Abstract
Available evidence supports the idea that muscle fibres provide retrograde signals that enable the expression of adult motoneuron electrical properties but the mechanisms remain unknown. We showed recently that when acetylcholine receptors are blocked at motor endplates, the electrical properties of rat motoneurons change in a way that resembles changes observed after axotomy. This observation suggests that receptor blockade and axotomy interrupt the same signalling mechanisms but leaves open the possibility that the loss of muscle fibre activity underlies the observed effects. To address this issue, we examined the electrical properties of axotomized motoneurons following reinnervation. Ordinarily, these properties return to normal following reinnervation and re-activation of muscle, but in this study muscle fibre activity and evoked acetylcholine release were prevented during reinnervation by blocking axonal conduction. Under these conditions, the properties of motoneurons that successfully reinnervated muscle fibres recovered to normal despite the absence of muscle fibre activity and evoked release. We conclude that the expression of motoneuron electrical properties is not regulated by muscle fibre activity but rather by a retrograde signalling system coupled to activation of endplate acetylcholine receptors. Our results indicate that spontaneous release of acetylcholine from regenerated motor terminals is sufficient to operate the system.
Activity of synaptic targets is thought to regulate presynaptic properties. In Drosophila, studies have shown that synaptic release properties at the glutamatergic neuromuscular junction (NMJ) are modulated by retrograde signals from muscle (Petersen et al. 1997; Davis et al. 1998; DiAntonio et al. 1999). These signals are initiated by glutamate receptor activation (DiAntonio et al. 1999) and help to establish homeostatic regulation of presynaptic glutamate release (Frank et al. 2006). Similar mechanisms may operate at the vertebrate NMJ (Rich & Wenner, 2007) where partial blockade of acetylcholine receptors (AChRs) at the rat NMJ produces an increase of motor terminal ACh release (Plomp et al. 1992, 1994).
Other evidence suggests that the influence of receptor-mediated retrograde signals derived from muscle may extend beyond regulation of transmitter release and include additional neuronal properties. In squid, the electrical properties of developing motoneurons can be altered by blockade of postsynaptic receptors on muscle (Nick & Ribera, 2000). In vertebrates, the importance of synaptic connections is indicated by observations that axotomy-induced changes of motoneuron electrical properties revert to normal when regenerating motoneurons reestablish synaptic contact and restore muscle activity (Kuno, 1976; Foehring et al. 1986a,b) but do not when synapse formation is inhibited (Pinter & Vanden Noven, 1989).
We recently showed that rat motoneurons exhibit axotomy-like changes of electrical properties after muscle AChRs are blocked for only a few days (Nakanishi et al. 2005). This evidence supports the idea that retrograde signalling enables expression of adult motoneuron properties and is mediated as a result of AChR activation. However, studies of postsynaptic neurotransmitter receptor blockade suffer from the complication that postsynaptic action potentials are also blocked by this manipulation. It is thus not known whether the observed effects of neurotransmitter block are due to loss of postsynaptic activity. The existing literature provides conflicting evidence on the possible role of muscle activity in determining motoneuron properties. Several previous studies have examined motoneuron properties after nerve conduction blockade (which prevents evoked ACh release and muscle activity), but the results vary between no apparent effect (Webb & Cope, 1992; Gardiner & Seburn, 1997) and axotomy-like changes (Czeh et al. 1978; Munson et al. 1997).
To address this issue in the present study, we used a different approach and reasoned that if muscle fibre activity plays an important role in maintaining motoneuron excitability properties, then fibre activity may also be required to enable the recovery of properties that normally occurs following reinnervation. However, previous studies have shown that several processes operate at the NMJ in the absence of muscle activity or evoked ACh release during reinnervation including synapse formation (Jansen et al. 1973; Cangiano et al. 1980; Pasino et al. 1996; Busetto et al. 2000; Costanzo et al. 2000) as well as synapse elimination (Thompson et al. 1979; Ribchester & Taxt, 1983; Callaway et al. 1987; Costanzo et al. 2000). In view of these observations, we hypothesized that motoneuron electrical properties might recover when axotomized motoneurons reinnervate vacant endplates but are prevented from activating muscle fibres by conduction blockade. Under these conditions, evoked release of ACh is also blocked but spontaneous release of ACh persists. Here we show that despite the absence of muscle fibre activity and evoked ACh release, motoneuron properties recover to normal following reinnervation. These results highlight the important role of AChR activation in enabling the expression of normal motoneuron properties and show that spontaneous ACh release is sufficient to enable retrograde signalling between motoneurons and muscle fibres. Some of these results have appeared in abstract form (Bichler et al. 2005).
Methods
Data for this study were collected from adult female Wistar rats (270–380 g, Charles River Laboratories, Wilmington, MA, USA). Animals were allocated to a control group that received no treatment and one of three main experimental groups. (1) The nerve supplying the medial gastrocnemius (MG) nerve was crushed with fine forceps 6 days or 1 month prior to a terminal experiment. (2) The posterior tibial nerve was fitted with a cuff connected to a saline-filled osmotic pump and the MG nerve was crushed or left intact 2 months prior a terminal experiment. (3) The posterior tibial nerve was fitted with a cuff connected to a tetrodotoxin (TTX)-filled pump and MG nerve was crushed after complete limb paralysis was verified. These animals were prepared 1 or 2 months prior to a terminal experiment. This design increases the ability to detect effects of activity. If, for example, muscle fibre activity is required for recovery of motoneuron properties during reinnervation, then properties would be expected to retain postaxotomy levels following reinnervation when nerve conduction is blocked and muscle activity is absent. These levels are significantly different from normal and thus easy to detect experimentally. Further details of the preparation of each data group are provided below.
