ultrahigh levels of wild type GABAA receptors, a high concentration (1 μm) of diazepam induced a small but detectable GABAergic current and proposed that this BDZ might act on GABAARs by affecting the opening transitions. In a recent study, we have reported that flurazepam and zolpidem affected both binding and gating of α1β2γ2 receptors (Mercik et al. 2007). The effect on receptor gating was deduced from a BDZ-induced decrease in amplitude and a moderate modulation of the time course of currents elicited by saturating [GABA]. These observations appeared peculiar as they were substantially different from what is commonly observed for synaptic currents. It is thus interesting to investigate whether the effects of flurazepam and zolpidem observed for α1β2γ2 receptors can be reproduced in neurons. In particular, it is appealing to compare the action of these drugs on currents evoked by exogenous GABA and on mIPSCs (miniature inhibitory postsynaptic currents). For this purpose, the effect of flurazepam and zolpidem has been examined on mIPSCs and on current responses to ultrafast GABA applications recorded from rat cultured hippocampal neurons. We found that flurazepam and zolpidem modulated both binding and gating of neuronal receptors. However, BDZ effects on amplitudes of mIPSCs and on responses to saturating [GABA] showed qualitative differences. To explore this discrepancy, we have modelled the synaptic currents by responses to short applications of non-saturating [GABA] and concluded that extreme non-equilibrium conditions, dictated by a fast agonist transient, render mIPSCs particularly susceptible to modulation by BDZs.
Methods
Neuronal primary cell culture
Primary cell culture was prepared as already described (Andjus et al. 1997). Briefly, P2–P4 Wistar rats were killed by decapitation. This procedure is in agreement with the Polish Animal Welfare Act and has been approved by the Local Ethical Commission. Hippocampi were dissected, sliced, treated with trypsin, mechanically dissociated and centrifuged twice at 40 g, plated in Petri dishes and cultured. Experiments were performed on cells between 10 and 15 days in culture.
Expression of recombinant receptors
The recombinant α1β2γ2S GABAA receptors were expressed in the HEK293 cell line (human embryonic kidney cells). HEK293 cells were cultured in Dulbecco's modified Eagle's medium with Glutamax-I (Gibco BRL) supplemented with 5% fetal bovine serum (Gibco BRL), 100 U ml−1 penicillin G, and 100 μg ml−1 streptomycin in an incubator at 37°C with 5.0% CO2. Transfection was performed using a standard calcium phosphate precipitation protocol (Chen & Okayama, 1987) with plasmids pGW1 ampicillin-resistant containing cDNA encoding for rat α1, β2, γ2S or GABAA receptor. Plasmids were cotransfected at individual concentrations of 1 μg ml−1 together with the pCVMCD4 plasmid to encode the CD4 receptor. Transfected cells were identified by markers (beads) covered with anti-CD4 antibodies (Dynabeads M–450 CD4, Dynal Biotech ASA, Oslo, Norway).
Electrophysiological recordings
Currents were recorded either from neurons or from HEK293 cells in the outside-out mode of the patch-clamp technique using the Axopatch 200B amplifier (Molecular Device Corporation, Sunnyvale, CA, USA) at −70 mV. The intrapipette solution contained: (mm) 137 CsCl, 1 CaCl2, 2 MgCl2, 11 BAPTA (tetracaesium salt), 2 ATP and 10 Hepes, pH 7.2 with CsOH. The external solution was composed of (mm): 137 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 20 glucose and 10 Hepes, pH 7.2 with NaOH. The current signals were filtered at 10 kHz with a Butterworth filter, sampled at 50–100 kHz, using the analog-to-digital converter Digidata 1322A (Molecular Device Corporation) and stored on the computer hard disk. pCLAMP 9.2 (Molecular Device Corporation) software was used for acquisition and data analysis. Miniature synaptic currents were recorded in the whole-cell configuration of the patch clamp technique in the presence of 1 μm tetrodotoxin (TTX). Current responses to 1 μm GABA were barely detectable in the excised patch configuration and for this reason were recorded in the whole-cell mode using a multibarrel system (RSC-200, Bio-Logic, Grenoble, France; exchange time ca 8–15 ms). In most cases, responses to 3 μm GABA obtained from excised patches were too small to be reliably analysed and therefore most of the responses to this GABA concentration were collected in the whole-cell mode as in the case of 1 μm GABA.
Rapid drug application
GABA was applied to excised patches using the ultrafast perfusion system based on a piezoelectric-driven theta-glass application pipette (Jonas, 1995). The piezoelectric translator was from Physik Instrumente (preloaded HVPZT translator 80 μm, Waldbronn, Germany) and theta-glass tubing was from Hilgenberg (Malsfeld, Germany). The open tip recordings of the liquid junction potentials revealed that 10–90% exchange of solution occurred within 50–80 μs. A minimum duration of drug application was 0.8–1 ms (at shorter pulses, often oscillations appeared). In experiments in which the effect of BDZ receptor agonists was examined, the tested modulator was present at the same concentrations in solutions supplied by both channels (wash and GABA-containing solution) of the theta-glass capillary. Before applying the agonist (in the presence or absence of BDZ) the patch was exposed to the washing solution for at least 2 min. Occasionally, in cells with high GABAAR expression, current responses to 3 μm GABA were studied in the excised patches using ultrafast perfusion. The current characteristics such as rise time and deactivation of responses recorded in the whole-cell and excised-patch modes were not clearly different. Due to extreme difficulty of experiments with ultrafast application system we have focused our study on testing the effects of flurazepam and zolpidem at micromolar concentrations that were close to saturation for both drugs (e.g. Tietz et al. 1999; Walters et al. 2000). For responses to low [GABA] (1–3 μm), 1 μm of flurazepam or zolpidem yielded a submaximal effect that reached saturation at 3 μm (zolpidem) or between 3 and 10 μm (flurazepam, data not shown).
Data analysis
The kinetics of the current rising phase was quantified as 10–90% rise time. Deactivation current was fitted with a sum of two exponential functions:
where A1 and A2 are the percentages while τfast and τslow are the time constants. For normalized currents, A1 + A2 = 1. The mean time constant was calculated as τmean =A1τfast + A2τslow. The desensitization kinetics was described by a sum of one exponential function and a constant value representing the steady-state current. Recovery parameter R was defined as R = (I2−I3)/(I1−I3), where I1 the first peak amplitude, I2 the second peak amplitude, and I3 is the current immediately before the second pulse. The effect of flurazepam and zolpidem on IPSCs and on current responses was assessed from the comparison between control and test recordings from the same cell (or excised patch). For this reason all the results are presented as relative values normalized to the respective controls.
The kinetic modelling was performed using the ChannelLab 2.0 software (developed by S. Traynelis for Synaptosoft, Decatour, GA, USA). This software converted the kinetic model into a set of differential equations and solved them numerically assuming, as the initial condition, that at t = 0 (where t is time), no bound or open receptors were present. The solution of such equations predicted the time courses of occupancies of all the states included in the model. The current time course was modelled as the time evolution of the sum of open state occupancies.
Data are expressed as mean ± s.e.m. and Student's t test was used for the comparison of data.
All experiments were performed at room temperature (22–24°C).
Results
Flurazepam and zolpidem enhance amplitude and slow down the decaying phase of mIPSCs
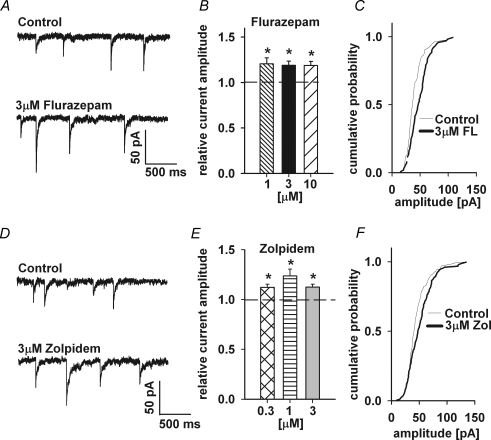
Miniature IPSCs were recorded in the whole-cell configuration at −70 mV in the presence of 1 μm TTX (Fig. 1A). On average, in control conditions, mIPSC amplitude was −41.21 ± 2.22 pA (n = 21). The averaged mIPSC frequency in control conditions was 0.26 ± 0.02 Hz (n = 21) and it was not significantly affected by either flurazepam or zolpidem (data not shown). Addition of flurazepam resulted in a significant increase in the mean mIPSC amplitude for the entire range of considered flurazepam concentrations (1–10 μm, Fig. 1B) and in a clear shift in the cumulative amplitude distribution (Fig. 1C). With 1 μm flurazepam, the relative amplitude was 1.20 ± 0.06 (n = 11). Similarly, zolpidem at concentrations of 0.3–3 μm, significantly enhanced mean mIPSC amplitudes (Fig. 1D and E; at 1 μm zolpidem, the relative amplitude increase was 1.23 ± 0.07, n = 6).
