Abstract
Receptors for extracellular ATP (both ionotropic and metabotropic) are widely expressed in the CNS both in neurones and glia. ATP can modulate neuronal activity in many parts of the brain and contributes to the central nervous control of several physiological functions. Here we show that during the systemic inflammatory response the extracellular concentrations of ATP increase in the anterior hypothalamus and this has a profound effect on the development of the thermoregulatory febrile response. In conscious rabbits we measured ATP release in real time with novel amperometric biosensors and monitored a marked increase in the concentration of ATP (4.0 ± 0.7 μm) in the anterior hypothalamus in response to intravenous injection of bacterial endotoxin – lipopolysaccharide (LPS). No ATP release was observed in the posterior hypothalamus. The release of ATP coincided with the development of the initial phase of the febrile response, starting 18 ± 2 min and reaching its peak 45 ± 2 min after LPS injection. Application of the ATP receptor antagonists pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulphonic acid, Brilliant Blue G or periodate oxidized ATP dialdehyde to the site of ATP release in the anterior hypothalamus markedly augmented and prolonged the febrile response. These data indicate that during the development of the systemic inflammation, ATP is released in the anterior hypothalamus to limit the magnitude and duration of fever. This release may also have a profound effect on the hypothalamic control of other physiological functions in which ATP and related purines have been implicated to play modulatory roles, such as food intake, hormone secretion, cardiovascular activity and sleep.
Development of the systemic inflammatory response during infection is accompanied by a number of behavioural and autonomic responses, such as a decrease in activity, sleepiness, malaise, fever and loss of appetite. The behavioural and autonomic symptoms of sickness represent a highly organized strategy to fight infection (Hart, 1988; Kluger, 1991; Dantzer, 2004). These adaptive responses are orchestrated by the central nervous system and are believed to be mediated by proinflammatory cytokines (such as interleukin (IL)-1β and others) that are temporarily expressed and produced in the CNS during infection. However, the exact mechanism that is responsible for triggering expression of cytokines in the brain during the development of the inflammatory process in the periphery is not entirely clear (for a discussion of the possible mechanisms see Dantzer, 2004 and Conti et al. 2004).
Extracellular ATP can induce release of IL-1β, tumour necrosis factor-α and other cytokines from glia (Hide et al. 2000; Inoue, 2002; Chakfe et al. 2002; Suzuki et al. 2004; Kucher & Neary, 2005). Ionotropic P2X7 receptors have been shown to mediate ATP-induced release of cytokines by activated glial cells (Hide et al. 2000; Chakfe et al. 2002; North, 2002; Suzuki et al. 2004; Khakh & North, 2006; Choi et al. 2007). Recently Choi et al. (2007) demonstrated that systemic inflammation induced in rats following intraperitoneal administration of bacterial endotoxin – lipopolysaccharide (LPS) – is accompanied by marked up-regulation in P2X7 receptor expression in the brain. In our recent study in rats we observed that systemic blockade of P2X7 receptors attenuates both fever and increases in plasma cytokine levels evoked by LPS in rats (Gourine et al. 2005c).
However, extracellular ATP is not just a cytokine-inducing signalling molecule. Specific ATP receptors, both ionotropic P2X and metabotropic P2Y, are widely expressed in the CNS by both neurones and glia. At some CNS synapses ATP acts as a fast neurotransmitter (for reviews see North, 2002; Khakh & North, 2006; Burnstock, 2007). ATP can modulate neuronal activity in many parts of the brain and contribute to the central nervous control of several physiological functions, such as body temperature regulation (Gourine et al. 2002b, 2004), cardiovascular (Spyer et al. 1997; Scislo & O'Leary, 2005) and respiratory control (Gourine et al. 2005a,b; Gourine, 2005) and others.
This clear functional role of extracellular ATP in the central nervous control of autonomic functions as well as its ability to induce cytokine release from glia, suggest that ATP-mediated signalling in the brain during systemic inflammation may play an important role (either directly or indirectly, via induction of cytokine release) in the manifestation of behavioural and autonomic symptoms of sickness. Of these symptoms, fever – a regulated rise in body temperature (Tb) – is one of the most fundamental. It represents an adaptive thermoregulatory response aimed at facilitating host resistance and slowing the growth of the pathogen. Fever is orchestrated by the preoptic area/anterior hypothalamic structures and is believed to be induced by the actions of proinflammatory cytokines (such as IL-1β) in the anterior hypothalamus (Klir et al. 1994; Kluger et al. 1995; Gourine et al. 1998). In experimental animals, the magnitude of fever usually correlates well with the severity of the underlying inflammatory process and can be easily monitored by measuring core Tb. To test our hypothesis we induced systemic inflammation in conscious rabbits and measured, in real time, changes in extracellular concentration of ATP and its breakdown product adenosine in the anterior and posterior hypothalamic regions. To ascertain the functional role of ATP released during fever, we applied ATP receptor antagonists to the sites of release and the effect of this treatment on the development of the febrile response was determined.
Methods
Animals
Adult male Chinchilla rabbits weighing 2.7–3.2 kg were used in this study (n = 136). They were housed in a room maintained at a constant temperature of 21 ± 1°C and in a 12 : 12 h light–dark cycle with light onset at 06.00 h. Drinking water and food were provided ad libitum. All studies on conscious rabbits were conducted in the facilities of the Institute of Physiology, National Academy of Sciences of Belarus and were approved and governed by the Institutional Animal Care and Use Committee.