During all surgical preparations, animals were initially anaesthetized in a closed chamber with 5% isoflurane (Novaplus) and maintained in an areflexive state with 3.5–4% isoflurane. Following completion of the procedure and recovery from anaesthesia, animals were housed individually and provided food and water ad libitum. All procedures were approved by the Emory University Institutional Animal Care and Use Committee.
MG nerve crush
The MG nerve was dissected free and crushed about 2 mm proximal to its entry into the muscle. The nerve was firmly grasped for 5 s with a pair of fine forceps (Fine Science Tools) taking care to avoid damage to the blood supply as much as possible.
TTX cuff placement
Conduction blockade of the posterior tibial nerve was produced by continuous superfusion with TTX delivered to the nerve by a system consisting of a mini-osmotic pump (Alzet, model 2004; Durect Corporation), a Sialstic cuff and a connecting tube (Sialstic tubing, Dow Corning). Under general anaesthesia, an incision was made at the popliteal fossa to expose the posterior tibial nerve and another small incision was made on the upper back. The nerve was separated from surrounding connective tissue and the cuff was gently placed around the dissected nerve and closed with two loops of suture. The connecting tube was passed beneath the skin and led proximally through the back incision into the midback region. The tube was filled with TTX solution (500 μg ml−1) and connected to the osmotic pump. All osmotic pump fluids included 200 U ml−1 of penicillin G potassium (Pfizer) and 200 μg ml−1 of gentamicin sulphate (Abbott Laboratories). The pump was placed beneath the skin and anchored by suture to subcutaneous connective tissue.
Successful tibial nerve blockade was indicated by the absence of left ankle extension. Following verification of complete paralysis (24–72 h following TTX cuff placement), animals were re-anaesthetized and the MG nerve was crushed as described above. Paralysis was maintained for about 1 month (28 days) or about 2 months (56 days). In the latter case, pumps were replaced after 28 days. Animals maintained for 1 month were only used for histological analysis of MG muscle innervation as described below.
Prior to the terminal experiments, the flow of TTX to the posterior tibial nerve was stopped. This enabled antidromic identification of MG motoneurons following MG nerve stimulation and use of intracellular stimulation of MG motoneurons for the recording of motor unit electromygraphic (EMG) potentials and electroneurograms (ENG). Animals were anaesthetized, the connecting tube was tied proximal to the cuff and TTX solution was flushed with saline from the tube below the tie. The incision was then closed using wound clips and the animal was allowed to recover from anaesthesia.
To monitor recovery from conduction blockade, animals were briefly re-anaesthetized and daily EMG recordings were obtained using fine concentric recording electrodes from the lateral gastrocnemius (LG) muscle during electrical stimulation of the sciatic nerve above the cuff site. For all animals in which motoneuron properties were studied, no EMG potentials were observed 24 h after TTX stoppage. This shows that conduction blockade was present just before the terminal experiment was performed. Nerve-evoked EMG potentials usually appeared between 1 and 2 days following stoppage of TTX flow (Pasino et al. 1996). Terminal experiments were performed immediately when evidence of nerve-evoked compound EMGs was observed. Given this recovery time, less than 1 day of MG muscle activity could have occurred before animals were re-anaesthetized for the terminal experiment.
To verify the long-term presence of TTX paralysis, soleus muscles were recovered from the TTX- and saline-treated animals bilaterally. The soleus muscles are innervated by axons within the posterior tibial nerve but soleus muscle nerves were not crushed. Analysis of these muscles thus allowed assessment of conduction blockade in the absence of nerve crush. Following immersion in fixative solutions, five cross-sections (30 μm thickness) were obtained from the muscle midbelly. Sections were stained using a standard haematoxylin/eosin procedure and visualized using transmitted light with an upright microscope. Images of each section were acquired with a video camera connected to the microscope. The soleus muscle cross-sectional area was measured using commercial imaging software (Image Pro, Media Cybernetics). Average soleus cross-sectional areas from TTX-treated sides were compared with areas from untreated sides.
Saline cuff placement
Procedures identical to those described above were used to place cuffs connected to saline-filled osmotic pumps.
Terminal recording experiments
For terminal experiments, rats were anaesthetized with pentobarbital (45 mg kg−1, i.p.) with supplemental doses (5–8 mg kg−1 h −1) administered as needed to maintain anaesthesia throughout the acute experiment. Details of animal preparation have been published previously (Nakanishi et al. 2005). Briefly, the left hindlimb was dissected to expose the MG nerve and muscle. The common peroneal and sural nerve branches were cut distally and removed as far proximally as possible. The MG muscle nerve was isolated from connective tissue and separated from the lateral gastrocnemius–soleus and the posterior tibial nerve. A laminectomy was performed to expose the lumbar spinal cord. All animals were placed in a frame that immobilized the vertebral column. Numbers of animals in each experimental group are provided in figure insets.