Figure 1.
Flurazepam and zolpidem enhance the amplitude of mIPSCs A, examples of mIPSCs recorded at −70 mV in control conditions (upper trace) and in the presence of 3 μm flurazepam (FL; lower trace). B, statistics of flurazepam effect on mIPSC amplitude. C, typical cumulative histogram for a record in control conditions (thin line) and in the presence of 3 μm flurazepam (thick line). D, examples of mIPSCs recorded at −70 mV in control conditions (upper trace) and in the presence of 3 μm zolpidem (Zol; lower trace). E, statistics of zolpidem effect on mIPSC amplitude. F, typical cumulative histograms for control conditions (thin line) and mIPSCs recordings in the presence of 3 μm zolpidem (thick line). Mean values were calculated from at least n = 5 cells. *Significant difference with respect to the control values.
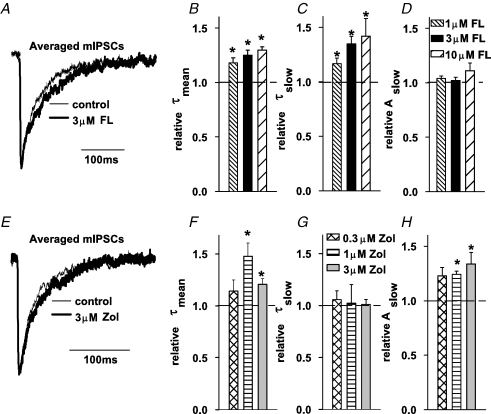
The flurazepam-induced increase in mIPSC amplitude was accompanied by a change in the current kinetics. In control conditions, the decaying phase of mIPSCs could be well fitted with a sum of two exponentials (τfast = 11.83 ± 1.06 ms, τslow = 61.37 ± 3.8 ms, Aslow = 0.46 ± 0.03, n = 11) and the mean decay time constant was τmean = 36.28 ± 1.92 ms. As shown in Fig. 2B, the value of τmean was increased by flurazepam in a dose-dependent manner. This effect was associated with a clear prolongation of the slower decay component (τslow, Fig. 2C) with no apparent effect on its percentage (Aslow, Fig. 2D). Zolpidem significantly prolonged the mean time constant of mIPSC decaying phase at concentrations of 1 and 3 μm (Fig. 2F) and this change was related to an increase in the percentage of the slow component with no effect on the slow time constant (Fig. 2G and H).
Figure 2.
Flurazepam and zolpidem prolong the decaying phase of mIPSCs A, typical averaged and superimposed mIPSCs recorded in control conditions (thin line) and in the presence of 3 μm flurazepam (thick line). B, statistics of the flurazepam effect on the mean decay time constant (τmean). Flurazepam prolongs the slow decay component (τslow, C) without affecting the percentage of this component (Aslow, D). E, typical averaged and superimposed mIPSCs recorded in control conditions (thin line) and in the presence of 3 μm zolpidem (thick line). F, statistics of the zolpidem effect on the mean decay time constant (τmean). Zolpidem does not affect the value of slow decay time constant (τslow, G) but increases the percentage of this component (Aslow, H). Mean values were calculated from at least n = 5 cells. *Significant difference with respect to the control values.
Flurazepam and zolpidem exert opposite effects on amplitudes of currents elicited by low and high [GABA]
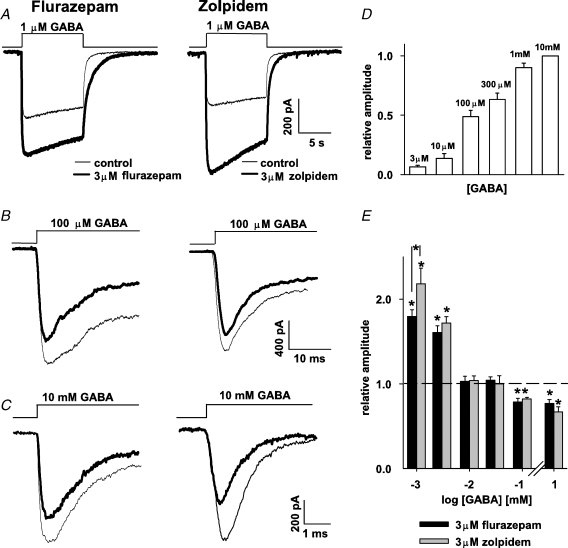
Results presented in Figs 1 and 2 confirmed that in our model both flurazepam and zolpidem enhanced amplitudes and prolonged decay of mIPSCs, although the effects of these drugs showed some differences. To explore the mechanism underlying such a modulation, current responses to rapid GABA applications were measured. Control responses to various GABA concentrations were recorded (examples in Fig. 3A–C thin lines) and a dose–response relationship was constructed from values of current amplitudes normalized to the amplitude of responses elicited by 10 mm GABA measured from the same patch (Fig. 3D, in control conditions, currents elicited by 10 mm GABA had the averaged amplitude of −789 ± 198 pA, n = 17). From these recordings we could estimate that the GABA concentration evoking a half-maximal amplitude was close to 100 μm (Fig. 3D). As expected, current responses to low [GABA] (1–3 μm) were strongly potentiated both by flurazepam and zolpidem but the effect of the latter was significantly larger at 1 μm GABA (Fig. 3A and E). However, when increasing [GABA] to 10 or 30 μm, the current enhancement was no longer present (Fig. 3E) and for 100 μm or 10 mm GABA both BDZ receptor agonists induced a significant current reduction (Fig. 3B, C and E). These data demonstrate that BDZ receptor agonists down modulate the responses of neuronal GABAARs to saturating or half-saturating [GABA], similarly to what was observed for recombinant α1β2γ2 receptors (Mercik et al. 2007).
Figure 3.
Effect of flurazepam and zolpidem on current amplitudes responses strongly depend on GABA concentration Left column shows examples of current responses evoked in control conditions (thin lines) and in the presence of 3 μm flurazepam (thick line) while middle column shows analogous examples for studies on the zolpidem effect. A, examples of currents evoked by 1 μm GABA in control conditions (thin lines), in the presence of 3 μm flurazepam (thick line, left panel) and 3 μm zolpidem (thick line, right panel). B and C, examples of currents elicited by 100 μm and by 10 mm GABA, respectively, in control conditions (thin line) and in the presence of flurazepam (thick line, left) and zolpidem (thick line, right). D, normalized dose–response relationship for current amplitudes in control conditions. Duration of GABA application was sufficient for current to reach the peak. Mean values were calculated from at least n = 4 cells. E, statistics on zolpidem and flurazepam effects on the amplitudes of currents evoked by different GABA concentrations. Mean values were calculated from at least n = 8 cells. Insets above current traces depict the time course of applied agonist. *Significant difference with respect to the control values.