Surgery
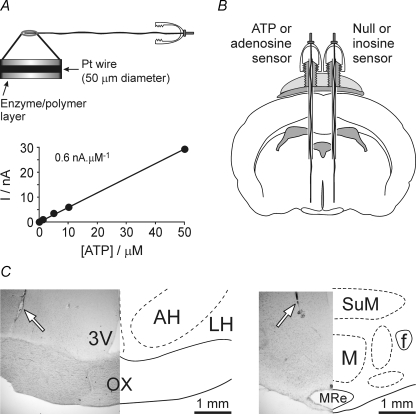
Rabbits were anaesthetized with a mixture of ketamine hydrochloride (45 mg kg−1, s.c.) and xylazine hydrochloride (10.0 mg kg−1, s.c.) with additional ketamine (20 mg kg−1s.c.) administered every 30 min if necessary. A miniature temperature-sensitive telemetry transmitter (model E-mitter, Minimitter, Sunriver, OR, USA) was implanted into the abdominal cavity of each animal for continuous monitoring of Tb. Then the head of the rabbit was placed in a David Kopf stereotaxic apparatus and two 20-gauge guide cannulae (Plastics One, Roanoke, VA, USA) were implanted either bilaterally into the anterior hypothalamus (stereotaxic co-ordinates: 0.5 mm rostral to bregma, 2.5 mm lateral to midline) or bilaterally into the posterior hypothalamus (2.5 mm caudal to bregma, 2.5 mm lateral to midline) according to the atlas of Sawyer et al. (1954). Each guide cannula was lowered 8 mm from the cortical surface so that the tip was positioned 6 mm dorsal to the desired recording/microinjection sites. Two small screws were placed into the skull, and the cannula was secured in place by dental acrylic. The guide cannula was closed with a dummy cannula that extended from the tip of the guide cannula by ∼1 mm. After surgery, rabbits were housed one per cage, were given penicillin and lidocaine and were allowed to recover for at least 7 days before any experiment. At the end of the experiment the animals were killed humanely by an overdose (200 mg kg−1) of pentobarbitone sodium injected intraperitoneally (i.p.), the brains were removed and the locations of the recording and injection sites were confirmed histologically (Fig. 1C).
Figure 1.
ATP biosensors and their placements in the rabbit hypothalamus A, scheme of the sensor assembly and calibration curve of the ATP biosensor. An expanded portion of the sensor is shown to indicate the enzymatic biolayer. Note that this biolayer completely surrounds the tip of the Pt wire. Calibration curve of the ATP biosensor demonstrates linearity of ATP detection in concentrations from 1 to 50 μm. B, schematic showing the placement of the sensors in the anterior hypothalamus of the rabbit. A dual recording configuration of ATP or adenosine sensor placed upon one side of the hypothalamus along with a null or inosine sensor that was placed in an equivalent position on the other side of the hypothalamus was used (see main text for details). C, histological identification of the sensor placements in the anterior (left) and posterior (right) hypothalamus. 3V, third cerebral ventricle; AH, anterior hypothalamic area; f, fornix; LH, lateral hypothalamic area; M, mammillary nuclei; MRe, mammillary recess of the third ventricle; OX, optic chiasm; SuM, supramammillary area. Arrows indicate track of the sensor.
ATP and adenosine biosensors
The principal design and operation of the enzyme-based ATP and adenosine biosensors have been described in detail elsewhere (Llaudet et al. 2003, 2005). ATP detection is based on the use of two enzymes, glycerol kinase and glycerol-3-phosphate oxidase, entrapped within a polymer matrix around a fine Pt wire. In the presence of glycerol these two enzymes convert ATP to H2O2 which is detected electrochemically (detection limit 100 nm ATP). To measure changes in adenosine concentration the biosensor's enzymatic cascade was comprised of adenosine deaminase, nucleoside phosphorylase and xanthine oxidase that successively converts adenosine to inosine, and then xanthine which is oxidized to uric acid with the evolution of H2O2. Thus, currents generated by the adenosine sensor may represent changes in local concentrations of either adenosine, inosine and/or hypoxanthine/xanthine. Therefore, in our experiments, inosine sensors (i.e. sensors containing just nucleoside phosphorylase and xanthine oxidase) were used in dual recording configurations (below) along with the adenosine sensors. The differences between adenosine and inosine sensor currents indicated changes in adenosine levels. The selectivity of the biosensors was improved by incorporation of an inner layer of 1,3 phenylene diamine, to screen out the majority of electroactive interferences such as ascorbate, urate and catecholamine transmitters.
In this study the sensor assembly was custom-made using dummy cannula supplied by Plastics One (Fig. 1A). This dummy cannula has a rigid stainless steel wire and rounded plastic cap which screws onto the guide cannula threaded post. Fine (125 μm) Teflon-coated Pt wire was spiralled around and glued to the stainless steel central wire of the dummy cannula. The very tip (∼1 mm in length) of the Pt wire was stripped of Teflon and covered with sensitive enzyme-polymer layer.
It is well known that electrochemical biosensors can respond – in addition to the analyte of interest – to many electroactive species in the millieu. Therefore, in all the experiments, we used a dual recording configuration of the ATP or adenosine sensor placed into one side of the hypothalamus along with a null sensor, lacking essential enzymes, that was placed in an equivalent position on the other side of the hypothalamus (Fig. 1B). Thus, the null sensor served as a control to determine whether any ‘non-specific’ electroactive interferents were released and could have confounded the measurements. Inosine sensors were used in dual recording configurations along with the adenosine sensors to detect changes in inosine levels. The development of a normal thermoregulatory febrile response during systemic inflammation in rabbits was found to be unaffected when biosensor recording have been made from either anterior or posterior hypothalamic regions.
Biosensors were calibrated in vitro immediately prior to and after the recordings. To convert changes in sensor current to changes in ATP and adenosine concentrations, the mean of the initial and final calibrations was used.
Injections into the hypothalamus
Animals were conditioned to handling once a day for 5–6 days prior to the experiment. Microinjections (2 μl volume) were made over a period of 1–2 min using an internal injection cannula connected to PE-50 tubing attached to a 10 μl syringe (Hamilton, Reno, NV, USA). The injection cannula was removed 2–3 min after the injection.
Induction of systemic inflammation accompanied by the febrile response
Purified LPS (Escherichia coli endotoxin 0111:B4, Sigma Chemical, St Louis, MO, USA) was dissolved in pyrogen-free saline and injected into an ear vein at a dose of 0.5 μg kg−1 and in a volume of ∼0.2 ml. These injections of small amounts of LPS did not cause any significant distress or lasting harm in rabbits. All animals recovered very well and appeared normal by 8 h after the injections.
