Abstract
The influence of the menstrual cycle on resting muscle sympathetic nerve activity (MSNA) remains controversial, and the effect of the menstrual cycle on MSNA responses to mental stress is unknown. We examined MSNA, mean arterial pressure (MAP), and heart rate (HR) responses to mental stress (via mental arithmetic) in 11 healthy females during the early follicular (EF) and mid-luteal (ML) phases of the menstrual cycle. The menstrual cycle did not alter resting MSNA (EF, 13 ± 3 bursts min−1 versus ML, 13 ± 2 bursts min−1), MAP (EF, 79 ± 3 mmHg versus ML, 81 ± 2 mmHg) and HR (EF, 66 ± 3 beats min−1 versus ML, 64 ± 2 beats min−1). 5 min of mental stress increased MSNA, MAP and HR during both the EF (Δ4 ± 2 bursts min−1, Δ12 ± 2 mmHg, Δ18 ± 2 beats min−1; P < 0.05) and ML (Δ4 ± 2 bursts min−1, Δ13 ± 3 mmHg and Δ20 ± 2 beats min−1; P < 0.05) phases. These responses were not different between phases. In contrast, MSNA responses were different between phases during the 10 min recovery from mental stress. MSNA remained elevated during the initial 5 min of recovery in both the EF (Δ6 ± 1 bursts min−1; P < 0.01) and ML (Δ7 ± 1 bursts min−1; P < 0.01) phases, but only remained elevated during the ML phase (Δ6 ± 1 bursts min−1; P < 0.01) during the final 5 min of recovery. Our results demonstrate that MSNA, MAP and HR responses at rest or during mental stress are not different during the EF and ML phases of the menstrual cycle in young, healthy females. However, MSNA activation during recovery from mental stress is prolonged during the ML phase compared to the EF phase.
Premenopausal women have a reduced risk for cardiovascular disease compared to age-matched men, a benefit that is often attributed to oestrogen. The potential mechanisms underlying the cardioprotective effects of oestrogen remain controversial, particularly whether oestrogens inhibit sympathetic nerve activity. Weitz et al. (2001) and Vongpatanasin et al. (2001) reported that oestrogen replacement therapy decreases muscle sympathetic nerve activity (MSNA) at rest in postmenopausal women, while others report that oestrogen replacement therapy does not change resting MSNA (Hunt et al. 2001; Vongpatanasin et al. 2001; Moreau et al. 2003). Some of these inconsistencies can be explained by the type of oestrogen used or the mode of delivery (Vongpatanasin et al. 2001). Others investigating MSNA during different hormonal phases of the menstrual cycle have also reported discrepancies. Ettinger et al. (1998) demonstrated no change in resting MSNA during high and low oestrogen phases of the menstrual cycle, while Minson et al. (2000) reported an increase in resting MSNA during the mid-luteal (ML) phase of the menstrual cycle compared to the early follicular (EF) phase. The findings of Minson et al. (2000) have prompted many investigators who examine MSNA to strictly control for the menstrual phase in their female subjects, yet these findings have not been confirmed.
In contrast to the numerous studies that have investigated the influence of oestrogen replacement therapy or the menstrual cycle on resting MSNA, no studies have examined the influence of oestrogens on MSNA responses to mental stress. This lack of information is surprising because mental stress has been linked to several cardiovascular diseases, including myocardial ischaemia (Deanfield et al. 1984; Rozanski et al. 1988), hypertension (Esler et al. 2003), and atherosclerosis (Yeung et al. 1991; Rozanski et al. 1999). Our laboratory has recently suggested that the increases in sympathetic neural activity during recovery from mental stress may precipitate cardiovascular problems associated with mental stress (Carter et al. 2005). The consistent and robust increases of MSNA after mental stress are mediated by the baroreflex (Anderson et al. 1991; Carter et al. 2005), thus factors that alter baroreflex function may potentially alter postmental stress MSNA responses. Minson et al. (2000) has reported an increase in baroreflex sensitivity during the ML phase of the menstrual cycle compared to the EF phase, yet the effects of the menstrual cycle on MSNA responses to mental stress have not been examined.
Based on previous findings that sympathetic arterial baroreflex sensitivity is increased during the ML phase of the menstrual cycle (Minson et al. 2000), we hypothesize that postmental stress MSNA will be augmented during the ML phase of the menstrual cycle. The primary goal of this study was to examine MSNA, arterial pressure, and heart rate responses to mental stress during the EF and ML phases of the menstrual cycle. A secondary goal was to re-examine resting MSNA responses during EF and ML phases.