Recording procedures
Bipolar stimulating/recording electrodes were placed on the MG muscle nerves for antidromic activation of motoneurons and for recording nerve potentials. A dorsal root entry zone electrode recorded afferent volleys that were used to set MG nerve stimulation intensity at 2.5 × threshold for the most excitable fibres. MG motoneurons were impaled using glass micropipettes (8–12 MΩ) filled with 2 m potassium acetate. MG motoneurons were identified by antidromic activation following MG nerve stimulation. Upon impalement, the antidromic action potential was sampled first. Rheobase current was determined as the minimal current (50 ms pulse) injected intracellularly needed to evoke an action potential. Averages of action potentials evoked by short intracellular stimulation (0.5 ms current pulse) were used to measure afterhyperpolarization (AHP) half-decay times by determining the time between the AHP peak amplitude and half this value during the AHP decay. Input resistance was measured from the voltage drop produced by small (1 or 3 nA), 50 ms current pulses injected through the micropipette. Data were collected from motoneurons with antidromic action potentials amplitude more than 60 mV. In the experiments reported in this paper, data from 7–19 motoneurons were obtained that met this criterion as well as the identification criteria described below.
To avoid uncertainties about motoneuron identification due to possible stimulus spread from the nerve stimulating electrode, each MG motoneuron was stimulated intracellularly with 0.5 ms suprathreshold current pulses while recording from the MG muscle nerve. Records of the ENG showed an all-or-nothing axonal action potential in the MG nerve linked to the intracellular stimulus, even in the proximal stump of previously crushed MG nerves. Because of the relatively small distance between the recording electrodes and the muscle, the ENG recording electrodes also provided records of motor unit potentials which were used to determine whether MG motoneurons could activate muscle fibres. In TTX-treated rats, a pair of fine-calibre EMG electrodes were also inserted into the MG muscle for recording motor unit EMG potentials following MG motoneuron intracellular stimulation. All recordings were digitized and analysed using custom software (LabWindows, National Instruments).
Immunolabelling
Following the conclusion of each electrophysiological experiment, MG muscles were harvested and fixed with 4% paraformaldehyde, washed in 0.1 m PBS and bathed in 20% sucrose solution overnight. Sections (50 μm thickness) containing endplate-rich regions were obtained using a cryostat (Leica). Motor endplate acetylcholine receptors (AChRs) were labelled with fluorescently conjugated α-bungarotoxin (Alexa Fluor 555; Molecular Probes). Axons and motor terminals were labelled with a mouse monoclonal antibody against the phosphorylated heavy fragment of neurofilament protein (SMI31, 1: 400, Sternberger Monoclonals Inc.). Labelling was visualized using fluorescein-conjugated secondary antibodies (1: 100, Jackson Immunoresearch Laboratories). Synaptic vesicles were labelled using a rabbit polyclonal antibody directed at synaptophysin (SYP (H93), 1: 100, Santa Cruz Biotechnology) and AMCA-conjugated secondary antibodies (1: 100, Jackson Immunoresearch Laboratories). MG muscle sections were imaged using an upright fluorescence microscope (Leica DMR) equipped with appropriate fluorescence filters and a CARV spinning-wheel confocal attachment (BD Biosciences). The images were used to tabulate the percentages of bungarotoxin-labelled endplates that were fully occupied by motor terminal immunostaining (completely reinnervated), partially occupied and completely unoccupied (Carrasco et al. 2004). The resulting data allowed quantification of the level of MG muscle reinnervation. For this analysis, an average of 143 NMJs were examined from each muscle.
Data analysis and statistics. Average values are presented ± 1 s.e.m. Nested ANOVA was used to examine of treatment effects. Tukey's HSD post hoc test was used to test the significance of comparisons of specific treatment effects. The statistical significance of side-to-side comparisons of percentages was determined using Fisher's two-tail exact test (2 × 2 tables) or the likelihood ratio Chi-square test (2 × 3 tables). Commercially available software was used to perform these statistical tests (Systat, Systat Software, Inc.).
Results
Motoneuron properties following nerve crush
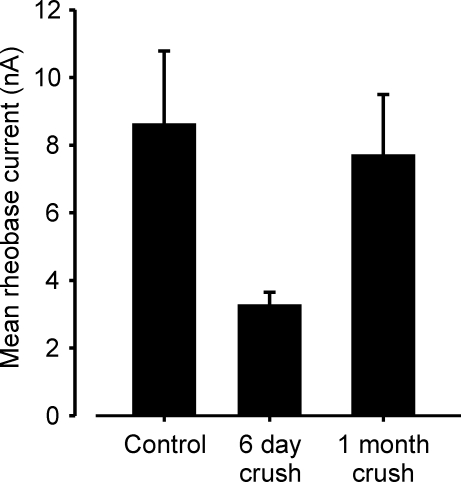
MG motoneuron properties were examined 6 days after MG nerve crush located near the entry of the nerve into the muscle. In 2/3 experiments, we found that nerve stimulation caused MG muscle contractions. Thus, MG motoneurons had begun to reinnervate the MG muscle by 6 days following peripheral nerve crush. This is consistent with previous reports that reinnervation commences rapidly following peripheral nerve crush in mice (Rich & Lichtman, 1989), in rats (McArdle & Albuquerque, 1973), and in cats (Fugleholm et al. 1994). Despite the evidence of reinnervation, average motoneuron properties (AHP, input resistance and rheobase current) were clearly altered from normal (P < 0.01) and had average values similar to those reported previously for axotomy by nerve crush (Nakanishi et al. 2005). The change in rheobase current is illustrated in Fig. 1. We did not study the normal time course of recovery of properties in detail. However, by 1 month following crush, average property values had recovered and were not significantly different from normal values (Fig. 1 for rheobase, P > 0.05). If 1 month approximates the end point of recovery and if an exponential time course for the recovery of average rheobase current is assumed, an estimate of 9–12 days is obtained for the time constant of recovery following initial reinnervation. A similar estimate using the data of (Foehring et al. 1986a) for recovery of average rheobase current in axotomized cat MG motoneurons yields a time constant value of about 60 days following initial reinnervation. The more rapid estimated time course of recovery in rats is likely to reflect the shorter distances within the rat MG muscle that need to be traversed by regenerating motor axons before reinnervation occurs.
