Abstract
The tolerance of breathing in neonates to oxygen depletion is reflected by persistence of inspiratory-related motor output during sustained anoxia in newborn rat brainstem preparations. It is not known whether lumbar motor networks innervating expiratory abdominal muscles are, in contrast, inhibited by anoxia similar to locomotor networks in neonatal mouse lumbar cords. To test this, we recorded inspiratory-related cervical/hypoglossal plus pre/postinspiratory lumbar/facial nerve activities and, sometimes simultaneously, locomotor rhythms in newborn rat brainstem–spinal cords. Chemical anoxia slowed 1 : 1-coupled cervical and lumbar respiratory rhythms and induced cervical burst doublets associated with depressed preinspiratory and augmented postinspiratory lumbar activities. Similarly, anoxia evoked repetitive hypoglossal bursts and shifted facial activity toward augmented postinspiratory bursting in medullas without spinal cord. Selective lumbar anoxia augmented pre/postinspiratory lumbar bursting without slowing the rhythm. This suggests a medullary origin of both anoxic inspiratory double bursts and preinspiratory depression, but a mixed medullary/lumbar origin of boosted postinspiratory lumbar activity. Lumbar respiratory rhythm is likely to be generated by the parafacial respiratory group expiratory centre as indicated by lack of normoxic and anoxic bursting following brainstem transection between the facial motonucleus and the more caudal pre-Bötzinger complex inspiratory centre. Opposed to sustained respiratory activities, anoxia reversibly abolished non-rhythmic spinal discharges and electrically or chemically evoked lumbar locomotor activities, followed by pronounced postanoxic spinal hyperexcitability. We hypothesize that (i) the anoxia tolerance of neonatal breathing includes pFRG-driven lumbar expiratory networks, (ii) the anoxic respiratory pattern transformation is due to disturbed inspiratory–expiratory centre interactions, and (iii) postanoxic lumbar hyperexcitability contributes to spasticity in cerebral palsy.
In neonatal mammals, breathing movements continue at a reduced rate for extended time periods during severe hypoxia or asphyxia leading to tissue anoxia (Fazekas et al. 1941; Selle & Witte, 1941; Gozal et al. 1996). In line with these findings, inspiratory-related motor rhythms of cranial and cervical spinal nerves slow substantially, but persist during anoxia periods of several tens of minutes in isolated brainstem–spinal cord preparations from newborn rats (Ballanyi et al. 1992, 1994; Kato et al. 2000; Cayetanot et al. 2001). This suggests that medullary neuronal respiratory networks driving respiratory muscles have a high tolerance to anoxia in newborns (Ballanyi et al. 1999; Ballanyi, 2004a,b; Ramirez et al. 2007). Inspiratory motor activity appears to ultimately originate from rhythmogenic interneurons of the pre-Bötzinger Complex (preBötC), a limited region of the ventral respiratory column within the lower brainstem (Smith et al. 1991) (Fig. 1). Findings from the above in vitro studies and more recent work on more mature mammals (Solomon, 2005; Paton et al. 2006) suggest that the preBötC also generates the drive for inspiratory motor activity during hypoxia/anoxia. The high tolerance to anoxia of the preBötC in neonates is, presumably, due primarily to oxygen-dependent stimulation and effective utilization of anaerobic metabolism (Ballanyi et al. 1992, 1996; Ballanyi, 2004a,b). Reduction of energy consumption by ‘functional inactivation’ of several classes of ventral respiratory column neurons appears to contribute to this tolerance to anoxia (Ballanyi et al. 1994, 1999; Ballanyi, 2004a,b). It is not known how anoxia affects neonatal motor networks in the first and second lumbar cord segment that control expiratory abdominal muscles (Monteau & Hilaire, 1991; Iscoe, 1998). Synaptic drive to these lumbar motoneurons appears to originate from premotoneurons that are activated by pre/postinspiratory active medullary neurons (Janczewski et al. 2002). These rhythmogenic interneurons comprise the parafacial respiratory group (pFRG), which is located several hundred micrometres rostral to the preBötC in newborn rats (Onimaru & Homma, 2003) (Fig. 1). Anoxia depresses the rhythmic activity of a major subpopulation of these cells (Ballanyi et al. 1994, 1999; Ballanyi, 2004a,b). Therefore, anoxia may impair lumbar expiratory motoneurons indirectly due to a reduced drive from the pFRG. However, the lumbar spinal cord may also be intrinsically more susceptible to oxygen depletion as hypoxia inhibits fictive locomotion in isolated newborn mouse lumbar spinal cords (Wilson et al. 2003).
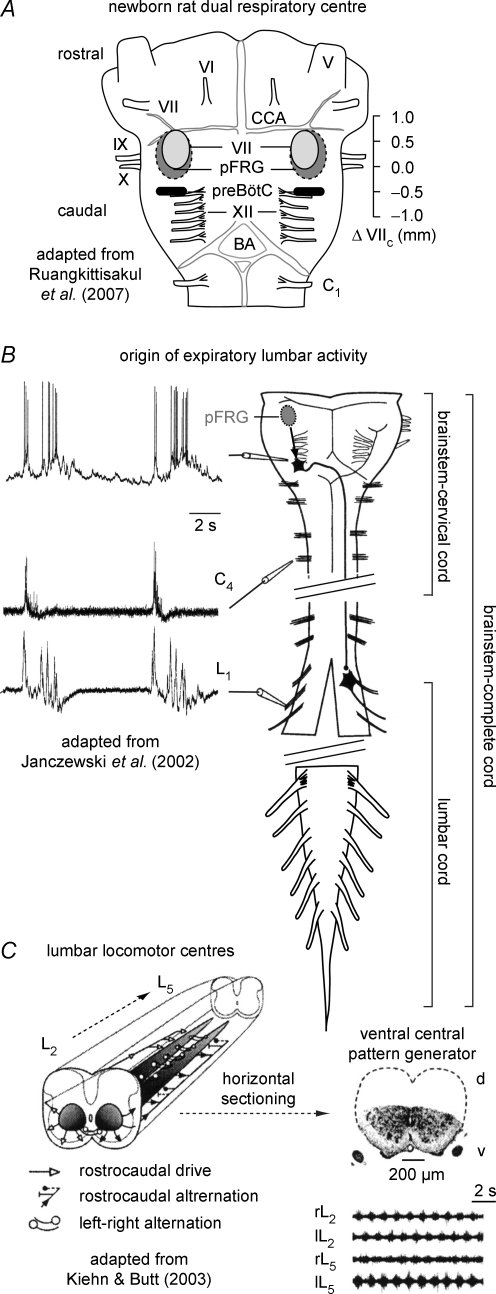
Figure 1. Motor networks in isolated newborn rodent brainstems and spinal cords.
A, schematic ventral view (adapted from Ruangkittisakul et al. 2007) on isolated newborn rat brainstem with the proposed locations of the hypothetical ‘dual respiratory centre’, comprised of the inspiratory pre-Bötzinger complex (preBötC) and the expiratory parafacial respiratory group (pFRG) (Mellen et al. 2003; Feldman & Del Negro, 2006). The presumptive rostrocaudal extensions of the preBötC and pFRG are referred to their distance in millimetres from the caudal end of the facial motor nucleus (VIIc) (Smith et al. 1991; Onimaru et al. 2006; Ruangkittisakul et al. 2006). The latter and related studies (Smith et al. 1991; Ballanyi et al. 1999) revealed that ventral cervical (e.g. C3–5) or cranial (e.g. hypoglossus (XII), vagus (X), or glossopharyngeus (IX)) nerves contain axons from motoneurons providing preBötC-driven inspiratory motor output, while pFRG-driven nerves (VII and lumbar (L), see B) show pre/postinspiratory motor output. Other abbreviations V, trigeminal nerve; VI, abducens nerve; CCA, caudal cerebellar artery, BA, basilar artery. B, pathway from the pFRG to expiratory abdominal muscles that are innervated by L1–2 roots (adapted from Janczewski et al. 2002 with permission of the authors). pFRG interneurons activate pre/postinspiratory premotoneurons (see ‘whole-cell’ membrane potential recording) projecting contralaterally to L1–2 motoneurons which show pre/postinspiratory activity (referred to simultaneously recorded C4 bursting). The schema of the brainstem–spinal cord (note interruption caudal to C4) shows the 3 major preparations used in the present study, specifically ‘brainstem–cervical cords’ (transection at C5), ‘brainstem-complete cords’ (containing the entire spinal cord with no transection at L2 as shown by Janczewski et al. 2002) and ‘lumbar cords’ (containing the complete cord caudal to the transection level between the 12th and 13th thoracic nerve). C, the central pattern generator for hindlimb locomotion appears to be a distributed network (indicated by the two grey columns), located in the ventromedial area of the lower thoracic and lumbar spinal cord (adapted from Kiehn & Butt, 2003 with permission of the authors and Elsevier). The taper and the grey rostrocaudal gradient of the columns indicate a high rostral and low caudal capability for locomotor rhythm, whereas the transverse grey gradient shows a greater ability of medial than lateral areas for rhythm. Note that both locomotor and respiratory motoneurons are located ventrolateral to the grey areas, specifically in the ventral horn. Evoked alternating locomotor rhythm persists in isolated lumbar cords following removal of the dorsal aspect. (For more information, see Kiehn & Butt, 2003.)