Drugs
Generic P2 receptor blocker pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) and the compounds generally regarded as P2X7 receptor antagonists – Brilliant Blue G (BBG) and periodate oxidized ATP dialdehyde (oATP) – were obtained from Sigma Chemical. The compounds were dissolved in artificial cerebrospinal fluid (aCSF) to the designed concentration in 2 μl volume. The aCSF used for injections consisted of (mm): 145.0 NaCl, 3.3 KCl, 1.3 CaCl2 and 1.0 MgCl2 dissolved in sterile pyrogen-free water.
Experimental protocols
Measurements of the changes in ATP and adenosine concentration in the anterior and posterior hypothalamic regions
Animals were conditioned to sit quietly in the restrainer for several hours each day for 5–6 days prior to the experiment. On the day of the experiment the rabbit was placed in the restrainer, the dummy cannula was unscrewed and 5 μl of aCSF containing glycerol (5 mm) essential for ATP biosensor operation was injected into the recording site. The whole length of the guide cannula was then filled with aCSF containing glycerol; the biosensor assembly was carefully lowered through the cannula into the desired recording site and firmly screwed onto the guide cannula threaded post. Preliminary studies did not reveal any effect of glycerol-enriched aCSF applied to the anterior or posterior hypothalamus on animal's behaviour, body temperature or ability to develop fever in response to LPS. A thin Ag–AgCl reference electrode was placed under local anaesthesia (1% lidocaine) into the soft tissue under the skin on the animal's forehead. The sensors were connected to a MicroC potentiostat (WPI, Sarasota, FL, USA) and a period of 20–30 min was allowed for sensors to polarize. To induce systemic inflammation, accompanied by fever, LPS was given intravenously as described above. In our initial experiments when recordings were conducted for 3–4 h after LPS challenge we noticed that ATP release in the anterior hypothalamus is relatively transient and occurs only during the initial phase of fever. Therefore, in all subsequent experiments we limited the duration of the recordings to the first 2 h following LPS injections. Changes in hypothalamic adenosine concentration were monitored for up to 4 h after induction of inflammation. At the end of the experiment the sensors were carefully removed and immediately calibrated to test whether they retained sensitivity. Recording sites were identified histologically and mapped using a stereotaxic atlas (Sawyer et al. 1954). Histological analysis of the sensor placements confirmed that recording sites were within the targeted regions of the anterior (anterior hypothalamic and medial preoptic areas) and posterior (supramammillary area and mammillary nuclei) hypothalamus (Fig. 1C).
Effect of ATP receptor blockade in the anterior and posterior hypothalamus on the febrile response during systemic inflammation
These experiments were conducted in unrestrained rabbits, which were kept in their cages. The animals were prepared as described above (see Surgical procedure). Body temperature was monitored using biotelemetry. ATP receptor antagonists (either PPADS (10 μg), BBG (10 μg) or oATP (10 and 100 μg)) were injected unilaterally into the designated hypothalamic site 5 min prior to LPS or saline treatment. Control animals were given aCSF (2 μl) also followed by intravenous administration of LPS or saline. Tb was monitored for 1 h before and 6 h after LPS or saline injections, which were performed between 09.00 and 10.00 h. At the end of the experiments the injection sites were marked by injections of Pontamine Sky Blue dye (2% in 0.2 m sodium acetate, 2 μl) and identified histologically.
Statistical analysis
Records of changes in ATP and adenosine levels were processed and analysed using Spike 2 software (Cambridge Electronic Design, Cambridge, UK). Changes in ATP levels are presented as means ± standard error (s.e.m.) of peak (in μm) and integral (in μm s) increases in concentrations. The integral increases in ATP levels were estimated by measuring the area under the curve relative to a straight line joining the sensor current before and after the response. Temperature data are reported as means ± s.e.m. Comparisons between experimental groups were made for each time point using analysis of variance (ANOVA) followed by the Tukey–Kramer's post hoc test to determine the main group effect. A value of P < 0.05 was considered to be significant.
Results
Measurements of the changes in ATP and adenosine concentrations in the anterior and posterior hypothalamic regions
The biosensors were polarized to their operating potential ( + 500 mV with respect to Ag–AgCl) immediately following their placement into the tissue through the guide cannula. This resulted in a quasi-exponential decaying current seen at the onset of the biosensor recordings (Figs 2 and 3). This decaying current is largely faradaic. The fastest components of the decay are usually completed by 15–20 min following polarization although some very slow decay will occur throughout the recordings (cf. Null sensor records, Fig. 2). It is important to note that this decay of the background current is inherent in the amperometric measurement technique and does not represent a drop in analyte concentration.
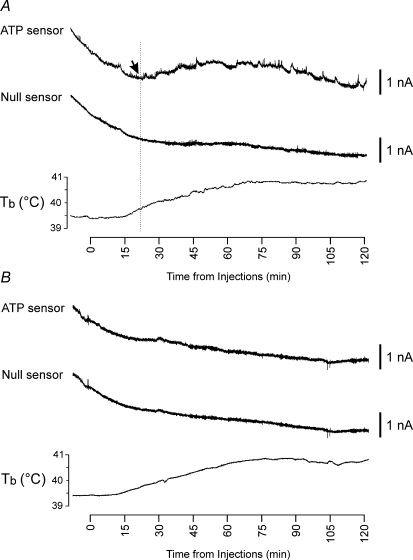
Figure 2.
Release of ATP in the anterior hypothalamus during the development of the systemic inflammatory response in rabbits Representative raw data illustrating changes in ATP and null sensor currents and development of fever (increase in core body temperature (Tb) shown in the bottom traces) following intravenous administration of bacterial endotoxin – lipopolysaccharide (LPS, 0.5 μg kg−1) when the sensors were placed in the anterior (A) and posterior (B) hypothalamic regions. Downward parallel shifts in the ATP and null sensor currents represent slow continuous polarization of the sensors during the whole experiment. X-axis denotes time after LPS injection. Arrow points to the moment when concentration of ATP starts to increase above the baseline.