Figure 1.
Motoneuron rheobase current after nerve crush Bar chart show the averages of mean values obtained from individual experiments for each group (control, n = 10; 6 day crush, n = 3; 1 month crush, n = 2). By 6 days following nerve crush, rheobase mean values have decreased to postaxotomy levels. After 1 month, mean values nearly recovered to control levels. Motor unit EMG recordings show that reinnervation after MG nerve crush commenced by 6 days (see text). In this and all following illustrations, error bars represent 1 s.e.m.
These data show that changes occur in motoneuron properties within 6 days following MG nerve crush, that reinnervation commences soon after crush and that recovery from the effects of nerve crush is accomplished by 1 month. Furthermore, these results demonstrate that recovery of motoneuron properties does not occur rapidly following recovery of evoked synaptic transmission and resumption of muscle activity.
Motoneuron properties following muscle reinnervation by inactive axons
In order to determine whether nerve terminal or muscle fibre activity is necessary for mediating recovery of motoneuron properties following reinnervation, MG motor axons were crushed below the site of a Sialstic cuff that delivered TTX to the posterior tibial nerve after TTX paralysis was confirmed. Motoneuron properties were determined during terminal experiments conducted after a delay of 2 months following nerve crush. As shown above, this is more than sufficient time for recovery of motoneuron properties following crush injury when motor axon conduction is not blocked.
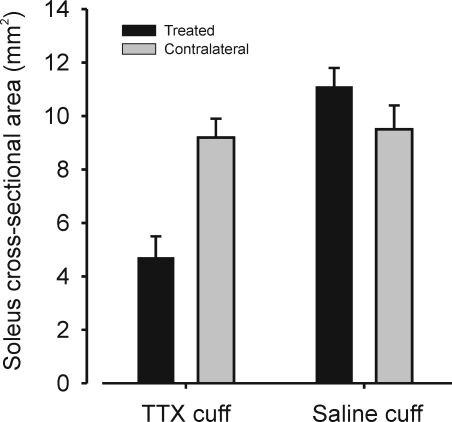
It was readily apparent that gastrocnemius muscles on the TTX-treated sides were significantly atrophied compared to contralateral gastrocnemius muscles. To quantify the extent of atrophy caused by TTX paralysis alone in the absence of nerve crush, soleus muscles, which are also innervated by axons in the posterior tibial nerve, were recovered and their cross-sectional areas measured. Figure 2 shows that the soleus muscles on the TTX-cuff side were, on average, about half the size of the untreated side (P < 0.01), while soleus muscles on saline-cuffed sides were slightly but not significantly (P > 0.05) larger than contralateral soleus muscles. This evidence of disuse atrophy in muscles subjected only to axonal conduction blockade by TTX but not nerve crush shows that an extended paralysis was accomplished by nerve conduction blockade of the posterior tibial nerve in TTX-treated animals. We thus infer that the MG muscle on TTX-cuffed sides remained paralysed during reinnervation following nerve crush.
Figure 2.
Disuse atrophy following 2 months of TTX blockade Bar chart illustrating average values of soleus muscle cross-sectional area from experiments in which TTX or saline cuffs were placed on the posterior tibial nerve of one side (for all bars, n = 4 animals). These data were obtained from the soleus muscles of the same animals used in the main series of experiments. In these experiments, motor axons supplying the soleus nerve remained intact because only the MG component of the posterior tibial nerve was crushed. Mean soleus muscle cross-sectional area was significantly decreased (P < 0.01) on sides on which TTX cuffs were placed compared with the contralateral muscle but not significantly different from normal on sides in which saline cuffs were placed. Cross-sectional areas for each muscle represent the average of 5 sections taken at midbelly.