It was the primary aim of our study to assess whether functionally expiratory lumbar motor activity in newborn rat brainstem–spinal cords is inhibited, as occurs in murine lumbar locomotor rhythms, or rather persists during anoxia, as in inspiratory-related nerve bursting. To achieve this, we used isolated lumbar spinal cords, brainstem–cervical spinal cords, brainstems without the spinal cord (but containing unilaterally a notable portion of the pons for recording from facial nerve) as well as brainstem (plus unilateral pons) preparations with the entire spinal cord attached (Fig. 1). We found that synchronous respiratory activity can be recorded bilaterally in vitro from the same lumbar roots that display alternating fictive locomotor activity. Within a few minutes, chemical anoxia abolished lumbar locomotor rhythms that were either electrically or pharmacologically induced (Smith & Feldman, 1987; Cazalets et al. 1992; Marchetti et al. 2001). In contrast, anoxia evoked persistent cervical burst doublets or triplets. Such repetitive anoxic cervical bursting was associated with pronounced lumbar respiratory nerve activity that showed a phase shift from pre/postinspiratory to augmented postinspiratory bursting.
Brainstem transections and selective anoxia of either the lumbar or supralumbar aspects of the diverse preparations in a partitioned recording chamber were carried out to determine the origin of the anoxic respiratory pattern changes. We present evidence that both normal lumbar expiratory activity and anoxic transformation of inspiratory and expiratory patterns depend on the pFRG and its interaction with the preBötC, respectively. We discuss these results in the context of the neural control of neonatal breathing during normoxia and its transformation to persistent ‘enforced breathing’ in anoxia. We also discuss whether anoxic inhibition of lumbar locomotor circuits, postanoxic depression of medullary locomotor control structures and/or postanoxic spinal hyperexcitability contribute to spasticity in cerebral palsy. We conclude that the preparations and recording techniques of the present study are valuable tools for studying mechanisms of respiratory disturbances associated with infancy such as apnoeas of prematurity or neuronal disfunction associated with perinatal hypoxic/ischaemic brain damage.
Methods
Ethical approval
All procedures were carried out in compliance with the guidelines of the Canadian Council for Animal Care and with the approval of the University of Alberta Health Sciences Laboratory Animal Services Welfare Committee.
Preparation and solutions
Experiments were carried out on brainstem–spinal cords and isolated lumbar cords from 0- to 2-day-old Sprague–Dawley (n = 68) or Wistar rats (n = 29). Animals were anaesthetized with isoflurane and decerebrated after the paw withdrawal reflex disappeared. The neuraxis was isolated at 19–22°C in the superfusate (for composition see below) (Brockhaus & Ballanyi, 1998, 2000) and the brainstem was cut rostrally between the trigeminal nerve roots and the caudal cerebellar artery (Fig. 1). This ensured that the entire facial nucleus, which overlaps rostrocaudally with the pFRG (Fig. 1), was included in these preparations (Onimaru & Homma, 2003; Onimaru et al. 2006; Ruangkittisakul et al. 2007). Either the complete spinal cord remained attached to the brainstem (‘brainstem-complete cords’) (Janczewski et al. 2002) or a transection was made at cervical root 5 (C5) (‘brainstem–cervical cords’). For tests of anoxia on fictive locomotion, ‘lumbar cords’ were generated by sectioning between thoracic roots 12 and 13 (Kiehn & Butt, 2003) (Fig. 1). To monitor facial nerve activity in one set of experiments, some portion of the pons was retained unilaterally in brainstem (spinal cord) preparations. Specifically, the brainstem was first completely transected with a razor blade rostrally, between the facial and trigeminal nerves. Subsequently, tissue between the facial nerve and the caudal cerebellar artery was removed on one side by an initial transverse section followed by a midline cut (compare Fig. 1 in present study with Fig. 1 in Onimaru et al. 2006). The spinal cord was removed in some of the latter cases by transection between C1 and the most caudal hypoglossal nerve root. Inspiratory-related hypoglossal nerve bursts were recorded as reference for respiratory facial nerve bursting (Fig. 1). For other experiments, brainstem-complete cords were transected between the caudal end of the facial nucleus (VIIc) and the rostral end of the preBötC that is presumably located ∼0.4 mm caudal to VIIc (Ruangkittisakul et al. 2006). A comparison of structures at the rostral surface of transected brainstem–spinal cords, or in sagittal sections of the cut rostral brainstem aspect (Ruangkittisakul et al. 2007), with a reference newborn rat brainstem atlas (Ruangkittisakul et al. 2006) revealed that the rostral brainstem boundary was 0.35–0.1 mm caudal to VIIc (mean 0.19 ± 0.05 mm, n = 5).
In the recording chamber (volume 2 ml), preparations were fixed (ventral side upwards) with insect pins at the brainstem. Lumbar cords were additionally stabilized with suction electrodes used for recording and/or stimulation. Preparations were superfused (flow rate 5 ml min−1) at 25–27°C with solution containing (mm): 118 NaCl; 4.5 KCl; 1.5 CaCl2; 1 MgCl2; 25 NaHCO3; 1.2 NaH2PO4 and 30 d-glucose (pH adjusted to 7.4 by gassing with 95% O2, 5% CO2). For some experiments, the recording chamber was partitioned with a Perspex barrier placed between thoracic roots 12 and 13 and sealed with a mixture of petroleum jelly and mineral oil for separate superfusion of the lumbar cord versus the brainstem plus cervical and thoracic cord (Beato & Nistri, 1999). After such experiments, phenol red was added to one compartment to check that the barrier was still leak-proof.
In newborn rat brainstem–cervical cords, hypoxic anoxia (by N2 gassing of the superfusate) and chemical anoxia (by block of aerobic metabolism with CN−) progressively slowed inspiratory nerve activities. Both procedures also elicited comparable effects on ion homeostasis and membrane properties of respiratory neurons (Ballanyi et al. 1992, 1999; Brockhaus et al. 1993; Ballanyi, 2004a,b). Thus, CN− (1 mm) was used here to evoke chemical anoxia for a time period of 20 min. In a subpopulation of brainstem–cervical cords, both types of anoxia accelerated cervical rhythms during the initial 1–4 min of their action (Ballanyi et al. 1992, 1999; Brockhaus et al. 1993; Völker et al. 1995; Ballanyi, 2004a,b). This effect was not studied here as the main objective was to analyse effects of long-term acute anoxia on lumbar expiratory activities. Combined administration of 8–10 μm serotonin and 4–6 μm of the glutamate receptor agonist N-methyl-d-aspartate was used to elicit fictive locomotion as previously reported (Cazalets et al. 1992; Marchetti et al. 2001). All agents were obtained from Sigma-Aldrich (Canada).
Nerve recording and stimulation
Discharge of respiratory active motoneurons, or motoneurons involved in fictive lumbar locomotion, was recorded extracellularly with suction electrodes from the proximal ends of ventral roots (C2–C4, L1–L2, VII, XII) (Fig. 1). Activity was bandpass-filtered (0.3–3 kHz) and integrated. The left or right L5 dorsal root was repetitively (10 Hz, 20–30 cycles, pulse duration: 0.1 ms) stimulated every 1.5–3 min via a suction electrode at voltages that were 3–4 times higher than threshold required to evoke alternating fictive locomotor activity between contralateral L1–L2 ventral nerve rootlets and ipsilateral L1-2 and L4-6 ventral roots (Marchetti et al. 2001; Taccola et al. 2004). Signals were sampled at a rate of 1 kHz into a personal computer via a digital recording system (Powerlab, ADInstruments, Castle Hill, Australia).
Data analysis
The statistical analysis regarding anoxia effects on respiratory activities used data obtained under control conditions (5 min prior to CN− application), at 10 and 20 min of chemical anoxia, and, finally, at 15 min after start of washout of CN−, when recovery of the cervical burst rate from anoxia was maximal. Since CN− blocked locomotor activity, data were analysed at the times at which block occurred. As the time course of recovery of locomotor rhythms from anoxia varied notably between preparations (partly because of the occurrence of spontaneous discharges), the effects were quantified when recovery was maximal. Respiratory burst rate was averaged over 2 min time windows and cervical burst duration was determined as the time period during which the signal was > 10% of their peak amplitude. Amplitudes of respiratory and pharmacologically induced locomotor bursts, and also those of the third locomotor burst following the end of tetanic electrical spinal cord stimulation, were expressed as the percentage change from control. The area between the integrated traces of either pre- or postinspiratory lumbar activity and the baseline was calculated using pCLAMP software (Molecular Devices Corp., Chicago, IL, USA). The area was calculated using a simple summation of points within the search region according to area = (Σa)t, with a corresponding to points (= amplitude) and t corresponding to sampling interval (= burst duration as defined above). For the latter analysis, five consecutive bursts were averaged from a single preparation, followed by averaging of such mean signals from seven and four preparations that were subjected to selective lumbar or supralumbar anoxia, respectively. No differences were observed between results from Sprague–Dawley and Wistar rats. Therefore, the data were pooled for statistical analyses. Values are means ± s.e.m. except for histology (mean ± s.d.). Significance (*P < 0.05, **P < 0.01) was assessed by one-sample Student's t test using SigmaPlot (Systat software Inc., Point Richmond, CA, USA).