Flurazepam and zolpidem differentially affect the deactivation kinetics of currents elicited by low and high [GABA]
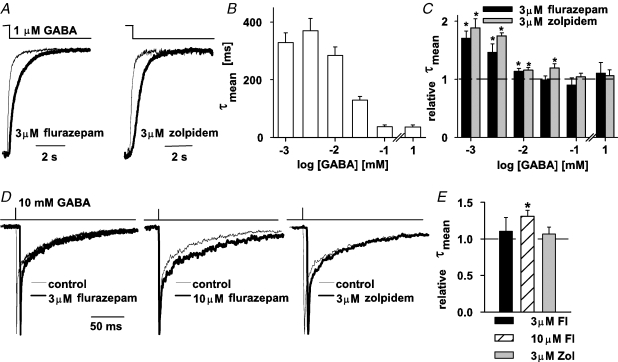
Recordings of synaptic currents confirmed that both flurazepam and zolpidem prolonged the decaying phase of mIPSCs (Fig. 2). Since GABA synaptic transient is very brief (Clements, 1996; Mozrzymas et al. 1999, 2003b; Overstreet et al. 2002; Mozrzymas, 2004) it is believed that mIPSC decay is largely determined by the deactivation process (time relaxation after agonist removal). It is thus interesting to check how flurazepam and zolpidem affect the deactivation kinetics of currents evoked by different GABA concentrations. For this purpose, currents were elicited by GABA pulse whose duration was sufficient for the current to reach its maximum (or plateau). For instance, at 1 μm GABA, a 2 s pulse was applied (Fig. 4A) and in these conditions, in 19 out of 28 cells, the deactivation kinetics was characterized by a biexponential time course (τfast = 191 ± 21 ms, τslow = 880 ± 82 ms, Aslow = 0.24 ± 0.03, n = 19) and in 9 out of 28 cells, a single exponential fit was made (τ = 288 ± 34 ms, n = 9). The mean deactivation time constant (1 μm GABA, 2 s application) was: τmean = 328 ± 28 ms (n = 28). The absolute values of τmean for currents recorded using this experimental protocol showed a tendency to decrease with GABA concentration (Fig. 4B) that, as explained in detail in the next section, appears to be related to an increasing contribution of rapid desensitization at high [GABA]. Interestingly, both BDZ receptor agonists significantly slowed down the deactivation time course (for 2 s application of 1 μm GABA, relative τmean = 1.70 ± 0.12, n = 5, for 3 μm flurazepam; 1.88 ± 0.15, n = 10, for 3 μm zolpidem, P < 0.05, Fig. 4A and C). The prolongation of deactivation kinetics by these drugs resulted mainly from the enhancement of percentage of the slow component (data not shown). However, as shown in Fig. 4C, when increasing [GABA] the effect of BDZ receptor agonists on deactivation kinetics progressively decreased. For flurazepam (3 μm), the prolongation of deactivation was significant for GABA concentrations up to 10 μm and for zolpidem (3 μm) up to 30 μm GABA (Fig. 4C). Since current responses to high [GABA] may provide essential information on receptor gating, it is important to describe in more detail the deactivation process for currents elicited by saturating [GABA] (see also sections below). Decaying phases of currents evoked by short pulses (1–3 ms) of 10 mm GABA were analysed to describe the deactivation kinetics (alteration of application time within 1–3 ms did not affect the time course of current responses to 10 mm GABA). Decay of these currents could be well fitted with a sum of two exponentials (τfast = 2.92 ± 0.87 ms, τslow = 125 ± 11.40 ms, Aslow = 0.26 ± 0.03, n = 20) and the weighted average deactivation time constant was τmean = 36.13 ± 5.91 ms (n = 20). Flurazepam, at concentration up to 3 μm, had no significant effect on deactivation kinetics (Fig. 4D and E). Interestingly, at 10 μm concentration, this BDZ significantly prolonged the deactivation kinetics (relative τmean = 1.31 ± 0.08, n = 8, P < 0.05, Fig. 4D, E) and this effect was related to a prolongation of the slow deactivation component (relative τslow = 1.30 ± 0.10, P < 0.05; relative τfast = 1.18 ± 0.10, P > 0.05; and relative Aslow = 0.81 ± 0.24, n = 8, P > 0.05). However, zolpidem (at concentrations up to 3 μm) had no significant effect on the deactivation kinetics of currents evoked by 10 mm GABA (Fig. 4D and E).
Figure 4.
Flurazepam and zolpidem affect the deactivation kinetics A, examples of normalized and superimposed traces evoked by 1 μm GABA (2 s application) in control conditions (thin lines) and in the presence of 3 μm flurazepam (thick line, left) or 3 μm zolpidem (thick line, right). B, dependence of mean deactivation time constant (τmean) on GABA concentration. Mean values were calculated from at least n = 5 cells. C, relative mean deactivation time constants in the presence of 3 μm flurazepam (black bars) and 3 μm zolpidem (grey bars) versus GABA concentration. Mean values were calculated from at least n = 5 cells. Note that a significant effect of both BDZ receptor agonists is observed only for GABA concentrations considerably below the EC50 value. D, examples of normalized responses elicited by short applications of saturating GABA (10 mm for 3 ms) in control conditions (thin line) and in the presence of flurazepam (thick lines, left and middle for 3 and 10 μm, respectively) and 3 μm zolpidem (thick line, right). Since at 3 μm flurazepam and 3 μm zolpidem there was no visible effect on the deactivation time course, the control traces were shifted to the left with respect to those recorded in the presence of flurazepam and zolpidem. E, statistics of the flurazepam and zolpidem effect on mean deactivation time constant (τmean). Insets above current traces depict the time course of the applied agonist. Mean values were calculated from at least n = 6 cells. *Significant difference with respect to the control values.
Notably, the deactivation kinetics observed following the application of 1 μm GABA (τmean = 328 ms, Fig. 4A and B) had a much slower time course than that for short and saturating GABA pulses (τmean = 36.13 ms, Fig. 4B) and also had a dramatically stronger sensitivity to flurazepam (compare Fig. 4C and E). Moreover, while deactivation of currents elicited by 1–3 ms pulse of 10 mm GABA was not affected by zolpidem, deactivation following a long pulse of 1 μm GABA was strongly prolonged by this drug (compare Fig. 4A and C with D and E). These results indicate that both flurazepam and zolpidem might be involved in modulation of deactivation kinetics but their effects were critically dependent on conditions in which the receptors were activated. In particular, it seems important to elucidate how the effects of flurazepam and zolpidem depend on the time duration of the GABA pulse and on the extent of receptor desensitization.
Flurazepam and zolpidem affect the deactivation–desensitization coupling differently
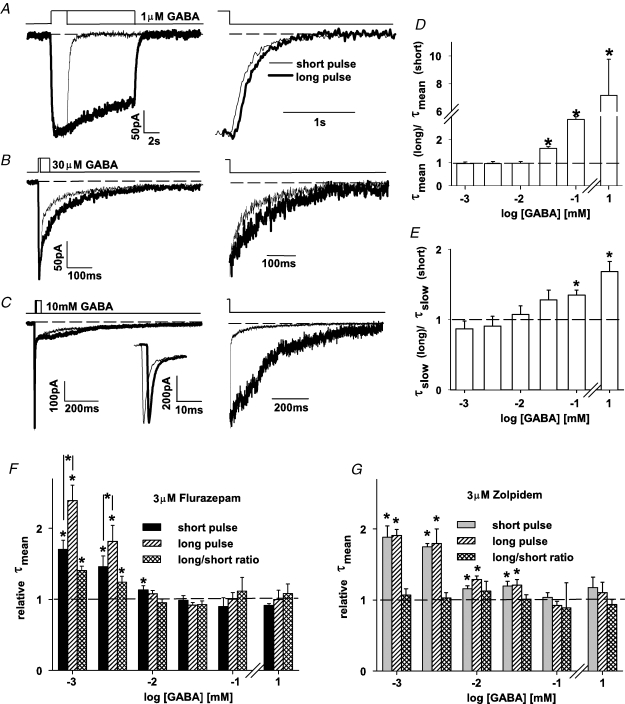
It is known that prolonged exposure of GABAARs to free agonist may result in a favoured entry into the desensitized state and therefore the time of agonist application may affect the deactivation kinetics (Jones & Westbrook, 1995). Taking into account this prediction, we have checked how time of agonist application affected deactivation kinetics of currents evoked by application at different [GABA] and how the ensuing deactivation–desensitization coupling is affected by flurazepam and zolpidem. To assess the deactivation–desensitization coupling at a given GABA concentration, currents were elicited by a GABA pulse of sufficient duration to reach the peak or a plateau value (a so called ‘short’ pulse; pulse durations are described in the legend of Fig. 5A–C) and then by a pulse at least five times longer (‘long’ pulses, Fig. 5A–C). The averaged deactivation time constants were measured for responses evoked by ‘long’ (τmean(long)) and ‘short’ pulses (τmean(short)) and the ratio of these time constants is shown in Fig. 5D. Interestingly, up to 10 μm GABA, the prolongation of agonist pulse did not affect the deactivation kinetics (Fig. 5D). On the contrary, starting from a GABA concentration of 30 μm, the larger [GABA] was, the larger the impact of time duration of the agonist pulse on the deactivation time course was (Fig. 5D). At high [GABA], a prolongation of the agonist pulse resulted in a reduction (or even disappearance) of the fast deactivation component (Fig. 5C) giving rise to a substantial increase in τmean (Fig. 5D). Indeed, while following 1 ms pulse of 10 mm GABA, there was a predominant fast component of ∼2.6 ms (see section above and Fig. 5C); after a longer pulse (10–30 ms), such a fast component was absent (Fig. 5C, right panel). Interestingly, the rapid decay component in currents evoked by 1 ms (10 mm GABA) and during a long pulse of the same [GABA] had indistinguishable fast decay components (see inset on the expanded time scale in Fig. 5C) indicating that the apparent fast deactivation is due to a rapid desensitization process. Indeed, as presented in details in the next section, the value of the fast desensitization time constant was not significantly different (P > 0.05) from the fast deactivation component. The view that the fast deactivation component is due to rapid desensitization is further supported by the fact that the recovery in the paired-pulse experiments with a short gap (two 1 ms pulses of 10 mm GABA separated by a 5 ms gap) yielded a recovery at the limit of detection level (below 5%, data not shown). Moreover, as already mentioned, the time courses of current responses elicited by 10 mm GABA pulses of duration between 1 and 3 ms were indistinguishable (data not shown) which is consistent with the mechanism in which even a very short pulse (e.g. 1 ms) of saturating [GABA] is sufficient to induce a profound desensitization that determines the fast component of apparent deactivation. However, the prolongation of deactivation process (at high [GABA]) by increasing the pulse duration was not only due to reduction or disappearance of the rapid component. For half-saturating or saturating [GABA], the slow deactivation components were significantly longer than the ones following the short pulses (Fig. 5E). Thus, at high [GABA], rapid desensitization appears to play a dual role in shaping the deactivation time course: (i) it largely determines the fast component of apparent deactivation and (ii) sojourns into the desensitized states prolong the slow component of deactivation process (Jones & Westbrook. 1995).