Methods
Subjects
Eleven healthy women (age 24.9 ± 1.1 years, height 164.3 ± 2.4 cm, weight 62.3 ± 2.0 kg, body mass index 23.2 ± 0.4 kg m−2; means ± s.e.m.) participated in the study. All subjects were non-smokers and non-diabetics, and were not taking oral contraceptives and/or other hormonal supplementations. They were all instructed to withhold from exercise for 24 h and caffeine for 12 h prior to laboratory testing. The experimental protocol was approved by the Human Subjects Committee of Michigan Technological University and all subjects gave written informed consent prior to the study. This study conformed to the provisions of the Declaration of Helsinki.
Experimental design
We examined MSNA, mean arterial blood pressure (MAP), and heart rate (HR) responses in healthy females before, during, and after mental stress. Only subjects with regular and consistent menstrual cycles were included (i.e. 26–30 days in length). Subjects were tested once during the EF phase (3.2 ± 0.4 days after start of menstruation) and again during the ML phase (22.0 ± 0.6 days after start of menstruation). The phase of the menstrual cycle was randomly selected for the initial testing day. ML phase was determined by waiting 8–10 days after the detection of the leuteinizing hormone surge by an ovulation prediction kit (OvuQuick, Quidel Corp., San Deigo, CA, USA).
On the days of testing, the subjects reported for a blood draw to document levels of oestrogen and progesterone. Following the blood draw, a lab technician recorded subject demographics and anthropometrics and prepared the subject for the study. The experiment was conducted with subjects lying prone with the face comfortably resting in a massage head piece. Each trial consisted of a 5 min baseline, 5 min of mental stress (induced by mental arithmetic), and a 10 min recovery.
During mental arithmetic, subjects continuously subtracted the number 6 or 7 from a two- or three-digit number. The subtraction number (6 or 7) was randomized for the two trials. Subjects answered verbally and were encouraged by an investigator to subtract as quickly as possible. An investigator provided a new number from which to subtract every 5–10 s. Subjects were asked to rate perceived stress using the following standard five-point scale: 0, not stressful; 1, somewhat stressful; 2, stressful; 3, very stressful; and 4, very, very stressful.
Measurements
Multifibre recordings of MSNA were directly measured via a tungsten microelectrode (Frederick Haer and Co., Bowdoinham, ME, USA) inserted into the peroneal nerve of the popliteal region behind the knee. A reference electrode was inserted 2–3 cm from the recording electrode. Both electrodes were attached to a differential preamplifier, then to an amplifier (80 000 gain). The nerve signal was band-pass filtered (700–2000 Hz) and integrated at a time constant of 0.1 s to obtain a mean voltage display of nerve activity. Satisfactory MSNA signals were determined if: (1) tapping of muscle and/or tendons evoked slight afferent mechanoreceptor discharges, but rubbing the skin did not elicit afferent activity, and (2) a loud noise did not arouse stimulus in sympathetic activity. Beat-to-beat arterial blood pressure was measured continuously via Finometer (Finapres Medical systems, Amsterdam, the Netherlands) at 500 Hz. Resting values of arterial blood pressure were taken three times (separated by one min) by an automated sphygmomanometer (Omron HEM-907XL, Omron Health Care, Inc., Vernon Hills, IL, USA) and the average of the three readings was calculated. Heart rate was recorded using a three-lead electrocardiogram. We were unsuccessful reproducing MSNA recordings over multiple days for one subject and we obtained a poor arterial blood pressure recording from the Finometer in one subject, and so we report a total of 10 subjects for MSNA and MAP. Additionally, blood analysis was not performed in two subjects, and so our oestradiol and progesterone data consist of nine subjects.