Results
It was the aim of our study to assess whether functionally expiratory lumbar nerve activity with a typically pre/postinspiratory burst pattern in isolated newborn rat brainstem–spinal cords persists during sustained anoxia similar to inspiratory nerve bursting, or is depressed as for locomotor rhythms in newborn mouse lumbar spinal cords.
Anoxia effects on rhythmic motor networks in brainstem–cervical and lumbar cords
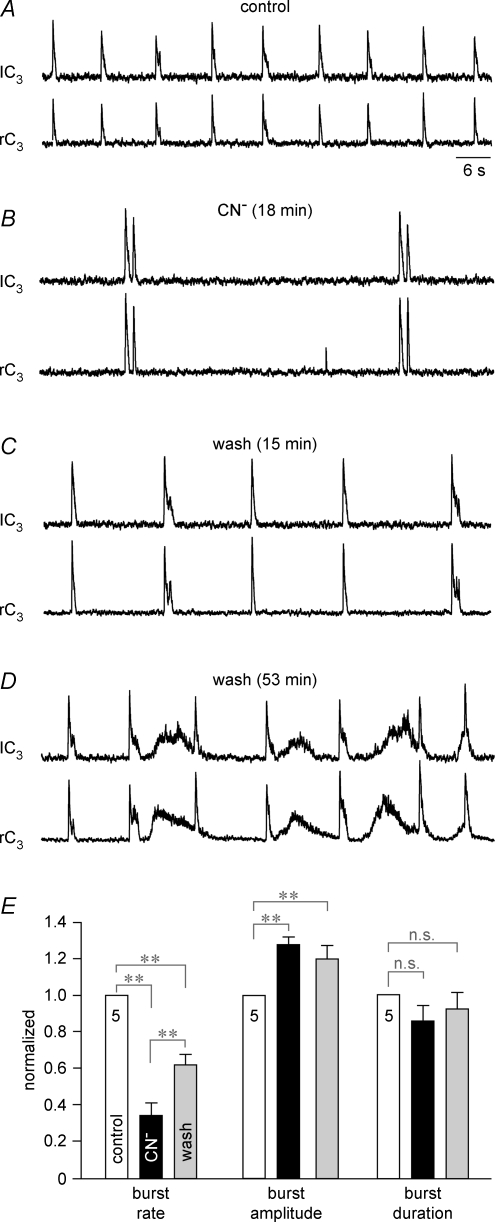
First, anoxia effects on endogenous cervical inspiratory rhythm in brainstem–cervical cords were compared with those on evoked locomotor rhythms in lumbar cords in identical recording solutions (Fig. 1). The brainstem–cervical cord in Fig. 2A generated bilaterally synchronous ventral cervical nerve bursts. CN−-induced chemical anoxia greatly slowed this rhythm, increased burst amplitude and induced burst doublets (Fig. 2B). These effects were partially reversible (Fig. 2C), but synchronous tonic discharge developed after > 30 min of washout of CN− (Fig. 2D). In five preparations with a mean burst rate of 6.0 ± 0.4 bursts min−1, CN− (1 mm) significantly slowed rhythm to 34% of control at the end of the 20 min application period (Fig. 2E). Concomitantly, burst amplitude increased significantly to 127% of control, whereas burst duration (0.78 ± 0.08 s in control) did not change (Fig. 2E). Anoxic cervical double bursts occurred in all preparations within 5–10 min after start of CN−. In two cases, one to two anoxic double bursts alternated with one single burst. Recovery of rhythm from anoxia was maximal 15 min after start of CN− washout. Cervical burst duration at this time did not differ from controls or anoxia (Fig. 2E). Burst rate recovered significantly, to 62% of control (but remained significantly lower than control), whereas burst amplitude was still significantly larger (120%) than the control at 15 min of recovery (Fig. 2E).
Figure 2. Anoxic frequency depression and inspiratory-related cervical nerve burst doublets in newborn rat brainstem–cervical cords.
A, suction electrode recording of bilaterally synchronous integrated extracellular activity in the left (lC3) and right (rC3) 3rd cervical ventral nerve roots containing axons of inspiratory active motoneurons. B, substantial slowing of inspiratory rhythm 18 min after the onset of chemical anoxia due to block of aerobic metabolism with 1 mm cyanide (CN−) was accompanied by a ∼30% increase in burst amplitude and occurrence of cervical double bursts. C, the frequency of respiratory rhythm partially recovered and double bursts disappeared 15 min after start of washout of CN−. D, at 53 min of recovery, inspiratory bursts were intermingled with synchronized, non-respiratory nerve discharge. Time scale identical for A–D. E, statistical analysis of the effects of chemical anoxia on burst rate (left group of bars, control 6.0 ± 0.4 bursts min−1), burst amplitude (centre group of bars, control 100%) and burst duration (right group of bars, control 0.78 ± 0.08 s) of cervical inspiratory rhythm that were normalized with reference to control. Numbers in control bars indicate the number of (identical) preparations; **P < 0.01; n.s., non-significant difference.
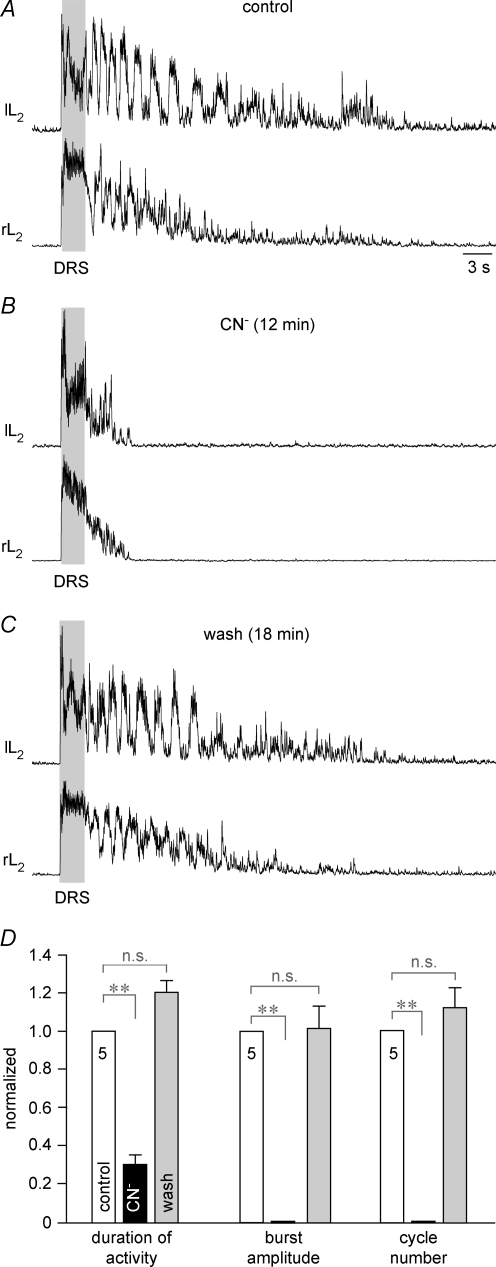
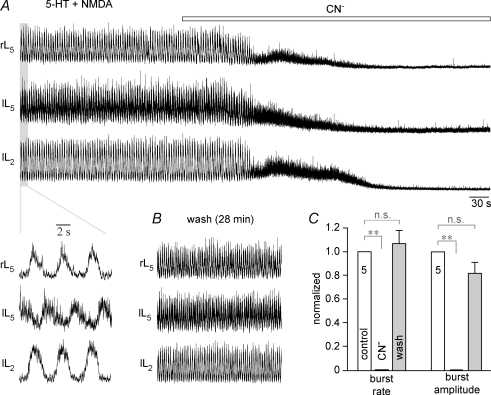
Under identical experimental conditions, anoxia blocked lumbar locomotor activity. In the lumbar cord of Fig. 3A, several cycles of bilaterally alternating fictive locomotion were revealed in L2 ventral roots after termination of repetitive (10 Hz, 2.5 s) dorsal root (L5) stimulation. This activity was abolished 12 min after the start of anoxia (Fig. 3B) and recovered fully 18 min after the start of CN− washout (Fig. 3C). In five preparations, 6.5 ± 0.5 locomotor cycles occurred during a 18.6 ± 3.7 s activity period following the end of stimulation. Locomotor activity was abolished within 7.4 ± 0.6 min after start of CN− and the duration of non-rhythmic activity was reduced to 31% of control (Fig. 3D). Upon washout of CN−, the duration of poststimulus activity, the number of locomotor cycles and the amplitude of the 3rd poststimulus locomotor burst returned to values that did not significantly differ from controls (Fig. 3D). The time for complete recovery varied notably, between 15 and 47 min (mean 27.4 ± 5.9 min), partly because spontaneous activity, including brief periods of locomotion, interfered with testing the electrically evoked rhythms. In the lumbar cord of Fig. 4A, combined application of serotonin (10 μm) and N-methyl-d-aspartate (6 μm) induced a stable locomotor rhythm that alternated between both L5 roots and the left L5 and L2 roots. This rhythm was blocked within < 2 min by CN−, whereas tonic activity (which was superimposed with locomotor rhythm in control) was abolished after a further 3.5 min. Locomotor rhythm recovered to control values 28 min after the start of CN− washout (Fig. 4B). In five preparations, pharmacologically evoked locomotor rhythm having a mean rate of 22.2 ± 2.4 cycles min−1 was abolished by CN− after 1.9 ± 0.5 min, whereas tonic activity was blocked after 6.7 ± 0.9 min. Recovery was maximal 20–55 min after the start of CN− washout and reached values, for both burst rate and amplitude, that did not significantly differ from controls (Fig. 4C).