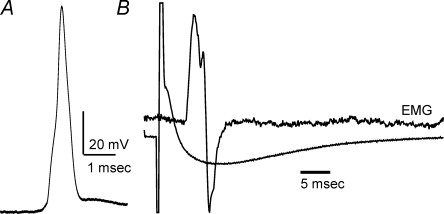
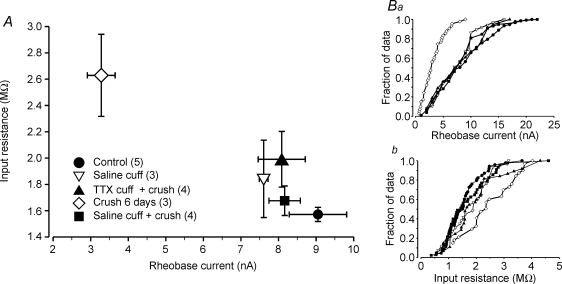
Despite evidence of extended MG muscle inactivity, MG motoneurons had reinnervated muscle fibres and were capable of activating motor units upon intracellular stimulation during terminal experiments (Fig. 3). Moreover, the average electrical properties of these motoneurons were indistinguishable from the properties of MG motoneurons that had regenerated below a saline-filled cuff and from normal MG motoneuron properties. This is illustrated in Fig. 4A which shows a plot of the mean values of averages for input resistance and rheobase current from individual animals in all treatments groups (symbols defined by inset). With the exception of the means for the 6 day nerve crush group, means for all other groups clustered near values obtained from normal animals. Nested analysis of variance showed that none of the means for the properties examined (rheobase, input resistance and AHP) in the latter groups differed significantly (P > 0.05). As shown in Fig. 4Ba and b, the average cumulative distributions of both rheobase and input resistance showed extensive overlap with average distributions from all other groups except that from the 6 day nerve crush group. These results indicate that recovery of normal motoneuron properties following crush injury and reinnervation does not require nerve-evoked muscle fibre activity (mechanical or electrical), axonal action potential invasion of motor terminals or evoked release of ACh.
Figure 3.
Recordings from TTX experiments Recordings were obtained from an MG motor unit after 2 months of axon conduction blockade and nerve crush. TTX flow to the implanted cuff was stopped 2 days before the terminal experiment was performed. A, motoneuron antidromic action potential. B, average records (10 sweeps) of motor unit EMG potential and membrane potential from the same motoneuron as in A following intracellular stimulation. The records show that this motoneuron had reinnervated muscle fibres and that motor terminals released sufficient ACh to activate muscle fibres.
Figure 4.
Motoneuron properties recover in TTX-treated animals A, plot of average rheobase current versus average input resistance for different experimental groups. Values represent the averages of mean values obtained from individual animals in each group (sample sizes shown in key). With the exception of data from the axotomy group (6 days), all other means show no significant differences from normal (control). B, average cumulative histograms of rheobase current (a) and input resistance (b) for different experimental groups (symbols as in A). Each histogram is the average of individual animal histograms obtained in each group. Number of animals in each group indicated in key in A. Symbols indicate points along the x-axes at which values were obtained during averaging of histograms of unequal sample size. For clarity, error bars have been omitted. Note that data from the TTX + crush group are intermingled with data from control and saline-treated groups. This shows that motoneurons in the TTX + crush group recovered property values throughout the normal range of variation and indicates non-selective and complete recovery.
MG muscle reinnervation
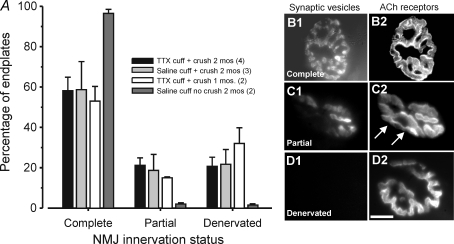
Previous studies of muscle reinnervation following crush injury have generally reported that all endplates in the denervated muscle eventually become reoccupied by nerve terminals (Rich & Lichtman, 1989). We examined the extent of reinnervation following crush injury in the MG muscles of animals that had TTX- and saline-filled cuffs placed on the posterior tibial nerve using fluorescent staining to visualize endplate acetylcholine receptors and nerve terminals. The results showed that in each of these animal groups, reinnervation of MG muscles was incomplete. This is illustrated in Fig. 5 which shows the percentages of endplates occupied by nerve terminal staining (complete or partial) and unoccupied (denervated) for TTX- and saline-treated animals and animals in which saline cuffs were placed but no MG nerve crush was performed. At 2 months, the mean percentages for saline and TTX cuff + crush groups were nearly identical (P > 0.05) indicating that the presence or absence of muscle or nerve terminal activity played no role in determining the extent of reinnervation. The data also show that placement of a saline cuff with no MG nerve crush had little or no effect on MG innervation. This indicates that the cuff itself did not provoke damage to axons in the MG nerve. It thus seems likely that the MG nerve crush may have caused localized damage that eventually prevented some MG motor axons from reinnervating the MG muscle. Our results demonstrate, however, that incomplete reinnervation of the MG muscle did not prevent recovery of normal properties among those MG motoneurons that successfully reinnervated fibres in the MG muscle (see above). This indicates that the overall innervation status of the muscle plays no role in determining the ultimate recovery of the normal properties of individual motoneurons that successfully reinnervate muscle fibres.
Figure 5.