Data analysis
Data were imported and analysed in a commercially available software program (WinCPRS, Absolute Aliens, Turku, Finland). R-waves were detected and marked in the time series. Muscle sympathetic bursts were automatically detected on the basis of amplitude using a signal-to-noise ratio of 3 : 1, within 0.5 s search window centred on the 1.3 s expected burst peak latency from the previous R-wave. Potential bursts were displayed and edited by one investigator. The integral of all sympathetic bursts occurring during the initial 5 min baseline period was calculated and divided by the number of bursts to derive an average control burst area that could be compared across time. Subsequent bursts that were equal to average bursts occurring during baseline were assigned a value of 100.0. Muscle sympathetic nerve activity was expressed as bursts per minute and total activity (total activity = total number of bursts multiplied by the averaged normalized burst area). We observed a slight shift in the MSNA recording during the transition from mental stress to recovery in two of our subjects. This slight shift did not affect our burst frequency data, but did make it inappropriate to examine total MSNA activity during the recovery period in those two subjects. Therefore, our total MSNA data represents eight subjects.
Statistical analysis
All data were analysed statistically using commercial software (SPSS 15.0, SPSS Inc., Chicago, Illinois). A two-way repeated measures ANOVA was used to compare MSNA (burst frequency and total activity), MAP and heart rate during the protocol interventions (mental stress and recovery) and across trials (menstrual cycle phases). Protected dependent t tests were performed for post hoc analyses. Resting variables were compared using paired t tests. Means were considered significantly different when P < 0.05 and results are expressed as means ± s.e.m.
Results
Resting values
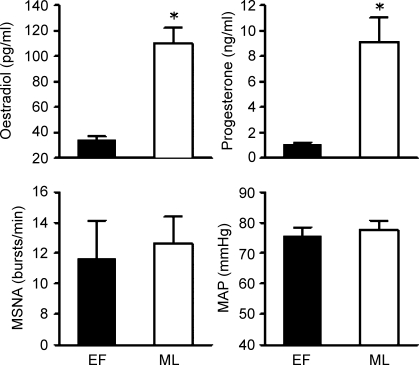
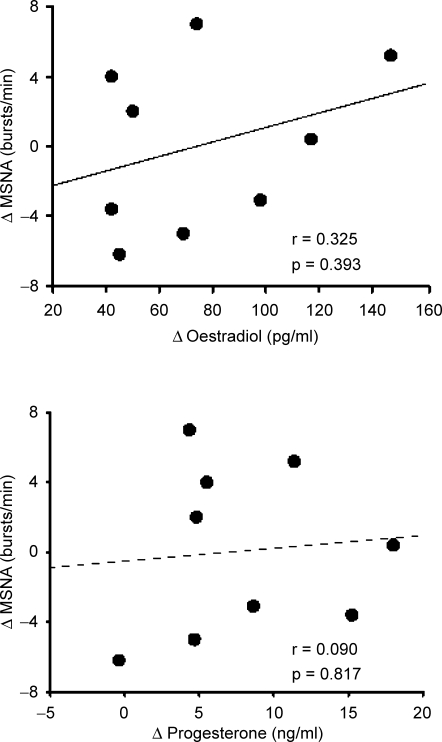
Resting levels of plasma oestradiol (110 ± 12 pg ml−1 verses 34 ± 3 pg ml−1; P < 0.001) and progesterone (9.1 ± 1.9 ng ml−1 verses 1.1 ± 0.l ng ml−1; P < 0.001; Fig. 1) were significantly elevated during the ML phase compared to the EF phase. Resting MSNA (EF, 13 ± 3 bursts min−1 versus ML, 13 ± 2 bursts min−1; n = 10), MAP (EF, 79 ± 3 mmHg versus ML, 81 ± 2 mmHg; n = 10) and HR (EF, 66 ± 3 beats min−1 versus ML, 64 ± 2 beats min−1; n = 11) were not different during high and low oestrogen levels. Changes in resting MSNA between the ML and EF phases were not correlated to changes in plasma oestradiol (r = 0.325, P = 0.393) or progesterone (r = 0.090, P = 0.817; Fig. 2).
Figure 1. Oestradiol, progesterone, muscle sympathetic nerve activity (MSNA), and mean arterial pressure (MAP) at rest during the early follicular (EF) and mid-luteal (ML) phases of the menstrual cycle.
Data are reported as means + s.e.m. *P < 0.05 versus EF phase.
Figure 2. Plots of the difference (mid-luteal minus early follicular phase) in resting muscle sympathetic nerve activity (MSNA) versus the difference in endogenous oestradiol (upper panel) or progesterone (lower panel).
Changes in endogenous sex hormones were not correlated to changes in resting MSNA.