Figure 3. Anoxic block of electrically evoked locomotion in newborn rat lumbar cords.
A, the dorsal nerve root of the 5th right lumbar cord segment was stimulated via a suction electrode to elicit epochs of alternating fictive locomotor activity in the left and right 2nd ventral lumbar nerve roots (lL2, rL2). Dorsal root stimulation (DRS) was done at 3× threshold, with 10 Hz for 2.5 s (grey area; single pulse duration 0.1 ms). Synaptic activity continued for several seconds after spontaneous arrest of poststimulus locomotor activity. B, anoxia abolished fictive locomotion within 12 min after start of CN− application, and also greatly reduced the time period of occurrence of non-rhythmic activities. C, locomotor rhythm and non-rhythmic discharges recovered to control levels 18 min after start of CN− washout. Time scale identical for A–C. D, statistical analysis of depressing effects of chemical anoxia, normalized with reference to control, revealed a complete recovery of the duration of evoked synaptic activity (left group of bars, control 18.6 ± 0.3.7 s), the amplitude of the 3rd poststimulus locomotor burst (centre group of bars, control 100%) and the number of locomotor cycles (right group of bars, control 6.5 ± 0.45 cycles).
Figure 4. Anoxic block of chemically evoked locomotion in lumbar cords.
A, combined bath-application of 10 μm serotonin (5-HT) and 6 μm N-methyl-D-aspartate (NMDA) induced a stable fictive locomotor rhythm that alternated between the left and right L5 ventral roots and between the left L5 and left L2 ventral roots (see inset at higher time resolution). Fictive locomotion was blocked within 2 min of start of CN− application, whereas tonic activity (on which locomotor rhythm was superimposed in control) was abolished after a further 3.5 min. B, locomotor rhythm recovered to control values 28 min after start of CN− washout. Time scale identical for A and B. C, statistical analysis of depressing effect of chemical anoxia on fictive locomotion, normalized with reference to control, revealed a complete recovery of locomotor burst rate (left group of bars, control 22 ± 2.4 cycles min−1) and amplitude (right group of bars, control 100%).
These results showed that spontaneous cervical inspiratory motor activity persisted at reduced frequency during anoxia (but recovered only partially), whereas evoked lumbar locomotor rhythm was rapidly abolished by anoxia (but the effect was fully reversible).
Anoxia effects on respiratory lumbar nerve bursts in brainstem-complete cords
Next, anoxia effects were tested on cervical and lumbar respiratory activities in brainstem preparations with a complete spinal cord. A previous study reported that a pre/postinspiratory burst pattern is typically recorded from L1-2 roots in such preparations (Janczewski et al. 2002). Here, we observed a stable biphasic activity pattern with a 0.2–0.5 s period of preinspiratory and a 0.3–0.8 s period of postinspiratory bursting in 14 of 34 preparations (Fig. 5). In eight different preparations, lumbar bursting occurred primarily during the postinspiratory phase (Fig. 6A), whereas preinspiratory lumbar bursts were followed solely by inspiratory activity in six other cases (Fig. 6B). Six further preparations showed an exclusively inspiratory lumbar burst pattern (Fig. 6C). Small amplitude inspiratory-related lumbar activity was also observed in preparations with either pre/postinspiratory (n = 4) or solely postinspiratory (n = 3) activity.
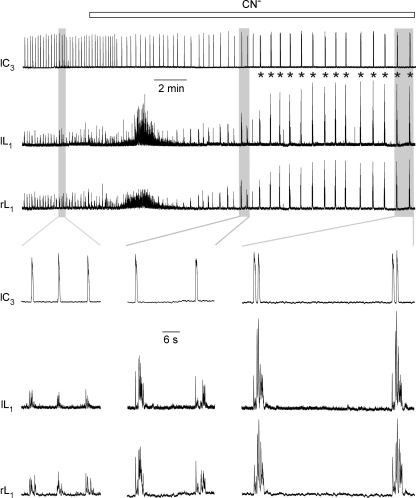
Figure 5. Anoxia effects on cervical and lumbar respiratory rhythms in brainstem-complete cords.
The upper 3 traces show simultaneous recordings from the left cervical (lC3) plus the left and right 1st lumbar (lL1, rL1) ventral nerve roots, whereas the lower 3 columns of traces illustrate parts of the continuous recording at higher time resolution. In control solution, inspiratory cervical nerve activity was accompanied by 1 : 1-coupled pre/postinspiratory lumbar nerve bursts. Chemical anoxia induced an identical reduction of the frequency of cervical and lumbar nerve bursting and evoked cervical double bursts (asterisks) after ∼10 min. Anoxia had no major effect on the amplitude of cervical bursting, but progressively augmented lumbar activity with a concomitant pattern transformation from pre/postinspiratory to inspiratory/postinspiratory. The progressive anoxic potentiation of lumbar bursting continued after the onset of inspiratory cervical double bursts. Note the short duration of the lumbar burst that appeared simultaneously with the first of the cervical double burst.
Figure 6. Anoxic postinspiratory augmentation of lumbar bursting in preparations with variable control lumbar burst patterns.
Traces on the left in A–C show overlayed recordings of 2 consecutive (black 1st, grey 2nd) bursts of cervical (C3-4) and lumbar (L1) nerve activities in brainstem-complete cords. Grey area indicates the inspiratory period. In all 3 preparations, anoxia slowed respiratory rhythm to < 40% of control (not shown). A, in a preparation with neither preinspiratory nor inspiratory lumbar activity in control, anoxia notably augmented the duration, but not the amplitude of lumbar postinspiratory activity. B, in a different preparation, anoxia shifted the control preinspiratory/inspiratory activity pattern toward the inspiratory/postinspiratory phases. Note that the 2nd anoxic cervical activity was a double burst (see asterisk). C, in a further preparation, anoxia changed the lumbar respiratory pattern from exclusively inspiratory to inspiratory/postinspiratory. Time scale identical for A–C.
As exemplified by a preparation with a pre/postinspiratory lumbar burst pattern, anoxia slowed rhythm and evoked cervical burst doublets (Fig. 5). In both L1 roots, a period of tonic discharge occurred between 2 and 5 min after onset of anoxia. During the next 5 min, the amplitude of lumbar bursts increased progressively with a concomitant change in burst pattern. Specifically, preinspiratory bursts were abolished, and a short inspiratory burst was followed by depressed activity for 0.5–1 s prior to the onset of a pronounced multipeak postinspiratory discharge. The augmented postinspiratory activity persisted throughout the recording and was accompanied by the occurrence of cervical double bursts after ∼10 min into anoxia (Fig. 5). Very similar effects of anoxia were seen in the other 13 cases with a control pre/postinspiratory lumbar burst pattern. Sustained anoxia augmented either the amplitude (n = 5) or duration (n = 3) of this activity in the eight preparations with an exclusively postinspiratory burst pattern (Fig. 6A). In the six cases with a preinspiratory/inspiratory control burst pattern, anoxia abolished preinspiratory activity and evoked postinspiratory discharge, but attenuated inspiratory bursts in only three cases (Fig. 6B). In the six preparations with exclusively inspiratory-related lumbar bursting, anoxia evoked postinspiratory discharge and augmented the amplitude of inspiratory activity in four cases (Fig. 6C). In 16 of 22 preparations with either pre/postinspiratory or solely postinspiratory bursts, the amplitude of postinspiratory activity increased by 100–600% in anoxia (Fig. 5), whereas postinspiratory burst duration was primarily augmented in the remaining cases (Fig. 6A). Within 5–12 min after start of CN−, cervical burst doublets occurred in all preparations and either persisted throughout the remaining time of anoxia (35% of 34 cases), or alternated with single bursts (47%) or bursts triplets (18%).