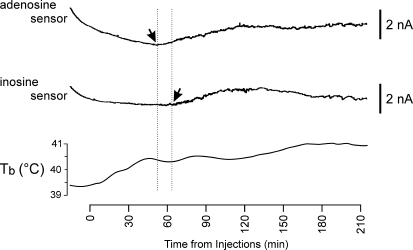
Figure 3.
Release of adenosine and inosine in the anterior hypothalamus during the development of the systemic inflammatory response in rabbits Raw data illustrating changes in adenosine and inosine sensor currents and development of fever (increase in core Tb) following intravenous administration of LPS (0.5 μg kg−1). The sensors were placed in the anterior hypothalamus. Downward shifts in sensor currents represent polarization of the sensors. X-axis denotes time after LPS injection. Arrows point to the moments when concentrations of adenosine and inosine start to increase above the baseline.
During the development of systemic inflammation ATP microelectrode biosensors placed into the anterior hypothalamic region responded with a large increase in current above the baseline, that started 18 ± 2 min after intravenous injection of LPS (mean signal: peak 1.1 ± 0.1 nA, integral 322 ± 70 nA·s; n = 9; Fig. 2A). Null sensors that lacked enzymes in the polymer coating displayed only small fluctuations of the current around the baseline. The changes in ATP sensor currents therefore indicate an increase in the extracellular concentration of ATP in the anterior hypothalamus that had a mean amplitude of 4.0 ± 0.7 μm (n = 9). The release of ATP reached its peak 45 ± 2 min after LPS injections and the total amount of ATP released in this part of the brain in the first 120 min of the development of the systemic inflammatory response was estimated to be 1179 ± 292 μm·s (n = 9). Biosensors placed into the posterior hypothalamus failed to detect any significant changes in extracellular concentration of ATP in response to intravenous injection of LPS (Fig. 2B).
In three out of six rabbits LPS also evoked marked increases in adenosine concentration in the anterior hypothalamus. Figure 3 depicts the time-course of LPS-induced adenosine release (top trace). The increase in adenosine concentration occurred 55 ± 7 min (n = 3) and peaked 191 ± 2 min (n = 3) after LPS challenge. Thus, the time-course of adenosine release during systemic inflammation was substantially different from that of ATP. This increase in adenosine sensor current had a mean amplitude of 1.8 ± 0.6 nA (n = 3) and was followed by a delayed increase in the inosine sensor current which was recorded in an identical position on the contralateral side of the anterior hypothalamus (Fig. 3). These data indicate that adenosine is released first and then converted to inosine. If this signal was purely due to the release of adenosine, it would be equivalent to 5.8 ± 0.2 μm. No adenosine (or inosine) release from the posterior hypothalamic structures was detected in a further five rabbits.
Effect of ATP receptor blockade in the anterior and posterior hypothalamus on the febrile response during systemic inflammation
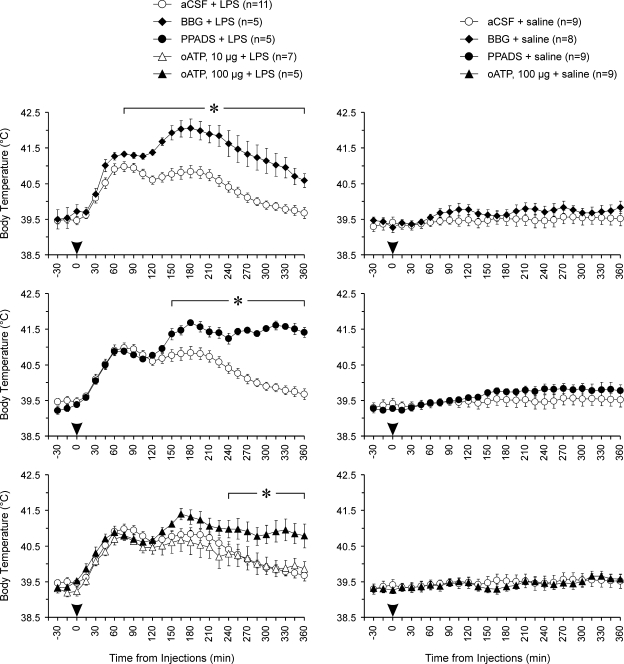
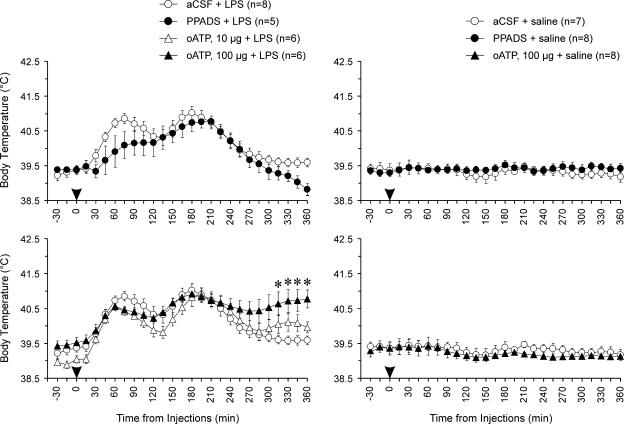
Systemic inflammation induced in rabbits following intravenous treatment with LPS is accompanied by the development of the profound febrile response (Figs 4 and 5). We tested the effects of a range of ATP receptor antagonists injected into the anterior hypothalamus immediately prior to LPS administration. Thus, by the time of LPS challenge ATP receptors have already been affected at the hypothalamic sites which exhibited ATP release during systemic inflammation. All ATP receptor antagonists used had a delayed effect on fever – the febrile response was prolonged and the magnitude of its late phase was significantly augmented (Fig. 4). Administration of BBG into the anterior hypothalamus potentiated fever 90–360 min after LPS challenge (P = 0.019, compared with the aCSF/LPS group). Tb of febrile rabbits that had PPADS injected into the anterior hypothalamus was also significantly higher compared with that of animals injected with aCSF 150–360 min following LPS administration (P = 0.014). oATP (at a higher dose of 100 μg) had a less profound, yet significant, effect on Tb during the late phase of the febrile response: 240–360 min after LPS injection the Tb of animals treated with oATP was significantly (P = 0.023) higher compared with controls. Thus, all of the animals treated with BBG, PPADS or oATP into the anterior hypothalamus remained febrile 6 h after induction of systemic inflammation by intravenous LPS challenge.