Mental stress
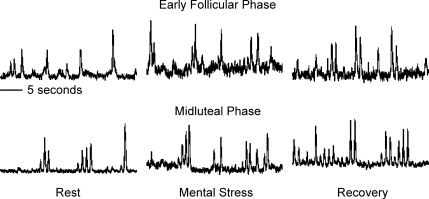
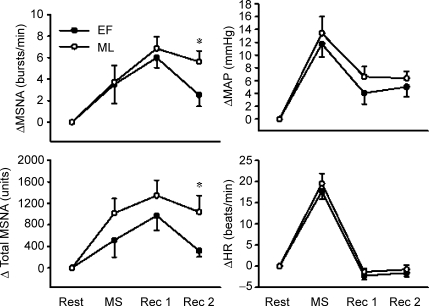
Figure 3 illustrates original recordings of MSNA responses to mental stress during the EF and ML phases of the menstrual cycle. Mental stress increased MSNA during both the EF (Δ4 ± 2 bursts min−1 and Δ516 ± 313 units; P < 0.05) and ML (Δ4 ± 2 bursts min−1 and 1017 ± 271 units; P < 0.05; Fig. 4) phases, but these responses were not different from one another. Mental stress also increased MAP and HR during both the EF (Δ12 ± 2 mmHg and Δ18 ± 2 beats min−1; P < 0.001) and ML (Δ13 ± 3 mmHg and Δ20 ± 2 beats min−1; P < 0.001; Fig. 4) phases, but responses were not different between phases. Perceived stress levels were not different between phases (EF, 3.1 ± 0.3 units. verses ML, 3.0 ± 0.2 units).
Figure 3. Representative neurograms from one subject obtained during rest, mental stress and recovery of the early follicular and mid-luteal phases of the menstrual cycle.
Figure 4. Change in muscle sympathetic nerve activity (MSNA), mean arterial pressure (MAP) and heart rate (HR) during mental stress (MS), the first 5 min of recovery (Rec 1), and the final 5 min of recovery (Rec 2).
Subjects were randomly studied during both the early follicular (EF) and mid-luteal (ML) phases of the menstrual cycle. Data are reported as means ± s.e.m. *P < 0.05 versus corresponding EF phase.
Recovery responses
MSNA remained elevated during the initial 5 min of recovery in both the EF (Δ6 ± 1 bursts min−1; P < 0.01) and ML (Δ7 ± 1 bursts min−1; P < 0.01) phases, but only remained elevated during the ML phase (Δ6 ± 1 bursts min−1; P < 0.01; Fig. 4) during the final 5 min of recovery. Although different MSNA responses were observed during the EF and ML phases, no phase differences were detected in the MAP and HR recovery responses. Mean arterial pressure remained elevated throughout the recovery in both the EF and ML phases, while HR returned to baseline during recovery in both phases (Fig. 4).
Discussion
The results of this study reveal three important and novel findings. First, resting MSNA is similar during the EF and ML phases of the menstrual cycle, a finding that is in conflict with a previous report (Minson et al. 2000). Second, mental stress increases MSNA, MAP and HR similarly during either the EF or ML phase of the menstrual cycle. Third, MSNA activation during recovery from mental stress is prolonged during the ML phase compared to the EF phase. The prolonged MSNA recovery responses during the ML phase may have cardiovascular implications for women.
Only two other studies have examined resting MSNA during different phases of the menstrual cycle (Ettinger et al. 1998; Minson et al. 2000). Ettinger et al. (1998) reported no change in resting MSNA between the EF (low oestrogen and low progesterone) and follicular phase (high oestrogen and low progesterone) of the menstrual cycle. More recently, Minson et al. (2000) reported significantly higher levels of resting MSNA during the ML phase (high oestrogen and high progesterone) compared to the EF phase. Our results conflict with the findings of Minson et al. (2000). We observed no change in resting MSNA in 10 healthy females during the EF and ML phases of the menstrual cycle.