In summary, anoxia depressed preinspiratory and augmented postinspiratory lumbar bursting, independent of the initial activity pattern. Most relevant for the primary aim of this study, lumbar respiratory rhythm persisted in 29 of 34 cases during the 20 min anoxia period. In only five cases (15%), the amplitude of lumbar respiratory activity started to decline 11–15 min after start of CN− followed by disappearance of bursts between 1.5 and 4 min before the end of chemical anoxia. In three of these cases, recovery from anoxia included a postanoxic potentiation of the amplitude and/or duration of lumbar respiratory activity. A transient postanoxic potentiation of lumbar respiratory activity was also seen between 4 and 15 min of recovery in 8 of the other 29 preparations.
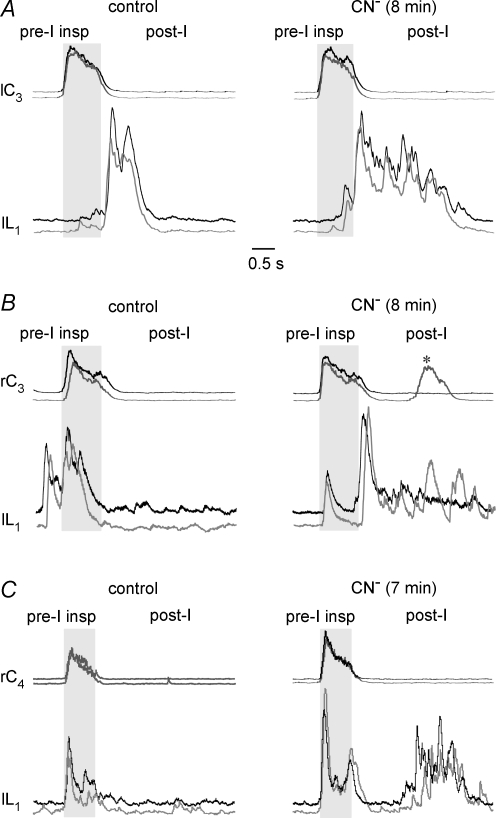
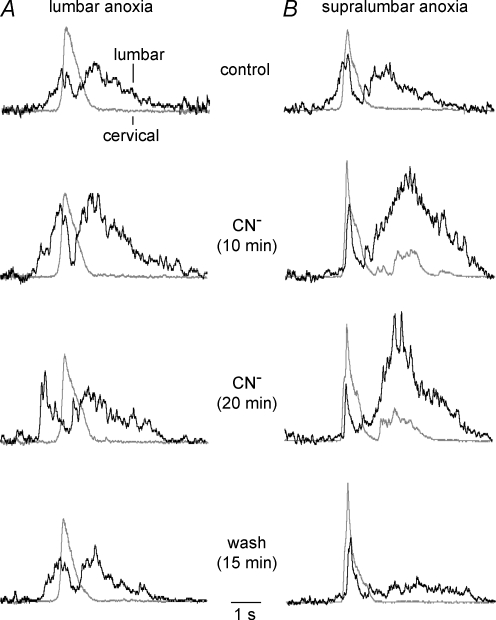
Effects of selective lumbar and supralumbar anoxia on respiratory activities
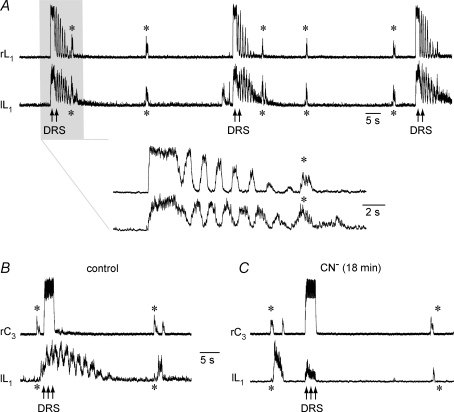
To identify the possible sites responsible for the observed anoxic transformation of the inspiratory and pre/postinspiratory activities in brainstem-complete cords, CN− was first applied exclusively to the lumbar cord in a partitioned bath chamber system. Figure 7A shows the average time course of five consecutive cervical and lumbar bursts from seven preparations with a pre/postinspiratory lumbar burst pattern in control. At 10 min of selective lumbar anoxia, the amplitudes of both the preinspiratory and postinspiratory component of lumbar bursting were notably enhanced. At the end of the 20 min period of CN− application, the anoxic augmentation of pre/postinspiratory lumbar activity was partly reversed and further recovered to control values within 15 min after the start of CN− wash. The average time course of cervical bursts did not change during any of these phases and no repetitive cervical bursts occurred (Fig. 7A). In contrast, selective anoxia of both the brainstem and the supralumbar spinal cord in four preparations induced cervical double bursts of modestly increased amplitude, blocked preinspiratory lumbar activity (revealing an inspiratory peak), and augmented the amplitude of postinspiratory lumbar bursting (Fig. 7B). Inspiratory bursting without recovery of preinspiratory activity was also seen 15 min after start of washout of supralumbar CN−, whereas the amplitude of postinspiratory lumbar bursting decreased below control (Fig. 7B).
Figure 7. Effects of selective lumbar or supralumbar anoxia on respiratory activities.
A, the top pair of traces shows the average time course of inspiratory cervical (grey) and pre/postinspiratory lumbar burst trajectories (black), resulting from 5 consecutive bursts in 7 brainstem-complete cords in standard solution. The 2nd pair of traces from the top, sampled 10 min after start of CN− administration to the lumbar spinal cord in a partitioned recording chamber, revealed a notable augmentation of both preinspiratory and postinspiratory lumbar activities. This augmentation was partially reversed at 20 min of anoxia (3rd pair of traces from the top), and fully reversed 15 min after start of CN− washout (bottom pair of traces). Under all conditions, the mean cervical burst remained unaffected. B, the top pair of traces shows the average time course of 5 consecutive cervical and lumbar bursts from 4 preparations in control solution. The 2nd pair of traces from top illustrates that CN− application to the brainstem plus supralumbar spinal cord in the partitioned recording chamber induced after 10 min an increase in the amplitude of the mean cervical burst and concomitant occurrence of cervical double bursts, which were accompanied by depression of the preinspiratory and augmentation of the postinspiratory component of lumbar bursts. The anoxic transformation by supralumbar anoxia of the lumbar respiratory pattern was maintained until the end of the 20 min period of CN− application (3rd pair of traces from top). Fifteen minutes after start of CN− washout, the anoxic augmentation of postinspiratory lumbar bursts reversed to levels notably smaller than in control, whereas inspiratory activities persisted in both the cervical and lumbar recordings (bottom pair of traces). Note that the second cervical burst upon supralumbar anoxia was attenuated by averaging due to a jitter in its onset compared to the first burst used as reference for averaging. Time scale identical for all traces.
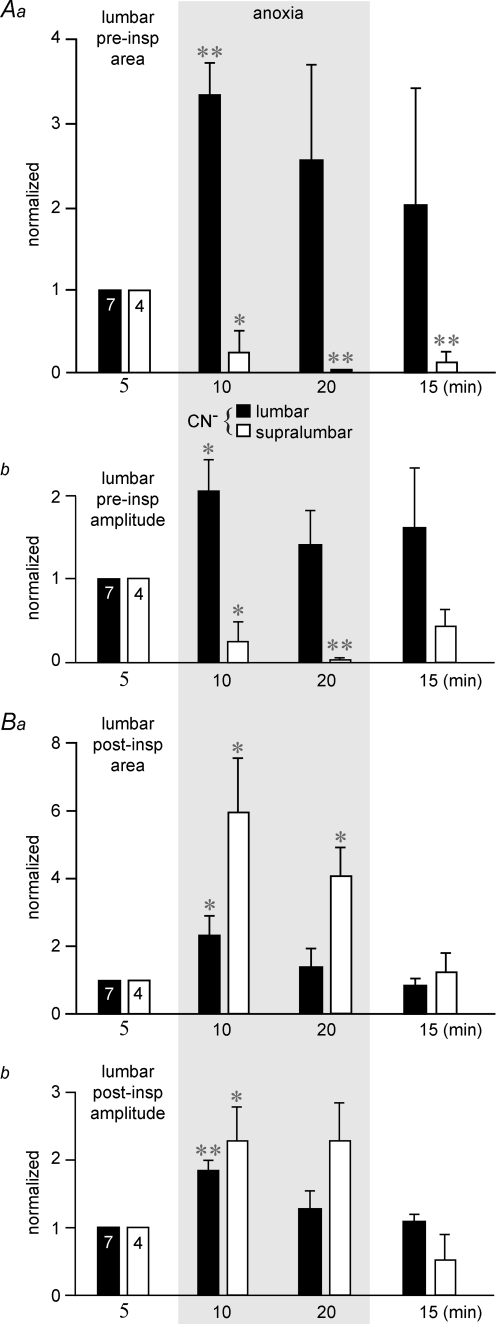
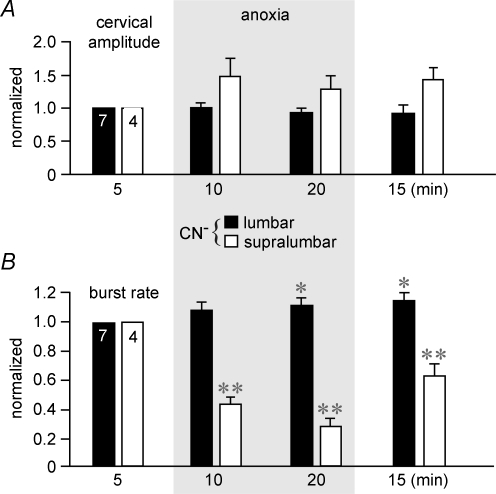
Quantification of anoxia effects in these preparations revealed first that lumbar anoxia significantly increased both the area between the integrated lumbar recording and baseline (Fig. 8Aa) and the amplitude (Fig. 8Ab) of preinspiratory bursts at 10 min (333 and 206% of control), but not 20 min (258 and 141% of control). Conversely, supralumbar anoxia strongly and significantly depressed both the preinspiratory area (Fig. 8Aa) and amplitude (Fig. 8Ab) at both 10 min (25 and 25% of control) and 20 min of anoxia (5 and 0.5% of control). Furthermore, both the area (Fig. 8Ba) and amplitude (Fig. 8Bb) of postinspiratory lumbar bursting were significantly increased by both supralumbar (594 and 230% of control) and lumbar anoxia (233 and 185% of control) at 10 min of anoxia. The augmenting effect on the postinspiratory area, but not amplitude, was also significant for supralumbar anoxia at 20 min (410 and 230% of control), but not for lumbar anoxia during that time (134 and 128% of control).