Reinnervation in cuffed and crushed animals is incomplete A, bar charts illustrate means for percentages of motor endplates visualized using fluorescently tagged α-bungarotoxin that are completely, partially or not stained for presynaptic motor terminal synaptic vesicles (synaptophysin – see Methods). Numbers of animals in each group indicated in figure key. When either TTX or saline cuffs were implanted on the posterior tibial nerve and the MG nerve was crushed, about 20–30% of MG endplates showed no motor terminal staining. Placement of a saline cuff in the absence of MG nerve crush had little effect on MG muscle innervation. B–D, examples of endplate innervation classification. Synaptic vesicles are shown immunolabelled for synaptophysin and ACh receptors are labelled by rhodamine conjugated α-bungarotoxin. Arrows in C2 show AChR rich endplate areas over which no presynaptic staining (C1) is evident. Innervated endplates show complete alignment while denervated endplate lack presynaptic staining.
We also examined two animals after 1 month of TTX application and found that the percentages of innervated and denervated endplates were nearly identical to those found after 2 months (Fig. 5). This suggests that most reinnervation of the MG was established by 1 month and did not change significantly thereafter. This is consistent with previous evidence that rat muscles become reinnervated by about 1 month following nerve crush near the muscle (McArdle & Albuquerque, 1973; Pasino et al. 1997; Leterme & Tyc, 2004).
Discussion
When axotomized but otherwise normal motoneurons regenerate, re-form synapses and re-activate muscle fibres, motoneuron properties eventually recover to normal levels (Kuno, 1976; Foehring et al. 1986a,b). In the present study, we examined whether muscle fibre activity as well as evoked release of ACh are required for this recovery by blocking axon conduction during regeneration and well after initial reinnervation occurs. Here we show that axotomized motoneurons that successfully reinnervate muscle fibres recover normal electrical properties despite the absence of evoked ACh release and muscle fibre activity. The synapses formed by regenerating motoneurons under these conditions are functional as shown by the ability of single-pulse intracellular stimulation of motoneurons to activate muscle fibres during the terminal experiments. Although we did not test whether re-formed synapses can sustain repetitive activation, the results indicate that the functional properties of these synapses are sufficient to mediate the recovery of motoneuron properties in the absence of action potential invasion of motor terminals, evoked release of ACh or muscle fibre activity.
Several possibilities exist for explaining how recovery of motoneuron properties occurred in the absence of muscle activity or evoked ACh release. As described in Methods, we waited for TTX conduction blockade to disappear before terminal experiments were performed so that MG motoneurons could be antidromically identified by nerve stimulation. Some MG muscle fibre activity probably occurred during this interval which, as described in Methods, comprised no more than 24 h before the terminal experiment. It is conceivable that this activity could have enabled the recovery of properties. However, placing the full responsibility of this recovery on the occurrence of muscle fibre activity in the 24 h interval before the terminal experiment would require that average motoneuron property values change from axotomized levels to normal levels in less than 1 day. Although we cannot exclude this possibility, it seems very unlikely based on several considerations. In the group of muscles in which the nerve was crushed 6 days before the terminal experiment, nerve-evoked EMG was detectible in 2/3 muscles studied, yet average motoneuron properties had not recovered from axotomy values. This shows that recovery of motoneuron properties does not occur immediately following regeneration of axons, recovery of synaptic transmission and re-activation of muscle. In this study, we obtained an estimate of 9–12 days for the time constant of recovery for average rheobase following initial reinnervation under normal conditions when regenerating motoneurons can activate muscle fibres during reinnervation. Thus, a full recovery from axotomized motoneuron property values mediated only by the reappearance of muscle activity over a 24 h interval immediately preceding the terminal experiment appears highly unlikely.
A second possible explanation for the recovery of motoneuron properties in the absence of muscle activity or evoked release involves activation of AChRs by spontaneous release of ACh from nerve terminals. Following AChR blockade, motoneuron properties exhibit axotomy-like changes which are as robust as those seen after axotomy itself and occur with a similar time course (Nakanishi et al. 2005). In order to explain this, we proposed that AChR blockade eliminates retrograde signalling that is normally activated by ACh binding with AChRs to maintain expression of normal motoneuron electrical properties. Since AChR blockade is capable of causing axotomy-like changes, recovery of motoneuron properties after reinnervation could depend on the resumption of AChR activation because this would presumably re-activate the putative signalling mechanisms and enable re-expression of normal motoneuron properties.
In the presence of a TTX blockade, however, evoked release of ACh is absent, and the only way that AChRs can be activated is by spontaneous release of ACh. The quantal version of spontaneous release is known to be present at regenerated but inactive motor terminals (Bray et al. 1979; Cangiano et al. 1980). It is not known whether non-quantal spontaneous release is present (Katz & Miledi, 1981), but if so, it would presumably add to any effects mediated by quantal spontaneous release. It thus appears that the best way to explain recovery of motoneuron properties in the present study is that spontaneous release of ACh reactivated AChRs and enabled re-expression of normal motoneuron properties. An extension of this idea is that muscle fibre activity played no role in the recovery of motoneuron properties that we observed and does not participate in retrograde signalling that enables expression of normal motoneuron properties.
The apparent ability of spontaneous ACh release to enable recovery of normal motoneuron properties during reinnervation adds to an increasing body of evidence that highlights the signalling capabilities and roles of spontaneous transmitter release (Fatt & Katz, 1952; McKinney et al. 1999; Frank et al. 2006; Sutton et al. 2006). An important signalling role of spontaneous release at the NMJ can help explain why several studies have observed that normal motoneurons do not exhibit axotomy-like property changes following TTX blockade of axonal conduction (Webb & Cope, 1992; Gardiner & Seburn, 1997).