Figure 5.
Flurazepam and zolpidem differentially affect the deactivation–desensitization coupling A, B and C, examples of current traces evoked by 1 μm, 30 μm and 10 mm GABA, respectively, applied for short (thin line) and long time (thick line). The following durations of agonist applications were used at different [GABA]: 1 μm, 2 s; 3 μm, 1 s; 10 μm, 50 ms; 30 μm, 10 ms; 100 μm, 5 ms; 10 mm, 1 ms and the respective long applications were five times longer (see text for explanation of how short and long application times were defined). Right panels in A, B and C show the same current traces but normalized to the current value at the end of GABA pulse. In C, the inset shows the rapid phases of current responses on an expanded time scale. Note that at high [GABA] the deactivation following brief GABA pulse is much faster than that after a long application (mainly due to a predominant rapid deactivation component in the former one). D, the ratio of τmean values measured for deactivation currents following long and brief GABA applications, respectively, versus GABA concentration. Note that pulse duration affects τmean only for GABA concentrations equal or above 30 μm at which rapid desensitization becomes prominent. E, ratio of time constants of slow deactivation components (τslow) measured following long and brief GABA applications, respectively, versus[GABA]. In D and E, mean values were calculated from at least n = 8 cells. F, relative τmean for deactivation currents in the presence of 3 μm of flurazepam (with respect to control conditions) for short pulses (black bars) and for long pulses (middle hatched bars). Cross-hatched bars show the ratios of relative τmean values measured for long and short pulses, respectively. G, analogous results as presented in F but obtained for 3 μm of zolpidem. In F and G, mean values were calculated from at least n = 5 cells. Note that at low [GABA] flurazepam enhances the deactivation–desensitization coupling and zolpidem does not. Insets above current traces depict the time course of applied agonist. *Significant difference with respect to the control values.
We next checked how flurazepam and zolpidem affected the deactivation–desensitization coupling described above. As shown in Fig. 5F, flurazepam exerted a significantly stronger action on deactivation kinetics for currents evoked by long agonist pulses but this effect was present only at low [GABA] (1 and 3 μm). On the contrary, zolpidem did not affect the deactivation–desensitization coupling over the entire considered range of [GABA] (Fig. 5G).
Effect of flurazepam and zolpidem on kinetics of current responses elicited by saturating [GABA]
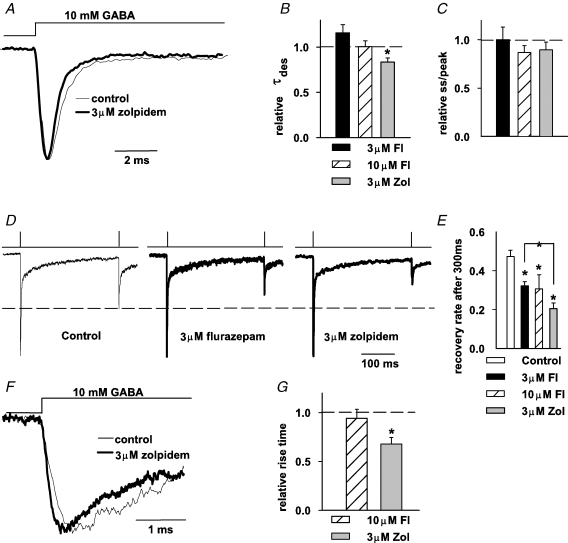
At saturating GABA concentrations, the binding step is expected to occur much faster than the conformational transitions between bound states. For this reason, the time course of current responses to saturating [GABA] is largely determined by gating properties of GABAAR. Thus, in order to assess the impact of flurazepam and zolpidem on GABAAR gating, a series of application protocols for saturating [GABA] was employed. The effect of flurazepam and zolpidem on deactivation kinetics of currents evoked by short pulses (1–3 ms) of 10 mm GABA was presented in previous sections (Figs 4 and 5). As already mentioned, the deactivation time course of GABAergic currents has been found to strongly depend on the desensitization kinetics (Jones & Westbrook, 1995) and therefore it is important to examine the effects of these BDZ receptor agonists on the desensitization time course. To this end, current responses were evoked by prolonged (30–100 ms) applications of saturating [GABA] (Fig. 6A). In control conditions, the desensitization time constant (τdes) was 2.05 ± 0.19 ms (n = 26) and the steady-state to peak (ss/peak) was 0.18 ± 0.023 (n = 26). Flurazepam at concentrations of 1–10 μm had no significant effect either on τdes or on the steady-state to peak ratio (Fig. 6B and C). In contrast, zolpidem was found to significantly increase the rate but not the extent of desensitization (at 3 μm zolpidem relative τdes: 0.83 ± 0.04, P < 0.05, and relative ss/peak: 0.89 ± 0.07, n = 10, P > 0.05, Fig. 6B and C). This result appears surprising as flurazepam (at 10 μm) prolonged current deactivation while zolpidem did not.
Figure 6.
Flurazepam and zolpidem affect the time course of responses elicited by saturating [GABA] A, normalized and superimposed current traces evoked by prolonged application of saturating (10 mm) [GABA] in control conditions (thin line) and in the presence of 3 μm zolpidem (thick line). B and C, statistics of flurazepam and zolpidem effects on the time constant of desensitization onset (τdes, B) and on the steady-state to peak parameter (ss/peak, C). In B and C, mean values were calculated from at least n = 6 cells. D, examples of normalized currents recorded in the paired-pulse experiments (3 ms applications of 10 mm GABA separated by 300 ms time interval) in control conditions (thin line, left) and in the presence of 3 μm flurazepam (thick like, middle) and 3 μm zolpidem (thick line, right). Dashed line indicates the amplitude of response to the second pulse in control conditions. E, statistics of flurazepam and zolpidem effects on the recovery parameter R (see Methods). Mean values were calculated from at least n = 6 cells. F, normalized and superimposed current traces evoked by saturating GABA in control conditions (thin line) and in the presence of 3 μm zolpidem (thick line). G, statistics of the flurazepam and zolpidem effects on 10–90% rise time. Mean values were calculated from at least n = 4 cells. Insets above current traces depict the time course of applied agonist. *Significant difference with respect to the control values.
It is known that GABAARs tend to strongly accumulate in the desensitized state even after a brief exposure to high [GABA] (Jones & Westbrook, 1995; see also section on deactivation–desensitization coupling). This phenomenon can be visualized in the paired-pulse experiments (Fig. 6D and E). The fact that the amplitude of response to the second pulse is smaller than that for the first GABA application, indicates that a considerable proportion of GABAARs accumulated in the desensitized state. As shown in Fig. 6D and E, both flurazepam and zolpidem significantly reduced the recovery parameter R (see Methods) but the effect of zolpidem was clearly stronger (P < 0.05). The reduction of recovery by these BDZ receptor agonists might be due to an increase in desensitization rate and/or the reduction of the resensitization rate constants but it cannot be excluded that other parameters (such as, for example, unbinding rate) could also be involved.
In control conditions, the 10–90% rise time of current responses evoked by rapid application of 10 mm GABA was 0.23 ± 0.01 ms (n = 16). Flurazepam at a concentration up to 10 μm did not significantly affect the current onset rate (Fig. 6G). In contrast, zolpidem at a concentration of 3 μm significantly accelerated the 10–90% rise time (relative 10–90% rise time 0.66 ± 0.06, n = 6, P < 0.05, Fig. 6F and G).
Altogether, recordings of currents elicited by various protocols of saturating [GABA] applications revealed that both flurazepam and zolpidem affected the time course of recorded responses although their effects showed qualitative differences.