Figure 4.
P2 receptor antagonists administered to the site of ATP release augment and prolong the febrile response during systemic inflammation in rabbits The graphs illustrate the effects of Brilliant Blue G (BBG), pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) and periodate-oxidized ATP (oATP) on body temperature during systemic inflammation induced in rabbits by intravenous injection of LPS (0.5 μg kg−1). BBG (10 μg), PPADS (10 μg), oATP (10 and 100 μg) or aCSF were injected unilaterally into the anterior hypothalamus 5 min prior to LPS administration. Data are presented as means ± s.e.m. Numbers in parentheses indicate sample sizes. Arrowheads indicate time of injections. For presentation purposes the temperature curves of BBG + LPS, PPADS + LPS, oATP + LPS, BBG + saline, PPADS + saline, and oATP + saline are shown on separate plots along with the temperature curves of the same respective control groups (aCSF + LPS or aCSF + saline). *BBG had significant (P < 0.05) increasing effect on body temperature 75–360 min, PPADS – 150–360 min and oATP (100 μg) – 240–360 min after initiation of the febrile response by intravenous LPS challenge.
Figure 5.
The effects of P2 receptor antagonists administered into the posterior hypothalamus on the febrile response during systemic inflammation in rabbits The graphs illustrate the effects of PPADS and oATP on body temperature during systemic inflammation induced in rabbits by intravenous injection of LPS (0.5 μg kg−1). PPADS (10 μg), oATP (10 and 100 μg) or aCSF were injected unilaterally into the posterior hypothalamus 5 min prior to LPS administration. Data are presented as means ± s.e.m. Numbers in parentheses indicate sample sizes. Arrowheads indicate time of injections. For presentation purposes the temperature curves of PPADS + LPS, oATP + LPS, PPADS + saline, and oATP + saline are shown on separate plots along with the temperature curves of the same respective control groups (aCSF + LPS or aCSF + saline). *oATP in a high dose of 100 μg had significant (P < 0.05) increasing effect on body temperature 315–360 min after LPS injection.
PPADS and oATP (10 μg) administered into the posterior hypothalamus had no effect on LPS-induced febrile response (Fig. 5). Injection of oATP in a higher dose of 100 μg resulted in a small potentiation of fever at the very late stages of the febrile response (comparison with controls at 315–360 min, P = 0.020, Fig. 5).
Injections of BBG, PPADS or oATP into either anterior or posterior hypothalamic regions had no effect on Tb in afebrile animals (Figs 4 and 5).
Discussion
This study performed in conscious animals has demonstrated for the first time that during the development of the systemic inflammatory response, extracellular concentration of the purine nucleotide ATP increases in the anterior hypothalamus and this has a profound effect on the profile of the accompanying thermoregulatory febrile response. This release of ATP coincides with the development of the initial phase of fever and appears to be also followed by increases in hypothalamic adenosine and then inosine concentrations. ATP receptor antagonists administered to the site of ATP release all markedly augmented and prolonged the febrile response. This finding suggests that ATP, released in the anterior hypothalamus during systemic inflammation, is acting locally as an endogenous antipyretic mediator limiting the magnitude and the duration of the febrile response. Notably, ATP release was not seen in the posterior hypothalamus.
Technical considerations
This is the first in vivo study in conscious animals to utilize enzyme-based biosensors to monitor in real-time the release of ATP and adenosine from the structures located deep in the brain. In our earlier studies in anaesthetized rats, we used biosensors to determine in various physiological conditions changes in ATP and adenosine concentrations in the structures located close to the exposed surface of the brain (Dale et al. 2002; Gourine et al. 2002a, 2005a,2005b). Here we have demonstrated that purine biosensors can be used for long-term measurements of ATP and adenosine levels in the deep brain structures in unanaesthetized experimental animals.
The ATP biosensor responds immediately to changes in ATP concentration and has a 10–90% response time of < 10 s (Llaudet et al. 2005). Thus, the sensors will quickly and accurately reflect the dynamic of changes in ATP concentration in the vicinity of the sensor. We found that during systemic inflammation the peak increase in anterior hypothalamic ATP concentration reaches ∼4 μm. This increase represents a dramatic elevation of extracellular ATP level over its basal concentration in the brain, which is estimated to be in the low nanomolar range (Phillis & O'Regan, 2002; Melani et al. 2005). Release of ATP coincides with the beginning of fever starting ∼18 min and reaching its peak ∼45 min after LPS injection. This increase in ATP concentration was found to be relatively transient, decreasing back to baseline within 120 min after LPS administration.
Although ATP biosensors require glycerol to operate, their responses are insensitive to variations in glycerol concentration over the range of 0.5–5 mm (Llaudet et al. 2005). To provide sensors with sufficient glycerol, recording sites were preinjected and the guide cannula was filled with glycerol-enriched aCSF. Although, ATP sensors retained > 70% of their initial sensitivity at the end of the experiment, the rate of glycerol washout from the recording site is unknown. If glycerol were to be rapidly diluted and washed away then the time to peak and the amount of ATP released may be significantly underestimated. However, this is unlikely because the enzyme–polymer layer of the biosensor has the ability to entrap and retain glycerol in amounts sufficient for the sensor to operate. Controls in which ATP biosensors soaked in 5 mm glycerol solution and then washed and repeatedly tested with ATP in glycerol-free media demonstrated that these biosensors retain their full sensitivity to ATP for up to 4 h (N. Dale, unpublished observations). These observations suggest that the time-course and the amount of ATP measured in our experiments represent quite accurately the dynamics of ATP release in the anterior hypothalamus.