The effectiveness of spontaneous release may, however, be related to the type of motoneuron involved. Previous studies in which no changes of motoneuron properties were observed after TTX blockade used motor nuclei composed primarily of fast motoneurons (as did the present study). In contrast (Czeh et al. 1978) observed changes of some electrical properties in soleus motoneurons (slow-type) following axonal conduction blockade. One factor that may be of importance in determining these apparent type-related differences is the balance between the total amount of ACh released at motor terminals as a result of evoked release versus spontaneous release. Available evidence shows that slow and fast motoneurons differ markedly in the total number of action potentials fired per day (Hennig & Lomo, 1985) and in the amount of ACh released per action potential (quantal content) but not in the rate of spontaneous ACh release (Reid et al. 1999). Calculations based on these data show that when considered over a 24 h interval, almost all AChR activation at the NMJs of normal slow motoneurons may be due to evoked ACh release while a substantial fraction of AChR activation at the NMJs of fast motoneurons is accomplished by spontaneous release (see Table 1). Blockade of axonal action potential conduction in normal slow motoneurons could produce a relatively large decrease in total AChR activation, while blockade of fast motoneurons would leave a substantial fraction of total AChR activation intact. Thus, at least in fast motoneurons, spontaneous ACh release following reinnervation in the absence of evoked activity could restore AChR signalling to efficacious levels. Interruption of evoked release at the NMJs of slow motoneurons would, however, introduce a major decrease in AChR-mediated signalling that might cause changes in slow motoneuron properties. This would imply that the level of ACh signalling may also be a factor in determining expression of motoneuron properties, but this is not known at present. It also remains possible that fast and slow motoneurons react differently to the loss of retrograde signals generated by AChR activation.
Table 1.
Estimates of total daily ACh release from motor terminals of fast and slow motoneurons*
| Motoneuron type | Action potentials per day** | Spontaneous release rate (Hz)*** | ACh quanta/action potential*** | Total daily ACh quanta | Percent by spontaneous release | Percent by evoked release |
|---|---|---|---|---|---|---|
| Slow | 500000 | 2 | 87 | 43845600 | 0.4 | 99.6 |
| Fast | 5000 | 2 | 100 | 845600 | 25.7 | 74.3 |
Note that this table does not take into account possible contributions from nonquantal spontaneous ACh release.
Data from Hennig & Lomo (1985).
Data from Reid et al. (1999).
Retrograde signalling
The results of this study provide further support for the idea that retrograde signalling based upon AChR activation is involved in enabling expression of adult motoneuron excitability properties. Other evidence suggests that such signalling may also modulate ACh release properties of motor terminals (Plomp et al. 1992, 1994) and play a role in determining the number or density of AChRs at the motor endplate (Akaaboune et al. 1999) and in synapse elimination at the NMJ (Balice-Gordon & Lichtman, 1994). Whether this evidence reflects the action of one or more signalling mechanisms is unknown. However, a common signalling mechanism enabling expression of both motorneuron properties and motor terminal ACh release properties could help explain the correlations that exist between motor terminal synaptic release properties and motoneuron type (Reid et al. 1999). It is conceivable that this AChR-based feedback system may serve to coordinate activities throughout the motor unit. The molecular identity of the responsible mechanisms awaits further investigation.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) (Grant NS24707). We wish to thank Drs Robert Burgess, Kevin Seburn and Pete Wenner for comments on earlier versions of this manuscript.
References
- Akaaboune M, Culican SM, Turney SG, Lichtman JW. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science. 1999;286:503–507. doi: 10.1126/science.286.5439.503. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- Bichler E, Rich MM, Carrasco DI, Pinter MJ. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Recovery of normal motoneuron properties following reinnervation in the absence of muscle activity. Program No. 396.18. [Google Scholar]
- Bray JJ, Hubbard JI, Mills RG. The trophic influence of tertrodotoxin-inactive nerves on normal and reinnervated rat skeletal muscles. J Physiol. 1979;297:479–491. doi: 10.1113/jphysiol.1979.sp013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busetto G, Buffelli M, Tognana E, Bellico F, Cangiano A. Hebbian mechanisms revealed by electrical stimulation at developing rat neuromuscular junctions. J Neurosci. 2000;20:685–695. doi: 10.1523/JNEUROSCI.20-02-00685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Soha JM, Van Essen DC. Competition favouring inactive over active motor neurons during synapse elimination. Nature. 1987;328:422–426. doi: 10.1038/328422a0. [DOI] [PubMed] [Google Scholar]
- Cangiano A, Lomo T, Lutzemberger L, Sveen O. Effects of chronic nerve conduction block on formation of neuromuscular junctions and junctional AChE in the rat. Acta Physiol Scand. 1980;109:283–296. doi: 10.1111/j.1748-1716.1980.tb06599.x. [DOI] [PubMed] [Google Scholar]