Effect of BDZ receptor agonists on current responses to brief applications of half-saturating [GABA]
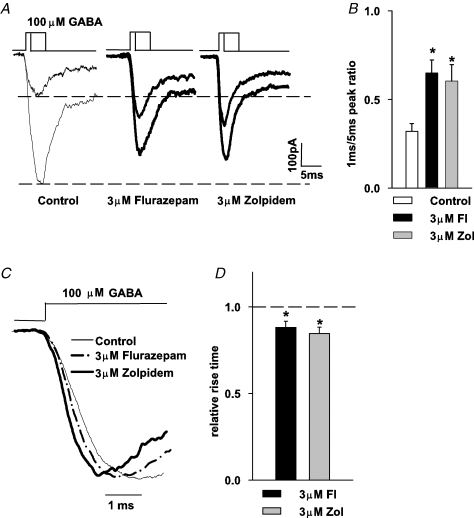
The results presented above show that both mIPSCs and current responses elicited by exogenous GABA applications are potently modulated by BDZ receptor agonists but a correlation between these effects is not straightforward. While these compounds markedly decreased the amplitude of current responses to 100 μm and 10 mm GABA (Fig. 3), the same concentrations of these drugs clearly increased the mIPSC amplitudes (Fig. 1). This observation is particularly puzzling because the peak of synaptic GABA transient is commonly believed to reach millimolar concentrations (Clements, 1996; Mozrzymas et al. 1999, 2003b; Overstreet et al. 2002; Mozrzymas, 2004). Moreover, while both flurazepam and zolpidem prolonged mIPSC decaying phase (Fig. 2), a moderate effect on deactivation of current responses was observed only for 10 μm flurazepam (Fig. 4). There are at least two possible explanations for these differences: (i) activation of synaptic receptors is evoked by a GABA transient that is considerably shorter than GABA pulses applied using our application system, and (ii) synaptic GABAARs are endowed with qualitatively different properties with respect to the extrasynaptic ones that are likely to be abundantly present in the excised patches (or whole cells). In the present section, we shall consider the first possibility while the second one is discussed in the next one. As mentioned in Methods, the shortest pulse duration that can be reliably applied with our perfusion system was ∼0.8–1 ms. Thus, the major problem with experimental modelling of synaptic transient is that it may be up to one order of magnitude shorter than 1 ms (Mozrzymas et al. 1999, 2003b; Overstreet et al. 2002; Mozrzymas, 2004). Most importantly, such extremely short duration of the agonist pulse makes a relatively high (millimolar) synaptic GABA concentration considerably distant from saturation. In practice, the amplitude of the postsynaptic current response to a synaptic agonist pulse is better correlated with the product of the peak concentration and time duration of neurotransmitter presence in the cleft rather than with the peak agonist concentration alone (see, for example, Mozrzymas, 2004; Barberis et al. 2004). Thus, as an attempt to qualitatively reproduce the synaptic conditions, we have applied the agonist for 1 ms while the concentration of GABA was set at 100 μm. In this way, the product of peak concentration and application time is similar to that estimated for synaptic transient (Overstreet et al. 2002; Mozrzymas et al. 2003b; Mozrzymas, 2004) while peak agonist concentration and time exposure were proportionally rescaled. It is expected that current responses to 1 ms application of 100 μm GABA would result in the activation of only a portion of receptors available in the patch because during such a short exposure to non-saturating [GABA], only a fraction of receptors would complete the binding step. To test this prediction, the amplitude of response to 1 ms application of 100 μm GABA was compared with that measured for 5 ms exposure to the same GABA concentration (Fig. 7A and B). The application of 100 μm GABA for 5 ms is sufficient for current to reach its maximum value (Fig. 7A, see also Mozrzymas et al. 2003a). On average, in control conditions, the ratio of peaks evoked by 1 and 5 ms was 0.31 ± 0.04 (n = 6, Fig. 7A and B) indicating that in this case the key mechanism of non-saturation is to ‘trim’ the receptor activation by agonist removal after a short exposure. As shown in Fig. 7A and B, the peak ratios (measured for 1 and 5 ms GABA applications) were clearly increased by both flurazepam and zolpidem (0.64 ± 0.07, n = 6 and 0.60 ± 0.04, n = 7, for 3 μm flurazepam and 3 μm zolpidem, respectively, P < 0.05). Interestingly, while both BDZ receptor agonists decreased the amplitudes of currents evoked by 5 ms application of 100 μm GABA (Fig. 7A and B, see also Fig. 3C and D), the amplitudes of currents evoked by 1 ms application of the same GABA concentrations were enhanced by flurazepam and zolpidem (Fig. 7A, see dashed line). This result shows that in conditions similar to those that presumably take place in the synapse, BDZ receptor agonists may potentiate the responses elicited by the same GABA concentration for which currents evoked by longer applications are down-regulated by these drugs (compare Figs 3 and 7).
Figure 7.
Timing of responses to non-saturating [GABA] determines the impact of BDZ receptor agonist on current amplitudes A, examples of superimposed currents evoked by 100 μm GABA applied for 1 and 5 ms in control conditions (thin lines, left) and in the presence of 3 μm flurazepam (thick line, middle) and 3 μm zolpidem (thick line, right). B, statistics of flurazepam and zolpidem effects on amplitude ratio (amplitude of current evoked by 1 ms pulse/amplitude of current evoked by 5 ms pulse). Mean values were calculated from at least n = 6 cells. C, examples of normalized current responses evoked by 100 μm GABA in control conditions (thin line) and in the presence of 3 μm flurazepam (thick dash–dotted line) or 3 μm zolpidem (thick line). D, statistics of flurazepam and zolpidem effects on the 10–90% rise time of currents evoked by 100 μm GABA. Mean values were calculated from at least n = 4 cells. Insets above current traces depict the time course of applied agonist. *Significant difference with respect to the control values.
The observed increase in peak ratios for responses to 1 and 5 ms of 100 μm GABA in the presence of flurazepam and zolpidem (Fig. 7) appears compatible with the increase in the binding rate by these drugs. Effective rate of agonist binding is assumed to be proportional to the agonist concentration (∼kon[GABA], where kon is the binding rate) and at a non-saturating [GABA] (100 μm GABA is far from saturating the onset rate, Mozrzymas et al. 2003a; Fig. 3D) the current onset is expected to depend on the binding rate. We therefore analysed the onset kinetics for responses evoked by 5 ms application of 100 μm GABA and found that in control conditions, the 10–90% rise time was 1.82 ± 0.06 ms (n = 9). The onset kinetics of these currents was significantly accelerated both by flurazepam and zolpidem (relative 10–90% rise time 0.88 ± 0.07, n = 5, and 0.85 ± 0.03, n = 4, for 3 μm flurazepam and 3 μm zolpidem, respectively, P < 0.05, Fig. 7C and D).
Responses of recombinant (‘synaptic’) α1β2γ2 receptors to brief applications of non-saturating [GABA] show similar zolpidem sensitivity as mIPSCs
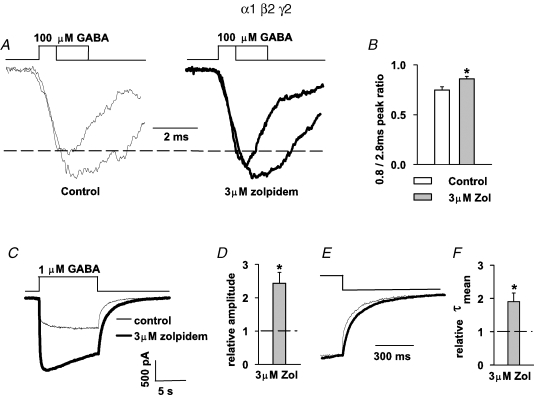
As already mentioned, mIPSCs and current responses to brief GABA applications may differ in their BDZ sensitivity because excised patches might contain a considerable proportion of extrasynaptic receptors characterized by different kinetics and pharmacology with respect to synaptic channels (e.g. Farrant & Nusser, 2005). A functional indication for such a difference is the fact that the time course of synaptic currents clearly differs from that observed for current responses to brief agonist applications (e.g. Jones & Westbrook, 1995; Mozrzymas et al. 1999, 2003b). In order to test whether different BDZ action on mIPSC and on current responses might result from differences between synaptic and extrasynaptic receptors, we have repeated key protocols for currents mediated by recombinant α1β2γ2 receptors. This receptor subtype is abundantly expressed in the CNS (Whiting, 2003) and, most importantly, in several brain regions is predominant in GABAergic synapses. As already reported in our previous study (Mercik et al. 2007), both flurazepam and zolpidem reduced the amplitudes of current responses to saturating [GABA] by 15 and 31%, respectively, which is close to what we report now for neurons (Fig. 3). In the context of the BDZ effect on synaptic currents, it is particularly interesting to repeat the protocol depicted in Fig. 7 (brief and long application of 100 μm GABA). As shown in Fig. 8A and B, the presence of 3 μm zolpidem significantly increased the peak ratio, similarly to the case of responses mediated by neuronal receptors (Fig. 7). The only difference between responses mediated by neuronal and recombinant receptors was that the activation of the latter by application of 100 μm GABA, yielded currents characterized by a faster onset rate (10–90% rise time for α1β2γ2-mediated currents was 1.33 ± 0.13 ms, n = 4) and for this reason the short application in the protocol in Fig. 8A was reduced to 0.8 ms.