In addition, if ATP were to be rapidly broken down to adenosine then measurements of the extracellular adenosine may also reflect the time-course of changes in ATP concentration. However, significant release of adenosine in the anterior hypothalamus during systemic inflammation was observed in only 3 out of 6 animals tested. It is not entirely clear why the remaining three animals showed no changes in adenosine concentration. All adenosine sensors retained ∼40% of their initial sensitivity at the end of the experiment and histological analysis of the sensor placements showed that the recording sites in all six cases were within the same general area of the anterior hypothalamus. It is likely that a high baseline concentration of extracellular adenosine might prevent detection of adenosine released in relatively small amounts during ‘normal’ physiological activity. It was shown previously that adenosine levels in the rat striatum were markedly higher (∼20-fold) shortly after implantation of the microdialysis probe as compared with 24 h after the surgery (Pazzagli et al. 1993). It is possible that the amount of cellular damage produced during sensor placement varied markedly between animals. Therefore, a higher baseline concentration of adenosine produced through greater tissue damage may be responsible for our inability to detect its release in some of the animals. Since adenosine sensors were gradually losing their sensitivity after placements into the hypothalamus (∼60% in 4 h) it was impossible to preimplant the sensors and conduct the experiments on the next day.
However, in the three animals that displayed adenosine release in response to LPS, the peak increase in concentration and time-course of release were remarkably consistent. The increase in adenosine concentration occurred some 55 min and peaked ∼3 h after LPS challenge. Thus, the increase in adenosine concentration commenced some 37 min after the onset of ATP release. As ATP is usually broken down to adenosine quite rapidly, this difference in time-course suggests that the production of adenosine during systemic inflammation is unlikely to originate entirely from prior release of ATP. It is not entirely surprising considering the findings by Frenguelli et al. (2007) who demonstrated independent and distinct mechanisms underlying release of ATP and adenosine during brain ischaemia. On the other hand, changes in inosine concentration closely followed changes in adenosine levels, strongly suggesting that released adenosine is rapidly converted to inosine.
Functional implications: ATP release in the anterior hypothalamus and its role in the febrile response during systemic inflammation
Fever is one of the most significant symptoms of sickness. It is induced and orchestrated by the central nervous system, specifically by the preoptic area/anterior hypothalamus. Pro-inflammatory cytokines such as IL-1β and others induce the febrile response by their actions in the anterior hypothalamus (Klir et al. 1994; Kluger et al. 1995; Gourine et al. 1998). Considering the vast amount of recent literature which implicates extracellular ATP in triggering P2X7-mediated release of proinflammatory cytokines (see introduction) we expected that ATP produced in this part of the brain would be responsible for local cytokine production and therefore would play an important role in the development of the febrile response during systemic inflammation. This hypothesis has received further recent support from evidence demonstrating a marked up-regulation in P2X7 receptor expression in the brain following peripheral LPS challenge (Choi et al. 2007) and our recent observation that systemic blockade of P2X7 receptors attenuates febrile and cytokine responses evoked by LPS in rats (Gourine et al. 2005c).
However, our data in the present study do not support this hypothesis. Although, ATP is indeed released in the anterior hypothalamus during systemic inflammation, localized blockade of P2 and specifically P2X7 receptors (at the site of ATP release in the anterior hypothalamus) did not reduce the febrile response. Instead, all three ATP receptor antagonists tested (PPADS, BBG and oATP) markedly augmented and prolonged the febrile response. Activation of different P2X and P2Y receptor subtypes is known to depend upon extracellular concentration of ATP (McLarnon, 2005). In this study the peak increase in ATP concentration (∼4 μm) recorded by the biosensors was well below the levels of ATP generally required to activate P2X7 receptors (in excess of 1 mm) (Hide et al. 2000; McLarnon, 2005). We therefore conclude that ATP released in the anterior hypothalamus is unlikely to play any major role in triggering production of proinflammatory cytokines and, by extension, is unlikely to be involved in facilitating the development of fever.
Conversely, our data suggest a different, but equally important, role for ATP-mediated signalling in the anterior hypothalamus during the development of the febrile response. The significantly augmented and prolonged fevers that occurred when the action of ATP was blocked by antagonists suggest that ATP normally acts in the anterior hypothalamus to limit the magnitude and duration of the febrile response.
However, ATP release is relatively transient (it reaches a peak some 45 min following the LPS challenge and decreases back to baseline within 120 min). The effects of the P2 receptor antagonists administered into the anterior hypothalamus on Tb for the most part occurred much later than this. Only BBG had a significant early effect on the febrile response – its effect on Tb was evident at 75 min after LPS injection. PPADS and oATP had significant effects on the febrile response starting from 150 min and 240 min after induction of systemic inflammation, respectively.
Actions of ATP receptor antagonists in the posterior hypothalamus had very little effect on the LPS-induced febrile response. Only oATP (in a higher dose of 100 μg) resulted in a small potentiation of fever at the very late stages of the febrile response. This small effect of the higher dose might be either due to a diffusion of the antagonist away from the injection site and its action at the anterior hypothalamic structures, or due to some non-specific action of the higher dose.
These data suggest that transient release of ATP in the anterior hypothalamus during the initial phase of systemic inflammation triggers a longer lasting mechanism that subsequently limits the febrile response. Interestingly, there is evidence that ATP in the low micromolar range (similar to that detected in this study) may inhibit cytokine (including IL-1β) production by cultured microglia via its actions at metabotropic P2Y receptors (Ogata et al. 2003). Inhibition of cytokine production by ATP released in the anterior hypothalamus could therefore be responsible for limiting the febrile response. We believe that this is unlikely. Although, PPADS may inhibit certain P2Y receptors, the effects of either BBG or oATP on P2Y receptors have not been described. In addition, our preliminary studies in rats revealed no significant effect of PPADS on IL-1β expression in the hypothalamus during LPS-induced systemic inflammation in rats (A. V. Gourine, D. M. Poputnikov, R. Gerstberger & V. N. Gourine, unpublished observations).