In the current study, we only report resting MSNA as burst frequency, while Minson et al. (2000) reported resting MSNA as burst frequency and total activity. The reason for this difference in reporting is due to a difference in the normalization procedures used to analyse MSNA. Minson et al. (2000) normalized MSNA by assigning the largest sympathetic burst under resting conditions an amplitude of 1000, and all other bursts were calibrated against this value. In the current study, the integral of all sympathetic bursts occurring during the initial 5 min baseline period was calculated and divided by the number of bursts to derive an average control burst area that could be compared across time. Subsequent bursts that were equal to average bursts occurring during baseline were assigned a value of 100. Both normalization procedures are well-documented and accepted throughout the literature, but our normalization period does not allow for a comparison of total MSNA activity baselines. The normalization procedure utilized by Minson et al. (2000) allowed for a comparison of total activity, but this procedure is debatable. We would argue that it is inappropriate to compare total activity at baseline regardless of the normalization procedure as the quality of signals and location of electrodes can be highly variable, and others support this view (Grassi & Esler, 1999; Kimmerly & Shoemaker, 2003; Vallbo et al. 2004). Total MSNA activity is a powerful indicator of sympathetic neural traffic, but it is best utilized when changes in total burst activity from a normalized baseline are compared.
Regardless of whether baseline total MSNA activity is a valid comparison, Minson et al. (2000) reported a significant increase in resting MSNA burst frequency during the ML phase, whereas we observed no change in resting MSNA burst frequency between the EF and ML phases of the menstrual cycle. Taken together, the findings of Ettinger et al. (1998) and Minson et al. (2000) imply that progesterone may be responsible for altered resting MSNA, yet our increase in progesterone during the ML phase was similar to that observed by Minson et al. (2000), suggesting that differences between our study and Minson et al. (2000) are not related to progesterone. Furthermore, the oestradiol levels were similar between our study and Minson et al. (2000). Therefore, differences in estadiol and progesterone levels do not appear to explain the discrepancy between our study and Minson et al. (2000).
The menstrual cycle is dynamic and includes complex fluctuations in other hormones, temperature, and circadian rhythm. Both the current study and the study by Minson et al. (2000) are limited to two discrete time points in the menstrual cycle. These time points were selected to maximize the differences in oestrogen and progesterone. We recognize that the intensity and magnitude of the oestrogen and progesterone fluctuations could potentially alter resting MSNA, but this could only be examined if repeated blood samples were taken multiple times throughout the menstrual cycle. Furthermore, our results demonstrate changes in resting MSNA during the EF and ML phases of the menstrual cycle were not correlated to changes in oestradiol or progesterone. Thus, it appears that resting MSNA is modulated by more than just relative changes in oestradiol and progesterone levels. Finally, it should be noted that the current study is not the only investigation to conflict with the findings of Minson et al. (2000). Hirshoren et al. (2002) reported no change in plasma noradrenaline between the EF and ML phases, while Minson et al. (2000) reported an increase in plasma noradrenaline during the ML phase when compared to the EF phase. It is clear that the influence of the menstrual cycle on resting sympathetic activity remains controversial. To date, no study has simultaneously measured both resting MSNA and plasma noradrenaline during the EF, late follicular and ML phases of the menstrual cycle.
Mental stress consistently increases heart rate and arterial blood pressure, but peripheral sympathetic neural responses remain equivocal. Most studies report an increase in leg MSNA during mental stress (Anderson et al. 1987, 1991; Callister et al. 1992; Ng et al. 1993; Middlekauff & Hui, 2001; Carter et al. 2002), but some report no change (Wasmund et al. 2002; Carter et al. 2005) or a decrease (Delius et al. 1972; Matsukawa et al. 1991) in MSNA during mental stress. Others report that mental stress can result in either an increase or no change in MSNA depending on whether it is reported as burst frequency or amplitude (Hjemdahl et al. 1989; Wallin et al. 1992; Kamiya et al. 2000). It is at present unclear why some individuals have a neural response to mental stress and others do not. Callister et al. (1992) has suggested it is related to the levels of perceived stress, demonstrating an increase in MSNA as the perception of stress increases. However, another potential explanation for the variability in MSNA responses to mental stress could be that women have altered neural responses to mental stress during different phases of the menstrual cycle. Many studies examining leg MSNA responses to mental stress have included both male and female subjects (Callister et al. 1992; Ng et al. 1993; Carter et al. 2002, 2005; Wasmund et al. 2002).