Figure 8.
Effects of BDZ receptor agonists on neuronal GABAA receptors are reproduced for recombinant α1β2γ2 receptors A, examples of normalized and superimposed currents evoked by 100 μm GABA applied for 0.8 and 2.8 ms in control conditions (thin lines, left) and in the presence of 3 μm zolpidem (thick line, right). B, statistics of the zolpidem effect on amplitude ratio (amplitude of current evoked by 0.8 ms pulse/amplitude of current evoked by 2.8 ms pulse). Mean values were calculated from at least n = 6 cells. C, examples of currents evoked by 1 μm GABA in control conditions (thin line) and in the presence of 3 μm zolpidem (thick line). D, statistics of zolpidem effect on amplitude of currents evoked by 1 μm GABA. Mean value was calculated from n = 7 cells. E, normalized and superimposed traces presented in C. A prolongation of deactivation by zolpidem (thick line) is clearly seen. F, statistics of zolpidem effects on the mean deactivation time constant (τmean). Mean value was calculated from n = 4 cells. Insets above current traces depict the time course of an applied agonist. *Significant difference with respect to the control values.
It is known that the kinetic and pharmacological properties of synaptic (e.g. α1β2γ2) and of extrasynaptic GABAARs can be substantially different. While synaptic GABAARs respond to rapid (phasic) pulses of agonist, extrasynaptic ones experience prolonged exposures to ambient [GABA] in a submicromollar range (e.g. Farrant & Nusser, 2005). It is thus interesting to confront the strong BDZ sensitivity of deactivation process in current responses to low [GABA] recorded from neurons to that measured from HEK cells expressing α1β2γ2 receptors. To this end we have investigated the zolpidem (α1-preferring BDZ receptor agonist) effect on current responses mediated by α1β2γ2 receptors and elicited by 1 μm GABA. As shown in Fig. 8C and D, zolpidem strongly enhanced the current amplitude (relative amplitude 2.42 ± 0.33, n = 7) and this effect was similar to that observed in neurons (Fig. 4A and C). Moreover, the mean deactivation time constant (τmean) was clearly prolonged by zolpidem (relative τmean = 1.90 ± 0.25, n = 4, Fig. 8E and F), nicely reproducing our observations in neurons (Fig. 4A and C). These experiments indicate that in the considered model of cultured hippocampal neurons, the BDZ sensitivity of currents elicited by exogenous GABA, qualitatively follows the pattern observed for responses mediated by recombinant α1β2γ2 receptors.
Model simulations
The effects of flurazepam and zolpidem on mIPSCs and on current responses to exogenous GABA applications suggest that these BDZ receptor agonists modulate both binding and gating of GABAAR. A robust enhancement of responses to 1–3 μm GABA (Fig. 3) confirms that BDZs strongly up regulate the agonist binding. It might be suggested that a similar effect could also be reproduced by increasing the receptor efficacy (Rusch & Forman, 2005; Downing et al. 2005; Campo-Soria et al. 2006). However, it would lead to an increase in amplitude at saturating [GABA], contrary to our experimental findings (Fig. 3, see also Discussion).
Both flurazepam and zolpidem clearly modulated the time course of current responses elicited by saturating [GABA] indicating that they affected gating properties of neuronal GABAARs. The reduction of amplitudes of responses evoked by saturating and half-saturating [GABA] (Fig. 3) suggests an increased occupancy of desensitized state(s). Enhancement of the desensitization time constant by zolpidem (Fig. 6A and B) together with a slower recovery observed for both compounds (Fig. 6D and E) further suggest this possibility. It is worth noting that strong reduction of the recovery by BDZ receptor agonists (Fig. 6E) could result in altered frequency dependence of IPSCs (Mellor & Randall, 1997). In our previous report (Mozrzymas et al. 2003b) we have proposed that modulation of desensitization may offer a potent mechanism of an amplitude regulation of responses evoked by saturating [GABA]. Clearly, interpretation of our macroscopic data based on such a mechanism requires a widely accepted notion that single channel conductance is not affected by these compounds (Twyman et al. 1989; Vicini et al. 1987; Rogers et al. 1994; Perrais & Ropert, 1999; but see Eghbali et al. 1997). A qualitatively similar enhancement of desensitization to that induced by zolpidem (Fig. 6A and B) was previously reported for other BDZs also by Lavoie & Twyman (1996) and Mellor & Randall (1997). The recovery process was slowed down by both flurazepam and zolpidem (Fig. 6D and E) and this effect could potentially involve enhanced desensitization rate, slower resensitization and/or a decrease in the unbinding rate. The enhancement of the onset rate for currents elicited by saturating [GABA] in the presence of zolpidem (Fig. 6) could suggest an increase in transition rate from closed to open bound states. However, as mentioned above, such an effect would result in enhancement of responses to saturating [GABA], contrary to our data. An alternative possibility could be that the acceleration of current onset was due to increased desensitization rate (Mozrzymas et al. 2003a). We suggest that in the case of zolpidem, a concomitant observation of accelerated desensitization onset, a decrease in current amplitude for responses to saturating [GABA], acceleration of current onset and reduced recovery in the paired-pulse experiments (Fig. 6) indicate that this BDZ receptor agonist does affect the desensitization kinetics. Although the impact of flurazepam was weaker, it is tempting to assume that the major effects of both drugs (a decrease in amplitude of current response to saturating [GABA] and slow down in recovery) resulted from increased occupancy in desensitized conformation(s). However, the present data are not sufficient to precisely indicate, for example, which of potentially several desensitized states are affected by the considered BDZ receptor agonists. Moreover, it needs to be born in mind that alteration of any current characteristics may potentially result from a change in any rate constant in the considered gating scheme because all receptor conformations are functionally coupled (Colquhoun, 1998; Mozrzymas et al. 2003a). Nevertheless, we made an attempt to use model simulations to interpret, at a qualitative level, our major findings. The main goal was to consider the impact of specific non-equilibrium conditions of synaptic receptor activation on the susceptibility of mIPSCs to modulation by BDZ agonists. Moreover, using this approach we tried to evaluate to what extent differences in BDZ modulation of mIPSCs and of current responses result from different conditions in which these currents are activated.
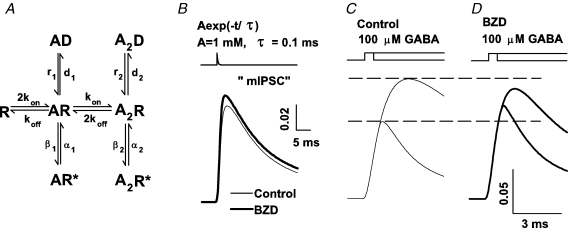
Taking into account the available experimental evidence, our strategy was to propose a minimum requirement for BDZ effects by adapting a previously published model. For this purpose we have used the model proposed in Mozrzymas et al. (2003b) (Fig. 9A) in which we have slightly increased the binding rate in control conditions because in the present study the currents elicited by 100 μm yielded currents with faster onset. The major simplifying assumption of this model is that it postulates only one set of fully bound states (open, closed, desensitized). In particular, it is known that besides the fast desensitized state included in the model, there could be several slower desensitized components which might additionally shape the current responses, especially those elicited by long GABA pulses. The major effect of BDZ was to increase (by nearly 80%, Fig. 3) the currents evoked by 1 μm GABA. Such a BDZ effect can be easily reproduced by increasing the binding rate kon from 8.0 to 12.0 ms−1. At saturating (10 mm) and at 100 μm[GABA], both BDZ receptor agonists considered here reduced the current amplitude (Fig. 3) indicating that, as mentioned above, both flurazepam and zolpidem favour the occupancy of the desensitized state. As an attempt to model this possibility, we assumed that BDZ increases the rate constant of entrance into the doubly bound desensitized state (d2 from 12 to 17 ms−1). The fact that BDZs do not affect opening/closing rates (Twyman et al. 1989; Rogers et al. 1994) provides additional, although indirect, evidence that the observed BDZs effects are due to their action on the desensitized state. It might be argued that a substantial modification of desensitization kinetics could affect the burst durations, the effect that has not been observed by Twyman et al. (1989) and Rogers et al. (1994). However, the impact of desensitization on the burst durations is expected to be best manifested at high agonist concentrations while single channel analysis in these studies was performed for low [GABA]. Thus, the effect of BDZs on burst durations at high [GABA] remains to be elucidated. The fact that the model considered in the present report is oversimplified leads to some predictions that were not supported by the experiment (e.g. an increase in d2 would lead to prolongation of deactivation kinetics that was not consistently confirmed in the experiment). The reason for this discrepancy is not clear. It may be speculated that real GABAARs are endowed with several desensitized conformations characterized by distinct kinetics and BDZs could affect a desensitized conformation with a slower kinetics, whose impact on deactivation would be minor.