Figure 8. Effects of selective lumbar or supralumbar anoxia on the area and amplitude of lumbar respiratory activities in brainstem-complete cords.
A, selective lumbar anoxia (filled bars) in the 7 preparations of Fig. 7A caused a significant increase at 10 min of anoxia in both the area between the integrated lumbar recording and baseline (a) and amplitude (b) of preinspiratory (preinsp) lumbar bursting. Grey area indicates valves during anoxia. This augmentation was still present, though not significant, at 20 min of chemical anoxia and decreased toward control values 15 min after start of washout of CN−. Conversely, supralumbar anoxia (open bars) in the 4 preparations of Fig. 7B significantly depressed (at 10 min) and almost abolished (at 20 min) both lumbar preinspiratory area (a) and amplitude (b). B, lumbar and supralumbar anoxia increased both the area (a) and amplitude (b) of postinspiratory (postinsp) lumbar activity. Note that the effect on postinspiratory area was not significant at 20 min of lumbar anoxia similar to the effect of both lumbar and supralumbar anoxia on postinspiratory burst amplitude. Significance values (*P < 0.05; **P < 0.01) were referred to controls.
Lumbar anoxia did not change the amplitude of cervical bursts, whereas supralumbar anoxia had a modest, though non-significant augmentating effect (Fig. 9A). Finally, 1 : 1-coupled cervical and lumbar respiratory burst rate was significantly lowered by supralumbar anoxia, from 7.3 ± 0.7 bursts min−1 to 43% (10 min) and 28% of control (20 min), whereas lumbar anoxia modestly raised burst rate above the control value (6.7 ± 0.4 bursts min−1). This effect was significant at 20 min of anoxia (111% of control) and at 15 min after start of recovery (115% of control) (Fig. 9B).
Figure 9. Effects of selective lumbar or supralumbar anoxia on cervical burst amplitude and respiratory rate in brainstem-complete cords.
A, lumbar anoxia (filled bars) did not change the amplitude of cervical nerve bursts, whereas supralumbar anoxia (open bars) had a modest, though insignificant augmenting effect. Grey area indicates valves during anoxia. B, lumbar anoxia did not change the relative 1 : 1-coupled cervical and lumbar respiratory frequency (referred to the pre-anoxic value of 6.7 ± 0.4 bursts min−1) in the 7 preparations of Fig. 7A, except for a slight progressive increase that was significant at 20 min of CN− application and 15 min after start of its washout. In contrast, supralumbar anoxia significantly decreased respiratory burst rate in the 4 preparations of Fig. 7B from the initial value of 7.3 ± 0.7 bursts min−1. Note that the effect was not fully reversible. Significance values (*P < 0.05; **P < 0.01) were referred to controls.
These results indicate that both lumbar and supralumbar structures contribute to the anoxic postinspiratory augmentation of lumbar bursts, whereas cervical double bursts and the decrease of preinspiratory activity appear to be mediated by supralumbar structures.
Medullary origin of the anoxic transformation of respiratory patterns
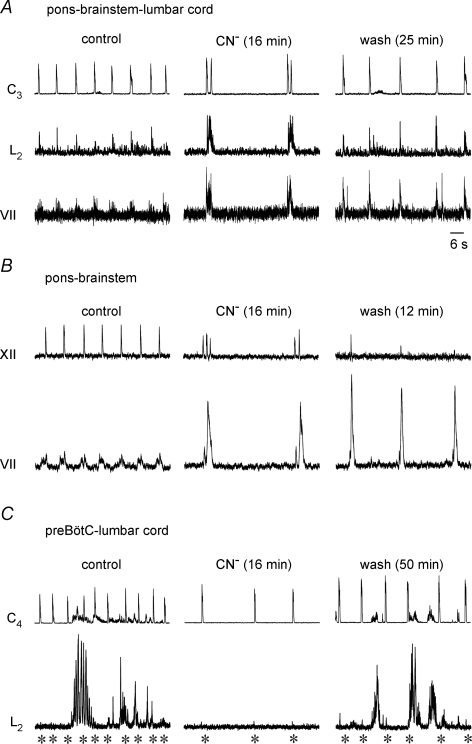
To investigate whether medullary structures contribute to the latter phenomena, anoxia effects were assessed on pre/postinspiratory bursting of the facial nerve (Onimaru et al. 2006). The recordings from a brainstem-complete cord with unilaterally extended pons in Fig. 10A show that respiratory lumbar and facial nerve bursts are very similar in anoxia. In control, inspiratory-related cervical and lumbar bursts were accompanied by facial activity that started during the preinspiratory and continued into the postinspiratory phase. Sixteen minutes after the start of anoxia, cervical double bursts were accompanied by a short inspiratory burst followed by pronounced postinspiratory activity in both lumbar and facial nerve roots. Single cervical bursts and the modest amplitude and duration of lumbar bursts, comparable to control, were restored 25 min after start of CN− washout, whereas facial bursts were still greatly potentiated.
Figure 10. Effects of brainstem transection on anoxia respiratory nerve bursts.
A, the left set of traces shows recordings of inspiratory cervical (C3) and lumbar (L2) plus pre/postinspiratory activity of facial (VII) nerve in a brainstem-complete cord with unilaterally extended pons for VII recording (Fig. 1). The middle pair of traces illustrates that anoxic slowing of rhythm and occurrence of cervical double bursts were accompanied by prominent postinspiratory lumbar and VII activities. The set of traces in the right panel shows the partial reversibility of these effects. B, recordings from a brainstem with unilaterally extended pons as in A, but cut caudally at the most caudal hypoglossal (XII) root shows single inspiratory-related XII bursts and VII activity which started in the preinspiratory phase and continued into the postinspiratory phase (left couple of traces). Anoxia transformed the pattern of VII bursting into greatly augmented postinspiratory activity and induced XII burst doublets and triplets (pair of traces in centre panel). Note that the postinspiratory augmentation of VII activity persisted until 12 min after start of CN− washout, whereas XII double bursts reversed into single bursts of very small amplitude (pair of traces in right panel). C, in a brainstem-complete cord cut 0.25 mm caudal to VIIc (Fig. 1), cervical inspiratory activity was not reflected in lumbar respiratory bursting (see asterisks for alignment) instead of tonic bursts that were synchronous with smaller tonic cervical activity (pair of traces in left panel). Anoxia did not evoke aervical burst doublets, but blocked lumbar non-respiratory activity and promoted short inspiratory lumbar bursts of small amplitude (pair of traces in centre panel). Recovery from anoxia included a minor augmentation of inspiratory lumbar bursting and reoccurence of non-respiratory activity (pair of traces in right panel). Time scale identical for A–C.
Similar effects of anoxia on respiratory-related facial nerve bursts were seen in two further preparations of this type and in four brainstem–cervical cords with unilaterally extended pons. Most importantly, five pons–brainstem preparations without the spinal cord also showed greatly enhanced postinspiratory facial bursts during anoxia. This effect was accompanied by doublets of inspiratory-related hypoglossal bursts that alternated with burst triplets in one case (Fig. 10B). In 2 of the 5 preparations, hypoglossal burst amplitude decreased progressively, until the activity disappeared (Fig. 10B). The progressive attenuation of hypoglossal burst amplitude was not caused by anoxia since it was also seen in 2 of 4 control preparations of this type and in brainstem–spinal cords with tissue rostral to VIIc (Ruangkittisakul et al. 2007). These results suggest that the anoxic phase shifts and a major portion of the persistent postinspiratory augmentation of bursting in pre/postinspiratory active lumbar (and cranial) nerves are generated by brainstem structures.