Alternatively, transient ATP release could conceivably trigger activation of one or more of the hypothalamic endogenous antipyretic systems which include release of the known central fever-reducing substances such as glucocorticoids, arginine-vasopressin, α-melanocyte stimulating hormone and nitric oxide (for recent review see Roth, 2006). For example, the ability of ATP to induce hypothalamic release of vasopressin – one of the most potent endogenous antipyretics – is well documented (Kapoor & Sladek, 2000). There is also evidence from the study involving our laboratory of a widespread co-localization of neuronal NO synthase and P2X receptors in the hypothalamus (Yao et al. 2003). Conceivably, ATP may trigger NO production which has been shown to have an antipyretic central action in rabbits (Gourine, 1995; Riedel, 1997) as well as in other species (for a review see Steiner & Branco, 2003). If there is an extended time delay in the action of these systems following their activation via ATP release, this may explain the delayed and long lasting effect of P2 antagonists on the development of the febrile response. Distinct mechanisms underlying the first and second phases of fever (see for example Steiner et al. 2006) may also account for the delayed effect of ATP receptor antagonists on the late phase of the febrile response.
Our data cast doubt on a role of P2X7 receptors in mediating the hypothalamic effects of ATP on fever. BBG and oATP were chosen for this study as they efficiently antagonize P2X7 receptors (Ralevic & Burnstock, 1998; North, 2002). P2X7 receptors are significantly less sensitive to blockade by the generic P2 antagonist PPADS (IC50∼50 μm), therefore this compound was used for comparison. However, the effects of BBG and PPADS on Tb during fever were not radically different. The effect of oATP was slightly less profound but qualitatively similar to that of PPADS. However, BBG and oATP are not just P2X7 receptor antagonists. BBG is effective at the rat P2X2 and human P2X4 receptors while oATP in the micromolar range partially (60%) reduces currents at P2X1 and P2X2 receptors (for review see North, 2002). Interestingly, BBG and PPADS appear to be equally potent in blocking P2X2 currents (North, 2002), suggesting that P2X2 subunit-containing receptors may be the most likely candidates to mediate the effects of ATP on the febrile response. Indeed P2X2 receptor subunits (along with P2X4 and P2X6) are the most abundant ATP receptors expressed by CNS neurones (North, 2002; Khakh & North, 2006) and have a widespread distribution throughout the hypothalamus (Xiang et al. 1998; Loesch et al. 1999; Kanjhan et al. 1999; Yao et al. 2003).
Perspectives
Receptors for extracellular ATP – both ionotropic P2X and metabotropic P2Y – are widely expressed in the CNS both in neurones and glia. ATP has been found to modulate neuronal activity in many parts of the brain and to contribute to the central nervous control of many physiological functions. In this study performed in conscious rabbits we observed that during development of the systemic inflammation the extracellular concentration of ATP markedly increases in the anterior hypothalamus. When released, ATP acts locally to limit the magnitude and duration of the febrile response. These data demonstrate the importance of directly measuring neurotransmitter release, rather than inferring it from the effect of antagonists on a particular physiological process – this study demonstrated that during fever, ATP release occurs significantly before any effects of P2 antagonists on Tb are observed.
Fever is just one of several behavioural and autonomic adaptations that occur during the systemic inflammatory response. Others include a decrease in locomotor activity, sleepiness, malaise and loss of food appetite. It is intriguing to speculate about the degree to which locally released ATP and adenosine (specific to the preoptic areas/anterior hypothalamus) play a role in the development of these responses. For example, the cholinergic basal forebrain and the ventrolateral preoptic area – both involved in the control of sleep (Saper et al. 2001; Basheer et al. 2004) – are close to the areas of ATP and adenosine release. As adenosine can act at these sites to promote sleep (Basheer et al. 2004; Morairty et al. 2004), this may partially explain why systemic inflammation is often accompanied by an increased sleep drive. In addition, it will be important to establish the cellular origin of ATP and adenosine release (neurones or glial) as well as the mechanisms that cause and mediate their release.
Acknowledgments
This study was supported by The Wellcome Trust and International Association for the promotion of cooperation with scientists from the New Independent States of the former Soviet Union (INTAS).