Figure 9.
Increase in binding and desensitization rates qualitatively reproduces BDZ effects in model simulations A, the frame of the model in Jones & Westbrook (1995). The rate constants were adapted from Mozrzymas et al. (2003b) with increased kon value as explained in text. Simulations of currents in control conditions were made with the following rate constants: kon = 8.0 ms−1 mm−1, koff = 1 ms−1, d2 = 12 ms−1, r2 = 0.07 ms−1, β2 = 3.0 ms−1, α2 = 0.4 ms−1, d1 = 0.045 ms−1, r1 = 0.014 ms−1; β1 = 0.15 ms−1, α1 = 1.5 ms−1. The effect of BDZ was modelled by increasing kon to 12.0 ms−1 mm−1 and d2 to 17 ms−1. Currents are simulated as a sum of open states occupancies. B, simulated synaptic currents in control conditions (thin line) and in the presence of BDZ (thick line). Synaptic GABA transient was modelled as exponentially decaying function: A exp(−t/τ) where A = 1.0 mm and τ = 0.1 ms. C and D show simulations of currents elicited by short (1 ms) and longer (5 ms) applications of 100 μm GABA in control conditions (thin lines, left) and in the presence of BDZ (thick lines, right). Note that response to short application is enhanced by BDZ while that elicited by longer GABA pulses was reduced. Insets above simulated traces described considered GABA application protocol.
Synaptic currents were simulated as responses to the exponentially decaying agonist waveform
where A = 1.0 mm and τtransient = 0.1 ms). As shown in Fig. 9B, modelling of BDZ action by increasing kon and d2 was sufficient to reproduce the increase in mIPSC amplitude. Using the same model, we have simulated the responses to 1 and 5 ms of 100 μm GABA. As shown in Fig. 9C, the above-mentioned assumptions were sufficient to qualitatively reproduce our finding that the peak ratio for currents evoked by 1 and 5 ms is clearly increased by BDZ (Fig. 7A and B). Moreover, while the absolute value of the response to 1 ms is increased by BDZ, the response to 5 ms is down regulated by this modulator (Fig. 9C and D). Thus, this simulation further indicates that there is no qualitative contradiction between observed enhancement of mIPSC by BDZ (Fig. 1) and a down-regulation of response to saturating or half-saturating [GABA] by this drug (Fig. 3).
Discussion
The major finding of the present work is that the non-equilibrium conditions of synaptic GABAAR activation, dictated by a very brief synaptic agonist transient (Clements, 1996; Mozrzymas et al. 1999, 2003b; Overstreet et al. 2002; Mozrzymas, 2004), have a crucial impact on IPSC sensitivity to BDZ receptor agonists. It was puzzling that mIPSCs were enhanced by flurazepam and zolpidem while responses evoked by saturating or half-saturating [GABA] were down-regulated by these drugs (Figs 1 and 3). This discrepancy could not result from different GABAAR subtypes in synapses and in excised patches (or whole cells) because a similar BDZ effect was observed for α1β2γ2 receptors (Fig. 8; Mercik et al. 2007) that are abundantly present in GABAergic synapses (e.g. Farrant & Nusser, 2005). Moreover, our experimental data (Figs 7 and 8) and model simulations (Fig. 9) show that for non-saturating [GABA], appropriate timing of GABA applications is sufficient to reproduce such apparently opposite BDZ effects. This reflects a general rule that a very brief agonist transient renders the IPSCs particularly sensitive to modulators affecting GABA binding and the larger the distance from saturation, the larger the impact of such modifiers (Mozrzymas et al. 1999, 2003b; Mozrzymas, 2004).
As explained above, the most parsimonious mechanism for observed effects of flurazepam and zolpidem is an up-regulation of binding and desensitization. Although modulation of GABAAR gating by both BDZ receptor agonists considered here appears most compatible with enhancement of desensitization, there were qualitative differences in modulation of gating by flurazepam and zolpidem. The impact of these drugs on deactivation–desensitization coupling (Fig. 5) and on desensitization (Fig. 6) was clearly different. Our data are insufficient to ascribe these differences to, for example, differential modulation of distinct desensitized conformations. Moreover, we cannot exclude that these compounds additionally affected conformational transitions other than desensitization (e.g. open/closed) but for reasons presented in ‘Model simulations’ we believe that their impact is minor. The mechanism proposed here differs from that recently suggested by Rusch & Forman (2005) and Downing et al. (2005), who postulated enhancement in efficacy by BDZs in a spontaneously active GABAAR mutant. However, mutations rendering the receptors spontaneously active could affect also their sensitivity to BDZs. More recently, however, Campo-Soria et al. (2006) have observed that in oocytes expressing ultrahigh levels of wild type GABAARs, diazepam induced a detectable current that was considered as further evidence for the enhancement of GABAAR efficacy by diazepam. Although our data are not sufficient to discriminate between these mechanisms, there are some points that are worth pointing out. A substantial enhancement of receptor efficacy by BDZs would be expected to result in a modification of open and/or closed time distributions that has not been observed in classical single channel studies (Twyman et al. 1989; Rogers et al. 1994). Since the peak of response depends on the occupancy balance of closed, open and desensitized states, it is expected that the BDZs-induced increase in efficacy would increase the amplitude of current evoked by saturating [GABA]. However, our experiments show the opposite (Fig. 3). While there is a general agreement that modulation of receptor desensitization may critically shape the time course of GABAergic currents, the impact of this conformation has not been considered in the papers suggesting BDZ-induced enhancement of efficacy. Finally, we have made an attempt to record currents evoked by up to 10 μm flurazepam or to 3 μm zolpidem but no detectable currents were observed (in some cultures GABAAR expression was quite high: whole-cell responses to 1 μm GABA were above 1 nA at −50 mV with current resolution of ∼5–10 pA). It is thus possible that diazepam might act differently than flurazepam or zolpidem. Taking altogether, in our view, the works discussed above (Rusch & Forman, 2005; Downing et al. 2005; Campo-Soria et al. 2006) raise an interesting possibility that BDZs might affect the receptor efficacy but do not exclude their effect on binding or desensitization and the precise contributions of these mechanisms remain to be assessed.
It is surprising that both flurazepam and zolpidem strongly slowed down the deactivation of currents evoked by low (1–30 μm) GABA (Fig. 4) while for responses to higher [GABA] this effect was not present (except for a subtle effect at 10 μm flurazepam). Importantly, this pattern was observed both for neuronal GABAARs and recombinant α1β2γ2 receptors (Figs 3 and 8). It is still more puzzling that the BDZ receptor agonists considered here prolonged mIPSCs (Fig. 2), while deactivation of currents elicited by 100 μm and higher [GABA] was not affected. Although we have no definite explanation for these discrepancies, we would like to propose a mechanism that appears plausible and is compatible with all our experimental findings. The fact that the major effects of BDZs on amplitude and on deactivation kinetics are observed at GABA concentration markedly lower than the EC50 value, suggests that they require conditions in which a considerable percentage of receptors are singly bound. Macdonald et al. (1989) have proposed that, at micromolar [GABA], a considerable proportion of channel activity is due to singly bound GABAA receptors. The increase in binding affinity by BDZ would increase the percentage of doubly bound receptors. This, in turn, would alter the deactivation kinetics because the rate constants of opening/closing and desensitization are different in doubly and singly bound receptors. This prediction is, to a smaller or larger extent, reproduced by each model in which singly bound open states are postulated (e.g. Macdonald et al. 1989; Jones & Westbrook, 1995). Such a mechanism predicts that at higher [GABA], at which fully bound GABAARs are predominant, BDZ impact on deactivation would be smaller. However, a clear effect of BDZs on mIPSCs decay kinetics (Fig. 2) remains puzzling, as synaptic [GABA] certainly exceeds micromolar concentrations. Assuming that the synaptic agonist efficiency is reasonably described by the product of peak concentration and duration of agonist exposure (Barberis et al. 2004), the action of synaptic GABA transient would be comparable to application of hundreds of micromoles for ∼1 ms. However, our experiments show that at 100 μm GABA, BDZs did not affect deactivation either for neuronal or recombinant α1β2γ2 receptors. An alternative possibility is that synaptically released agonist spills over from the cleft and activates perisynaptic GABAARs. Clearly, GABA spilling over from a synapse reaches perisynaptic receptors at concentrations lower than in the cleft and therefore the ensuing current is more susceptible to modulation by BDZs. The enhancement of this current would appear as an up-regulation of the ‘slow’ IPSC component because of prolonged diffusion and slower kinetics due to low [GABA]. A similar proposal that IPSCs are partially shaped by subsaturating [GABA] has been put forward by Hill et al. (1998) who studied modulation of GABAergic synaptic currents by diethyl-lactam that affected currents evoked by non-saturating [GABA] but had no effect on responses to saturating [GABA]. There is a general agreement that the phenomenon of GABA spill-over may have a pronounced impact on GABAergic currents, especially in the case of intense synaptic activity (e.g. Isaacson et al. 1993; Overstreet & Westbrook, 2003) although blockade of GABA uptake exerts only a weak, if any, effect on mIPSCs. Interestingly, Hill reported that the contribution of subsaturating [GABA] to IPSCs was insensitive to blockade of the GABA uptake system. Altogether, we propose that the major mechanisms of mIPSCs modulation by BDZs are related to: (i) enhancement of the binding rate that has a particularly strong effect in conditions of non-saturation and fast agonist transient, and (ii) enhancement of current evoked by low [GABA] spilling over from the synapse that is manifested as a slow down of mIPSC. Although the latter proposal is compatible with our observations, we have no direct evidence for it and therefore, at the present stage, it remains speculative. Moreover, kinetic behaviour of synaptic and extrasynaptic GABAA receptors (even of the same type) might differ because of possible modulatory post-translational processes mediated by, for example, phosphorylation/dephosphorylation or binding to regulatory proteins (e.g. GABARAP or gephyrine).