Our subjects reported similar levels of perceived stress and significant increases in MSNA during mental stress during both the EF and ML phases of the menstrual cycle. These increases in MSNA during the EF and ML phases were not different from one another. Likewise, MAP and HR responses were similar between phases. Thus, it appears that women have similar physiological responses to mental stress during high and low oestrogen/progesterone levels, at least with regard to MSNA, arterial pressure, and heart rate. To our knowledge, this is the first study to examine MSNA responses to mental stress during different phases of the menstrual cycle. Previous studies examining catecholamine responses to mental stress during different phases of the menstrual cycle (Mills et al. 1996; Komesaroff et al. 1999) or during oestrogen supplementation (Komesaroff et al. 1999) report alterations in sympathetic outflow during mental stress. Mills et al. (1996) reported an augmented catecholamine response to mental stress during the follicular phase of the menstrual cycle in black, but not white, women. Komesaroff et al. (1999) reported attenuated catecholamine responses to mental stress in premenopausal women during oestrogen supplementation.
In contrast to the similar responses observed during mental stress, postmental stress MSNA responses were different during the EF and ML phases. We observed a prolonged elevation of MSNA during the ML recovery compared to the EF recovery. Specifically, MSNA was significantly elevated compared to baseline during the entire 10 min of recovery during the ML phase, but was only elevated during the initial 5 min of the EF recovery and returned to resting baseline values during the final 5 min of the EF recovery. The increase in MSNA after mental stress is mediated, in part, by the arterial baroreflex (Anderson et al. 1991; Carter et al. 2005). Therefore, factors that influence the arterial baroreflex may be capable of modulating the postmental stress increase in MSNA. One factor that has been reported to influence the arterial baroreflex is the menstrual cycle. Minson et al. (2000) reported an increased sensitivity of the sympathetic arterial baroreflex during the ML phase of the menstrual cycle. An increased sensitivity of the sympathetic arterial baroreflex results in a more rapid and potent augmentation of MSNA outflow when arterial blood pressure drops. Mental stress causes robust increases in arterial blood pressure, but cessation of the mental stressor leads to prompt decreases in arterial blood pressure. We hypothesized that the increased sympathetic baroreflex sensitivity observed during the ML phase of the menstrual cycle could lead to either a more pronounced or prolonged increase of MSNA during the ML mental stress recovery. Our data demonstrate a prolonged increase in MSNA during the ML mental stress recovery. We cannot determine weather oestrogen or progesterone were responsible for this prolonged MSNA response during mental stress recovery because we only tested during low oestrogen/low progesterone and high oestrogen/high progesterone.
A prolonged increase of MSNA during the ML recovery phase of the menstrual cycle may have clinical significance. The prolonged increased in sympathetic neural traffic during the ML recovery phase occurs at a time when heart rates are low. Some studies have even reported a decrease in resting heart rate after mental stress (Callister et al. 1992). Myocardial ischaemia often occurs at low heart rates (Schang & Pepine, 1977; Deanfield et al. 1983) and sympathoexcitation is a well-recognized trigger for myocardial ischaemia (Rozanski et al. 1999). We recognize that our data are limited to young, healthy females, a population at low risk for serious cardiac events. However, it is possible that other populations of premenopausal females (i.e. older and/or overweight) may also experience this prolonged sympathoexcitation during the ML recovery phase and may be at higher risk for sudden cardiac death or myocardial infarction. Future studies examining the influence of menstrual cycle on sympathetic neural responses to mental stress appear warranted in at risk populations.
In summary, this study demonstrates that resting MSNA and mental stress-induced increases of MSNA are not different during the EF and ML phases of the menstrual cycle. However, the MSNA activation during recovery from mental stress is prolonged during the ML phase compared to the EF phase of the menstrual cycle. This prolonged increase in MSNA after mental stress is likely due to an increased sympathetic baroreflex sensitivity during the ML phase (Minson et al. 2000). A prolonged increase in postmental stress MSNA during the ML phase of the menstrual cycle may have important cardiovascular implications for premenopausal women.
Acknowledgments
The authors thank Angela Lucas, John Durocher, and Alexandra Tepkasetkul for their technical assistance. This project was supported by a grant from the Michigan Tech Research Excellence Fund (REF-060605; JR Carter).