- Carrasco DI, Rich MM, Wang Q, Cope TC, Pinter MJ. Activity-driven synaptic and axonal degeneration in canine motor neuron disease. J Neurophysiol. 2004;92:1175–1181. doi: 10.1152/jn.00157.2004. [DOI] [PubMed] [Google Scholar]
- Costanzo EM, Barry JA, Ribchester RR. Competition at silent synapses in reinnervated skeletal muscle. Nat Neurosci. 2000;3:694–700. doi: 10.1038/76649. [DOI] [PubMed] [Google Scholar]
- Czeh G, Gallego R, Kudo N, Kuno M. Evidence for the maintenance of motoneurone properties by muscle activity. J Physiol. 1978;281:239–252. doi: 10.1113/jphysiol.1978.sp012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Foehring R, Sypert G, Munson J. Properties of self-reinnervated motor units of medial gastrocnemius of cat. I. Long-term reinnervation. J Neurophysiol. 1986a;55:931–946. doi: 10.1152/jn.1986.55.5.931. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J Neurophysiol. 1986b;55:947–965. doi: 10.1152/jn.1986.55.5.947. [DOI] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugleholm K, Schmalbruch H, Krarup C. Early peripheral nerve regeneration after crushing, sectioning, and freeze studied by implanted electrodes in the cat. J Neurosci. 1994;14:2659–2673. doi: 10.1523/JNEUROSCI.14-05-02659.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner PF, Seburn KL. The effects of tetrodotoxin-induced muscle paralysis on the physiological properties of muscle units and their innervating motoneurons in rat. J Physiol. 1997;499:207–216. doi: 10.1113/jphysiol.1997.sp021921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig R, Lomo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Jansen JKS, Lomo T, Nicolaysen K, Westgaard RH. Hyperinnervation of skeletal muscle fibers: Dependence on muscle activity. Science. 1973;181:559–561. doi: 10.1126/science.181.4099.559. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. Does the motor nerve impulse evoke ‘non-quantal’ transmitter release? Proc R Soc Lond B Biol Sci. 1981;212:131–137. doi: 10.1098/rspb.1981.0029. [DOI] [PubMed] [Google Scholar]
- Kuno M. Responses of spinal motor neurons to section and restoration of peripheral motor connections. Cold Spring Harbor Symposia on Quantitative Biology. 1976;XL:457–463. doi: 10.1101/sqb.1976.040.01.043. [DOI] [PubMed] [Google Scholar]
- Leterme D, Tyc F. Re-innervation and recovery of rat soleus muscle and motor unit function after nerve crush. Exp Physiol. 2004;89:353–361. doi: 10.1113/expphysiol.2004.027151. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Albuquerque EX. A study of the reinnervation of fast and slow mammalian muscles. J Gen Physiol. 1973;61:1–23. doi: 10.1085/jgp.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Munson JB, Foehring RC, Mendell LM, Gordon T. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. II. Motoneuron properties. J Neurophysiol. 1997;77:2605–2615. doi: 10.1152/jn.1997.77.5.2605. [DOI] [PubMed] [Google Scholar]
- Nakanishi ST, Cope TC, Rich MM, Carrasco DI, Pinter MJ. Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation. J Neurosci. 2005;25:2226–2232. doi: 10.1523/JNEUROSCI.5065-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Ribera AB. Synaptic activity modulates presynaptic excitability. Nat Neurosci. 2000;3:142–149. doi: 10.1038/72082. [DOI] [PubMed] [Google Scholar]
- Pasino E, Buffelli M, Arancio O, Busetto G, Salviati A, Cangiano A. Effects of long-term conduction block on membrane properties of reinnervated and normally innervated rat skeletal muscle. J Physiol. 1996;497:457–472. doi: 10.1113/jphysiol.1996.sp021780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasino E, Buffelli M, Busetto G, Cangiano A. Use of dexamethasone with TTX block of nerve conduction shows that muscle membrane properties are fully controlled by evoked activity. Brain Res. 1997;770:242–247. doi: 10.1016/s0006-8993(97)00881-0. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Pinter MJ, Vanden Noven S. Effects of preventing reinnervation on axotomized spinal motoneurons in the cat. I. Motoneuron electrical properties. J Neurophysiol. 1989;62:311–324. doi: 10.1152/jn.1989.62.2.311. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in α-bungarotoxin-treated rats. J Physiol. 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. The upregulation of acetylcholine release at endplates of α-bungarotoxin-treated rats: its dependency on calcium. J Physiol. 1994;478:125–136. doi: 10.1113/jphysiol.1994.sp020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R, Slater CR, Bewick GS. Synaptic vesicle dynamics in rat fast and slow motor nerve terminals. J Neuroscience. 1999;19:2511–2521. doi: 10.1523/JNEUROSCI.19-07-02511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester RR, Taxt T. Motor unit size and synaptic competition in rat lumbrical muscles reinnervated by active and inactive motor axons. J Physiol. 1983;344:89–111. doi: 10.1113/jphysiol.1983.sp014926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Lichtman JW. In vivo visualization of pre- and postsynaptic changes during synapse elimination in reinnervated mouse muscle. J Neurosci. 1989;9:1781–1805. doi: 10.1523/JNEUROSCI.09-05-01781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007;30:119–125. doi: 10.1016/j.tins.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Thompson W, Kuffler DP, Jansen JK. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4:271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Webb CB, Cope TC. Modulation of Ia EPSP amplitude: The effects of chronic synaptic inactivity. J Neurosci. 1992;21:338–344. doi: 10.1523/JNEUROSCI.12-01-00338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]