References
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Seguela P. ADP and AMP induce interleukin-1β release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. 2002;22:3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- Dale N, Gourine AV, Llaudet E, Bulmer D, Thomas T, Spyer KM. Rapid adenosine release in the nucleus tractus solitarii during defence response in rats: real-time measurement in vivo. J Physiol. 2002;544:149–160. doi: 10.1113/jphysiol.2002.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;101:1400–1413. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV. Pharmacological evidence that nitric oxide can act as an endogenous antipyretic factor in endotoxin-induced fever in rabbits. Gen Pharmacol. 1995;26:835–841. doi: 10.1016/0306-3623(94)00240-n. [DOI] [PubMed] [Google Scholar]
- Gourine AV. On the peripheral and central chemoreception and control of breathing: an emerging role of ATP. J Physiol. 2005;568:715–724. doi: 10.1113/jphysiol.2005.095968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Dale N, Gourine VN, Spyer KM. Fever in systemic inflammation: roles of purines. Front Biosci. 2004;9:1011–1022. doi: 10.2741/1301. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005a;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci. 2005b;25:1211–1218. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Thomas T, Dale N, Spyer KM. Adenosine release in nucleus tractus solitarii does not appear to mediate hypoxia-induced respiratory depression in rats. J Physiol. 2002a;544:161–170. doi: 10.1113/jphysiol.2002.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Melenchuk EV, Poputnikov DM, Gourine VN, Spyer KM. Involvement of purinergic signalling in central mechanisms of body temperature regulation in rats. Br J Pharmacol. 2002b;135:2047–2055. doi: 10.1038/sj.bjp.0704679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Poputnikov DM, Zhernosek N, Melenchuk EV, Gerstberger R, Spyer KM, Gourine VN. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br J Pharmacol. 2005c;146:139–145. doi: 10.1038/sj.bjp.0706287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Rudolph K, Tesfaigzi J, Kluger MJ. Role of hypothalamic interleukin-1β in fever induced by cecal ligation and puncture in rats. Am J Physiol Regul Integr Comp Physiol. 1998;275:R754–R761. doi: 10.1152/ajpregu.1998.275.3.R754. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-α release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- Inoue K. Microglial activation by purines and pyrimidines. Glia. 2002;40:156–163. doi: 10.1002/glia.10150. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol. 1999;407:11–32. [PubMed] [Google Scholar]
- Kapoor JR, Sladek CD. Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci. 2000;20:8868–8875. doi: 10.1523/JNEUROSCI.20-23-08868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Klir JJ, McClellan JL, Kluger MJ. Interleukin-1β causes the increase in anterior hypothalamic interleukin-6 during LPS-induced fever in rats. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1845–R1848. doi: 10.1152/ajpregu.1994.266.6.R1845. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Leon LR, Soszynski D, Conn CA. Cytokines and fever. Neuroimmunomodulation. 1995;2:216–223. doi: 10.1159/000097199. [DOI] [PubMed] [Google Scholar]
- Kucher BM, Neary JT. Bi-functional effects of ATP/P2 receptor activation on tumor necrosis factor-α release in lipopolysaccharide-stimulated astrocytes. J Neurochem. 2005;92:525–535. doi: 10.1111/j.1471-4159.2004.02885.x. [DOI] [PubMed] [Google Scholar]
- Llaudet E, Botting NP, Crayston JA, Dale N. A three-enzyme microelectrode sensor for detecting purine release from central nervous system. Biosens Bioelectron. 2003;18:43–52. doi: 10.1016/s0956-5663(02)00106-9. [DOI] [PubMed] [Google Scholar]
- Llaudet E, Hatz S, Droniou M, Dale N. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem. 2005;77:3267–3273. doi: 10.1021/ac048106q. [DOI] [PubMed] [Google Scholar]
- Loesch A, Miah S, Burnstock G. Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamo-neurohypophysial system. J Neurocytol. 1999;28:495–504. doi: 10.1023/a:1007009222518. [DOI] [PubMed] [Google Scholar]
- McLarnon JG. Purinergic mediated changes in Ca2+ mobilization and functional responses in microglia: effects of low levels of ATP. J Neurosci Res. 2005;81:349–356. doi: 10.1002/jnr.20475. [DOI] [PubMed] [Google Scholar]
- Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Morairty S, Rainnie D, McCarley R, Greene R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123:451–457. doi: 10.1016/j.neuroscience.2003.08.066. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ogata T, Chuai M, Morino T, Yamamoto H, Nakamura Y, Schubert P. Adenosine triphosphate inhibits cytokine release from lipopolysaccharide-activated microglia via P2y receptors. Brain Res. 2003;981:174–183. doi: 10.1016/s0006-8993(03)03028-2. [DOI] [PubMed] [Google Scholar]
- Pazzagli M, Pedata F, Pepeu G. Effect of K+ depolarization, tetrodotoxin, and NMDA receptor inhibition on extracellular adenosine levels in rat striatum. Eur J Pharmacol. 1993;234:61–65. doi: 10.1016/0014-2999(93)90706-n. [DOI] [PubMed] [Google Scholar]
- Phillis JW, O'Regan MH. Evidence for swelling-induced adenosine and adenine nucleotide release in rat cerebral cortex exposed to monocarboxylate-containing or hypotonic artificial cerebrospinal fluids. Neurochem Int. 2002;40:629–635. doi: 10.1016/s0197-0186(01)00113-9. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Riedel W. Antipyretic role of nitric oxide during endotoxin-induced fever in rabbits. Int J Tissue React. 1997;19:171–178. [PubMed] [Google Scholar]
- Roth J. Endogenous antipyretics. Clin Chim Acta. 2006;371:13–24. doi: 10.1016/j.cca.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Sawyer CH, Everett JW, Green JD. The rabbit diencephalon in stereotaxic coordinates. J Comp Neurol. 1954;101:801–824. doi: 10.1002/cne.901010307. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Purinergic mechanisms of the nucleus of the solitary tract and neural cardiovascular control. Neurol Res. 2005;27:182–194. doi: 10.1179/016164105X21959. [DOI] [PubMed] [Google Scholar]
- Spyer KM, Lambert JH, Thomas T. Central nervous system control of cardiovascular function: neural mechanisms and novel modulators. Clin Exp Pharmacol Physiol. 1997;24:743–747. doi: 10.1111/j.1440-1681.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Branco LG. Fever and anapyrexia in systemic inflammation: intracellular signaling by cyclic nucleotides. Front Biosci. 2003;8:s1398–s1408. doi: 10.2741/1188. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Ivanov AI, Serrats J, Hosokawa H, Phayre AN, Robbins JR, Roberts JL, Kobayashi S, Matsumura K, Sawchenko PE, Romanovsky AA. Cellular and molecular bases of the initiation of fever. PLoS Biol. 2006;4:e284. doi: 10.1371/journal.pbio.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Bo X, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamus. Brain Res. 1998;813:390–397. doi: 10.1016/s0006-8993(98)01073-7. [DOI] [PubMed] [Google Scholar]
- Yao ST, Gourine AV, Spyer KM, Barden JA, Lawrence AJ. Localisation of P2X2 receptor subunit immunoreactivity on nitric oxide synthase expressing neurones in the brain stem and hypothalamus of the rat: a fluorescence immunohistochemical study. Neuroscience. 2003;121:411–419. doi: 10.1016/s0306-4522(03)00435-4. [DOI] [PubMed] [Google Scholar]