Effects of brainstem transection on anoxic cervical and lumbar respiratory activities
To test whether the anoxic respiratory pattern shifts depend on structures rostral to the preBötC, brainstem-complete cords were cut between VIIc and the preBötC (Fig. 1). In five such preparations with the mean rostral boundary 0.19 ± 0.05 mm caudal to VIIc, no respiratory lumbar activity was seen except in two cases short and small amplitude inspiratory bursts were present during some respiratory cycles. In contrast, 4 of the 5 preparations showed pronounced non-respiratory lumbar activities that sometimes included short periods of fictive locomotion (Fig. 10C). Anoxia abolished this non-respiratory activity within < 5 min and revealed, in 3 of the 5 preparations, small amplitude inspiratory lumbar bursts, whereas neither postinspiratory lumbar nor repetitive cervical bursts were seen (Fig. 10C). In four cases, inspiratory bursting with slightly increased amplitudes occurred during recovery from CN−, before such respiratory bursting was again masked by non-respiratory discharges (Fig. 10C). These findings suggest that structures rostral to the preBötC are important for the anoxic shift of both cervical and lumbar respiratory patterns.
Anoxia effects on simultaneously recorded lumbar respiratory and locomotor bursts
In a final approach, the effects of anoxia were tested during simultaneously recorded lumbar respiratory and locomotor activities. In the experiment shown in Fig. 11, two suction electrodes were first used to monitor respiratory rhythm from both L1 roots (Fig. 11A). During the interval between respiratory bursts, fictive locomotion was induced by dorsal root stimulation (compare Fig. 3). Subsequent repositioning of one lumbar recording electrode to the right C3 root revealed that the lumbar respiratory pattern was primarily postinspiratory-related (Fig. 11B). Similar to the above findings, anoxia potentiated the amplitude of lumbar postinspiratory activity during cervical double bursts, but not single bursts, whereas locomotor activity was abolished (Fig. 11C). A very similar block of electrically evoked locomotion and persistence of (postinspiratory augmented) lumbar respiratory bursting was seen in further four preparations, in which both types of activity were monitored simultaneously.
Figure 11. Effects of anoxia on spontaneous respiratory and electrically evoked locomotor activities in the same lumbar root of a brainstem-complete cord.
A, recording from the left and right ventral L1 roots revealed endogenous synchronous respiratory bursting (asterisks). In the interval between respiratory bursts, the left dorsal L5 root was stimulated (3× threshold, 10 Hz, 2.5 s) to induce epochs of fictive locomotion (see inset at higher time resolution). B, repositioning of one recording suction electrode from the right L1 to the right ventral C3 rootlet showed that lumbar respiratory activity was primarily postinspiratory-related. C, postinspiratory lumbar activity persisted during 18 min of anoxia and was even potentiated at the occurrence of cervical double bursts, whereas evoked locomotor activity was abolished.
Discussion
We report that expiratory lumbar activity in isolated newborn rat brainstem–spinal cord preparations persists with a changed pattern during sustained anoxia, but shows an incomplete recovery, similar to inspiratory rhythm. In contrast, although fictive locomotion recorded from the same lumbar roots is abolished, this shows full recovery. Recovery from anoxia of in particular electrically evoked locomotor rhythm and endogenous respiratory activities interfered with persistent spinal discharges.
Anoxia effects on inspiratory cervical and expiratory lumbar activities
As a major finding of our study, the activity of lumbar respiratory motoneurons, innervating a subgroup of expiratory abdominal muscles (Iscoe, 1998; Janczewski et al. 2002), persisted in 85% of preparations during sustained anoxia. Thus in neonatal rats, isolated pre/postinspiratory active lumbar (and also facial) motor networks have a high tolerance to anoxia similar to inspiratory active cranial or cervical spinal motor networks (Ballanyi et al. 1992, 1999; Ramirez et al. 1998, 2007; Kato et al. 2000; Ballanyi, 2004a,b). Anoxia induced a modest increase in the amplitude of cervical bursting. The present study did not clarify whether this stimulatory effect of anoxia originates from phrenic (pre)motoneurons or rhythmogenic preBötC interneuronal networks. A similar modest augmentation of the amplitude of phrenic nerve activity was associated with either mild hypoxia or anoxia in non-denervated, vagotomized, chemo-denervated, decerebrate or anaesthetized animals (Fregosi et al. 1987; St John et al. 1989; St John 1990; Richter et al. 1991; Fukuda, 2000). Also asphyxia augmented phrenic nerve bursting only modestly, but induced a > 10-fold increase in the amplitude of inspiratory-related intercostal nerve activity (Macefield & Nail, 1987). The authors proposed that this difference is due primarily to a greater activation of high-threshold intercostal motoneurons. Recruitment of motor units also contributes 40% to hypoxic augmentation of abdominal expiratory activity in spontaneously breathing cats, while the remaining 60% are presumably due to increased rate coding (Mateika et al. 1996). Recruitment of motor units may also be involved in the anoxic augmentation of postinspiratory lumbar respiratory bursting observed in the present study. Local lumbar respiratory networks seem to contribute to this phenomenon as the augmentation was seen upon selective lumbar anoxia. In addition, medullary structures appear to be involved as indicated by the augmented amplitude of facial nerve bursts in anoxic pons–brainstem preparations without the spinal cord.
Similar to our study, very pronounced postinspiratory activity was revealed in abdominal nerves of adults cats in vivo, but only in the initial phase of anoxia (St John et al. 1989). Both severe hypoxia and CN− also substantially increased (by 657% and 242%, respectively) abdominal expiratory activity in 1 of 3 ketamine-anaesthetized cats (Fregosi et al. 1987). In the remaining two ketamine-anaesthetized animals, and in decerebrated cats of the latter report, hypoxia strongly depressed expiratory lumbar nerve bursts, whereas such activity was augmented when the vagi were intact. Hypoxic augmentation of abdominal expiratory muscle activity, depending on vagal inputs, was also seen in conscious dogs (Yasuma et al. 1993) and anaesthetized cats (Fregosi, 1994). All this suggests that a major portion of augmented respiratory activity in newborns is due to a facilitatory action of anoxia on vagal stimuli, although the augmented postinspiratory lumbar bursting in our study shows that a central component is also involved.
In contrast to the potentiation of postinspiratory bursting, anoxia blocked the preinspiratory component of lumbar rhythm (if present in control), and thus shifted its activity pattern toward the postinspiratory phase. Similarly, moderate hypoxia augmentated the postinspiratory component of diaphragm activity in adult cats (Lovering et al. 2003). Also in cats, severe arterial hypoxia abolished postinspiratory phrenic nerve activity, transformed its normally incrementing inspiratory pattern to a sharp decrementing (typically shorter) trajectory of activity, and abolished expiratory activities including those of abdominal nerves (St John et al. 1989). The latter scenario of respiratory changes, resulting in primarily synchronized inspiratory interneuronal and nerve bursting, has been defined as ‘gasping’ (St John 1990). It should be noted, though, that subclasses of preBötC neurons in juvenile rat working heart–brainstem preparations showed augmented preinspiratory activity during hypoxic gasping (Paton et al. 2006).
In summary, it remains to be determined whether the persistence of lumbar expiratory activity in neonatal rat brainstem-complete cords is a further characteristic feature of the extreme tolerance to anoxia of breathing in newborn mammals, probably contributing to the pronounced increase in both the strength and duration of single breaths in intact animals (Macefield & Nail, 1987; Fung et al. 1996; Gozal et al. 1996; Fewell et al. 2000; Ballanyi, 2004a). The latter findings suggest that the term ‘enforced breathing’ (Ballanyi, 2004a) is appropriate to describe hypoxic/anoxic breathing efforts in newborns, opposed to gasping in more mature mammals (St John 1990).
Involvement of the pFRG in anoxic respiratory pattern transformation
In addition to the shift toward the postinspiratory phase of lumbar bursting, anoxia also changed the cervical burst pattern, specifically by inducing burst doublets and triplets. These events resembled closely spaced double or triple hypoxic gasps in human infants (Sridhar et al. 2003). Burst doublets were also seen in phrenic nerve recordings from adult cats upon selective chemostimulation of the preBötC (Solomon et al. 2000). Similar to repetitive cervical bursts, anoxia induced inspiratory hypoglossal nerve burst doublets/triplets, and evoked a shift toward the augmented postinspiratory phase of pre/postinspiratory facial nerve activity in brainstems without the spinal cord. This excludes the possibility that the anoxic transformation of cervical or lumbar respiratory patterns originates from putative respiratory rhythm generators within the cervical spinal cord (Aoki et al. 1980; Dubayle & Viala, 1996). A contribution of the pontine respiratory group (Okada et al. 1998; Ballanyi et al. 1999; Potts et al. 2005) to this phenomenon is also not likely as brainstem-complete cords did not contain this more rostral area.