References
- Anderson EA, Sinkey CA, Mark AL. Mental stress increases sympathetic nerve activity during sustained baroreceptor stimulation in humans. Hypertension. 1991;17:III43–49. doi: 10.1161/01.hyp.17.4_suppl.iii43. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension. 1987;9:III114–119. doi: 10.1161/01.hyp.9.6_pt_2.iii114. [DOI] [PubMed] [Google Scholar]
- Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol. 1992;454:373–387. doi: 10.1113/jphysiol.1992.sp019269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol. 2005;564:321–327. doi: 10.1113/jphysiol.2004.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Ray CA, Cooke WH. Vestibulosympathetic reflex during mental stress. J Appl Physiol. 2002;93:1260–1264. doi: 10.1152/japplphysiol.00331.2002. [DOI] [PubMed] [Google Scholar]
- Deanfield JE, Maseri A, Selwyn AP, Ribeiro P, Chierchia S, Krikler S, Morgan M. Myocardial ischaemia during daily life in patients with stable angina: its relation to symptoms and heart rate changes. Lancet. 1983;2:753–758.. doi: 10.1016/s0140-6736(83)92295-x. [DOI] [PubMed] [Google Scholar]
- Deanfield JE, Shea M, Kensett M, Horlock P, Wilson RA, de Landsheere CM, Selwyn AP. Silent myocardial ischaemia due to mental stress. Lancet. 1984;2:1001–1005. doi: 10.1016/s0140-6736(84)91106-1. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972;84:82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275–284. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol. 1998;85:2075–2081. doi: 10.1152/jappl.1998.85.6.2075. [DOI] [PubMed] [Google Scholar]
- Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, Jacob G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab. 2002;87:1569–1575. doi: 10.1210/jcem.87.4.8406. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P, Fagius J, Freyschuss U, Wallin BG, Daleskog M, Bohlin G, Perski A. Muscle sympathetic nerve activity and norepinephrine release during mental challenge in humans. Am J Physiol Endocrinol Metab. 1989;257:E654–E664. doi: 10.1152/ajpendo.1989.257.5.E654. [DOI] [PubMed] [Google Scholar]
- Hunt BE, Taylor JA, Hamner JW, Gagnon M, Lipsitz LA. Estrogen replacement therapy improves baroreflex regulation of vascular sympathetic outflow in postmenopausal women. Circulation. 2001;103:2909–2914. doi: 10.1161/01.cir.103.24.2909. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Iwase S, Michikami D, Fu Q, Mano T. Head-down bed rest alters sympathetic and cardiovascular responses to mental stress. Am J Physiol Regul Integr Comp Physiol. 2000;279:R440–R447. doi: 10.1152/ajpregu.2000.279.2.R440. [DOI] [PubMed] [Google Scholar]
- Kimmerly DS, Shoemaker JK. Hypovolemia and MSNA discharge patterns: assessing and interpreting sympathetic responses. Am J Physiol Heart Circ Physiol. 2003;284:H1198–H1204. doi: 10.1152/ajpheart.00229.2002. [DOI] [PubMed] [Google Scholar]
- Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab. 1999;84:606–610. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Uneda S, Miyajima E, Shionoiri H, Tochikubo O, Ishii M. Augmented sympathetic nerve activity in response to stressors in young borderline hypertensive men. Acta Physiol Scand. 1991;141:157–165. doi: 10.1111/j.1748-1716.1991.tb09064.x. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Yu JL, Hui K. Acupuncture effects on reflex responses to mental stress in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1462–R1468. doi: 10.1152/ajpregu.2001.280.5.R1462. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Nelesen RA, Ziegler MG, Parry BL, Berry CC, Dillon E, Dimsdale JE. Menstrual cycle effects on catecholamine and cardiovascular responses to acute stress in black but not white normotensive women. Hypertension. 1996;27:962–967. doi: 10.1161/01.hyp.27.4.962. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol. 2003;547:309–316. doi: 10.1113/jphysiol.2002.032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton-Chalfen S, Hestrin L, Bietendorf J, Berman DS. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Schang SJ, Jr, Pepine CJ. Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol. 1977;39:396–402. doi: 10.1016/s0002-9149(77)80095-7. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103:2903–2908. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmund WL, Westerholm EC, Watenpaugh DE, Wasmund SL, Smith ML. Interactive effects of mental and physical stress on cardiovascular control. J Appl Physiol. 2002;92:1828–1834. doi: 10.1152/japplphysiol.00019.2001. [DOI] [PubMed] [Google Scholar]
- Weitz G, Elam M, Born J, Fehm HL, Dodt C. Postmenopausal estrogen administration suppresses muscle sympathetic nerve activity. J Clin Endocrinol Metab. 2001;86:344–348. doi: 10.1210/jcem.86.1.7138. [DOI] [PubMed] [Google Scholar]
- Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]