Contrary to our findings, Mellor & Randall (1997) and Krampfl et al. (1998) reported that BDZs prolonged deactivation kinetics of currents evoked by high [GABA]. The reason for this discrepancy is not clear. However, in these studies the fast deactivation component was at least one order of magnitude slower than that reported here. This may suggest that in the protocols applied by Mellor & Randall (1997) and Krampfl et al. (1998) the fast component was not detected. Other factors, such as differences in benzodiazepine type, cellular model or GABA application protocols could also underlie these different observations.
The observation that BDZ receptor agonists might affect different GABAAR properties (binding and gating) is not surprising. Walters et al. (2000) provided evidence for two functionally distinct BDZ binding sites on α1β2γ2 receptors. It is thus possible that different sensitivities to flurazepam of currents evoked by 1 μm GABA (Fig. 3) and on deactivation of currents evoked by saturating [GABA] (a significant effect only at 10 μm, Fig. 4) might involve the presence of different BDZ binding sites.
An important conclusion of this work is that the proposed mechanisms of BDZ receptor agonist action implicates them as potent modulators of both tonic and phasic GABAergic currents. Qualitatively, their effect on the tonic component appears larger, which seems important as it is believed that tonic currents mediate considerably larger charge transfer than the phasic ones (Farrant & Nusser, 2005). On the other hand, it needs to be born in mind that the tonic and phasic forms of inhibition are functionally coupled. Indeed, ambient [GABA] depends on network excitability while shunting (tonic) GABAergic conductance has a major impact on the neuronal firing and therefore on synaptic signalling.
Acknowledgments
This work was supported by Wellcome Trust International Senior Research Fellowship in Biomedical Science (grant no. 070231/Z/03/Z). The authors are thankful to Piotr Brzeźnicki for his help in preparing and performing a part of experiments on synaptic currents.
References
- Andjus PR, Stevic-Marinkovic Z, Cherubini E. Immunoglobulins from motoneurone disease patients enhance glutamate release from rat hippocampal neurones in culture. J Physiol. 1997;504:103–112. doi: 10.1111/j.1469-7793.1997.103bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A, Petrini EM, Cherubini E. Presynaptic source of quantal size variability at GABAergic synapses in rat hippocampal neurons in culture. Eur J Neurosci. 2004;20:1803–1810. doi: 10.1111/j.1460-9568.2004.03624.x. [DOI] [PubMed] [Google Scholar]
- Campo-Soria C, Chang Y, Weiss DS. Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol. 2006;148:984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Conti F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24:155–162. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- Clements JD. Transmitter time course in the synaptic cleft: its role in the central synaptic function. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing SS, Lee YT, Farb DH, Gibbs TT. Benzodiazepine modulation of partial agonist efficacy and spontaneously active GABAA receptors supports an allosteric model of modulation. Br J Pharmacol. 2005;145:894–906. doi: 10.1038/sj.bjp.0706251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali M, Curmi JP, Birnir B, Gage PW. Hippocampal GABAA channel conductance increased by diazepam. Nature. 1997;388:71–75. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Frerking M, Borges S, Wilson M. Variation in GABA mini amplitude is the consequence of variation in transmitter concentration. Neuron. 1995;15:885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci. 2000;12:810–818. doi: 10.1046/j.1460-9568.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Hill MW, Reddy PA, Covey DF, Rothman SM. Contribution of subsaturating GABA concentrations to IPSCs in cultured hippocampal neurons. J Neurosci. 1998;18:5103–5111. doi: 10.1523/JNEUROSCI.18-14-05103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Jonas P. Fast application of agonists to isolated membrane patches. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York and London: Plenum Press; 1995. pp. 231–243. [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Krampfl K, Lepier A, Jahn K, Franke C, Bufler J. Molecular modulation of recombinant rat α1β2γ2 GABAA receptor channels by diazepam. Neurosci Lett. 1998;256:143–146. doi: 10.1016/s0304-3940(98)00767-8. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Twyman RE. Direct evidence for diazepam modulation of GABAA receptor microscopic affinity. Neuropharmacology. 1996;35:1383–1392. doi: 10.1016/s0028-3908(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Rogers CJ, Twyman RE. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor JR, Randall AD. Frequency-dependent actions of benzodiazepines on GABAA receptors in cultured murine cerebellar granule cells. J Physiol. 1997;503:353–369. doi: 10.1111/j.1469-7793.1997.353bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercik K, Piast M, Mozrzymas JW. The benzodiazepine receptor agonists affect both binding and gating of recombinant α1β2γ2 GABAA receptors. Neuroreport. 2007;18:781–785. doi: 10.1097/WNR.0b013e3280c1e2fb. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW. Dynamism of GABAA receptor activation shapes the ‘personality’ of inhibitory synapses. Neuropharmacology. 2004;47:945–960. doi: 10.1016/j.neuropharm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Barberis A, Mercik K, Zarnowska ED. Binding sites, singly bound states and conformation coupling shape GABA-evoked currents. J Neurophysiol. 2003a;89:871–883. doi: 10.1152/jn.00951.2002. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Barberis A, Michalak K, Cherubini E. Chlorpromazine inhibits miniature GABAergic currents by reducing the binding and by increasing the unbinding rate of GABAA receptors. J Neurosci. 1999;19:2474–2488. doi: 10.1523/JNEUROSCI.19-07-02474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozrzymas JW, Zarnowska ED, Pytel M, Mercik K. Modulation of GABAA receptors by hydrogen ions reveals synaptic GABA transient and a crucial role of the desensitization process. J Neurosci. 2003b;23:7981–7992. doi: 10.1523/JNEUROSCI.23-22-07981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23:2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL, Jones MV. Measuring and modeling the spatiotemporal profile of GABA at the synapse. In: Quick M, editor. Transmembrane Transporters. New York: Wiley; 2002. pp. 259–275. [Google Scholar]
- Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Ropert N. Altering the concentration of GABA in the synaptic cleft potentiates miniature IPSCs in rat occipital cortex. Eur J Neurosci. 2000;12:400–404. doi: 10.1046/j.1460-9568.2000.00957.x. [DOI] [PubMed] [Google Scholar]
- Rogers CJ, Twyman RE, Macdonald RL. Benzodiazepine and β-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol. 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rusch D, Forman SA. Classic benzodiazepines modulate the open-close equilibrium in α1β2γ2L γ-aminobutyric acid type A receptors. Anesthesiology. 2005;102:783–792. doi: 10.1097/00000542-200504000-00014. [DOI] [PubMed] [Google Scholar]
- Tietz EI, Kapur J, Macdonald RL. Functional GABAA receptor heterogeneity of acutely dissociated hippocampal CA1 pyramidal cells. J Neurophysiol. 1999;81:1575–1586. doi: 10.1152/jn.1999.81.4.1575. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of γ-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1989;25:213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- Vicini S, Mienville JM, Costa E. Actions of benzodiazepine and β-carboline derivatives on γ-aminobutyric acid-activated Cl− channels recorded from membrane patches of neonatal rat cortical neurons in culture. J Pharmacol Exp Ther. 1987;243:1195–1201. [PubMed] [Google Scholar]
- Wafford KA. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Walters RJ, Hadley SH, Morris KD, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci. 2000;3:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- Whiting PJ. GABAA receptor subtypes in the brain: a paradigm for CNS discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]