Instead, the pFRG, which overlaps with the facial nucleus and, presumably, extends even further caudally by ∼0.25 mm (Onimaru et al. 2006; Ruangkittisakul et al. 2007), may be involved in the anoxic respiratory pattern transformation and postinspiratory augmentation. This view is based on our finding that pre/postinspiratory lumbar activity in control as well as augmented anoxic postinspiratory lumbar bursts and repetitive cervical bursts were absent in preparations transected between VIIc and the preBötC (compare Ruangkittisakul et al. 2007). This finding was similar to the lack of pre/postinspiratory abdominal muscle activity in normoxic juvenile rats in vivo after brainstem transection close to VIIc (Janczewski & Feldman, 2006). Although the interpretation of results from transection experiments is limited (Wilson et al. 2006), our findings support the view of an important role of the pFRG in respiratory control (Jacquin et al. 1996; Chatonnet et al. 2003; Onimaru & Homma, 2003). In extension of the latter studies, we hypothesize that anoxia induces a major reorganization of pFRG activity, and/or of pFRG interactions with the preBötC, which presumably constitute a dual respiratory centre (Mellen et al. 2003; Ballanyi, 2004a; Feldman & Del Negro, 2006). Presumably, only ∼11% of pFRG neurons drive ipsilateral medullary premotoneurons, which are located caudal to the preBötC and activate contralateral lumbar motoneurons innervating expiratory abdominal muscles (Fig. 1) (Janczewski et al. 2002). Our results raise the possibility that anoxia depresses preinspiratory and augments postinspiratory bursting in particular in these pFRG neurons, while other subclasses of pFRG cells are ‘functionally inactivated’ by anoxia (Ballanyi et al. 1994, 1999; Ballanyi, 2004a,b).
Cellular mechanisms of augmented anoxic postinspiratory lumbar bursting
The cellular mechanisms of the anoxic potentiation of lumbar respiratory activity remain unknown. Possibly, anoxic depression of anion-mediated inhibitory synaptic processes within respiratory networks disinhibits excitatory inputs to lumbar respiratory motoneurons during the inspiratory/postinspiratory phases (Richter et al. 1991; Ballanyi et al. 1994, 1999; Ramirez et al. 1998; Pena & Ramirez, 2005). Such anoxic disinhibition may already occur at the level of the pFRG and reconfigure the activity pattern of a subpopulation of these presumptive hypoxia-sensing neurons (Voituron et al. 2006). Important to note, anion-mediated postsynaptic potentials appear to be inhibitory in newborn rodent respiratory interneurons including pFRG cells (Arata et al. 1998; Brockhaus & Ballanyi, 1998), and also in spinal motoneurons (Parkis et al. 1999; Hübner et al. 2001), in contrast to other neonatal rat brain structures. As an alternative mechanism, anoxia-related factors such as acidosis of the ventral respiratory column (Völker et al. 1995; Voipio & Ballanyi, 1997) may augment the activity of presumptive anoxia-resistant pFRG neurons (see above). Indeed, CO2/H+ stimulates bursting of newborn rat pFRG neurons (Kawai et al. 2006) and unmasks, or augments activity in abdominal expiratory muscles in brainstem-complete cords (Iizuka, 2003). Such chemostimulation may be due to CO2/H+-evoked block of (leak) K+ channels, including inward-rectifiers or TASK channels (Mulkey et al. 2004). Anoxia-induced acidosis may also stimulate central chemoreceptors of the retrotrapezoid nucleus, which may overlap (or be identical with) the pFRG (Mulkey et al. 2004; Feldman & Del Negro, 2006).
The view that neuromodulation by CO2/H+ is involved in normoxic (and anoxic) pre/postinspiratory lumbar activities is supported by in vivo findings showing that the strength of expiratory activities correlates positively with arterial CO2 (Fregosi et al. 1987; Yasuma et al. 1993; Mateika et al. 1996). In that context, expiratory activity is not detectable (even with CO2 stimulation) in cats anaesthetized with urethane, α-chloralose or nembutal, whereas it is seen in ketamine-anaesthetized or decerebrate animals (Fregosi et al. 1987). This suggests that the (abdominal) expiratory component of the medullary respiratory network is very sensitive to experimental conditions (Iscoe, 1998). Accordingly, slight variations in preparation techniques might account for the variable patterns of control lumbar respiratory activities in our study.
Effects of anoxia on lumbar locomotor activity
It is not known which neurons constitute the primary central pattern generator of hindlimb locomotion. Though, several lines of evidence suggest that the ability to generate locomotor output in isolation is highest in a spinal region between thoracic root 11 and L2 (Kiehn & Butt, 2003) (Fig. 1). The latter study also showed that locomotor activity persists upon removal of the dorsal aspect of the isolated lower thoracic-lumbar spinal cord. This indicated that the central pattern generator is located in ventromedial regions (Fig. 1). Conversely, locomotor neurons are located within the ventral horn, likely in close anatomical association with the lumbar respiratory motoneurons. We showed that anoxia abolished both electrically and chemically evoked lumbar locomotor rhythms similar to previous findings in isolated newborn mouse spinal cords (Wilson et al. 2003). In a different study, hypoxia first potentiated and later attenuated ventral root evoked potentials in newborn rat cervical cords (Ataka et al. 1996). It was concluded from findings in that report and related work on adult spinal cats (Kolenda et al. 2003) that interneurons of excitatory pathways are less sensitive to hypoxia/ischaemia than motoneurons, and that motoneurons are less sensitive than interneurons of inhibitory pathways. Based on the present results, it cannot be decided whether lumbar locomotor neurons are more susceptible to anoxia than their respiratory counterparts, or whether they do rather not receive drive from a presumptive anoxia-sensitive central locomotor pattern generator. However, because lumbar respiratory activity persisted during sustained anoxia neuronal functions are not principally blocked by anoxia in newborn rat lumbar cords.
In contrast to the persistence of cervical and lumbar respiratory bursts during anoxia, their recovery was incomplete in contrast to full recovery of locomotor rhythms. It is possible, though, that the recovery of locomotor activity is only transient and that anoxic inactivation of the central locomotor pattern generator induces a sequel of events leading to damage later during postnatal development. In line with this assumption, one study reported that severe hypoxia of newborn rats results in shorter dendrites of lumbar, but not cervical, motoneurons by the end of the second postnatal week (Takahashi et al. 1999). The authors hypothesized that this phenomenon may contribute to the more pronounced occurrence in lower limbs of spastic motor deficits, which are the major neurological manifestation of hypoxic brain injury in preterm infants with cerebral palsy (Volpe, 1994).
In that context, we found that the recovery of both (electrically induced) locomotor and respiratory rhythms from anoxia interfered with the occurrence of persistent and massive tonic lumbar activity. Such postanoxic discharge may cause long-term changes in lumbar motor circuits which may result, for example, in the above mentioned shortening of lumbar dendrites and chronic spinal hyperexcitability. Pronounced spontaneous lumbar activity was also observed in control solution in brainstem-complete cords that were transected between the preBötC and VIIc. This activity is likely to be due to removal of descending tonic inhibitory influences on spinal locomotor circuits from rostral brainstem structures (Smith & Feldman, 1987; Zaporozhets et al. 2006). It is possible that the postanoxic spinal hyperexcitability in preparations with more rostral medullary tissue is, at least partly, due to long-term depression of these inhibitory brainstem structures. This raises the possibility that also long-term impairment of medullary structures involved in locomotor control may contribute to spasticity in cerebral palsy. We suggest that the brainstem–spinal cord preparations of the present study are potent models for studying such clinically relevant questions.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR) and the Alberta Heritage Foundation for Medical Research (AHFMR) (to K.B.) and FIRB (to G.T.). We thank Araya Ruangkittisakul and Paul Blackburn for participation in some experiments and analysis of some of the data. We also thank Dr A. Nistri for valuable discussions and comments on an earlier version of the manuscript.
References
- Aoki M, Mori S, Kawahara K, Watanabe H, Ebata N. Generation of spontaneous respiratory rhythm in high spinal cats. Brain Res. 1980;202:51–63. [PubMed] [Google Scholar]
- Arata A, Onimaru H, Homma I. Possible synaptic connections of expiratory neurons in the medulla of newborn rat in vitro. Neuroreport. 1998;9:743–746. doi: 10.1097/00001756-199803090-00033. [DOI] [PubMed] [Google Scholar]
- Ataka H, Murakami M, Goto S, Moriya H, Hayashi F, Fukuda Y. Effects of hypoxia on the ventral root motor-evoked potential in the in vitro spinal cord preparation. Spine. 1996;21:2095–2100. doi: 10.1097/00007632-199609150-00007. [DOI] [PubMed] [Google Scholar]
- Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharmacol. 2004a;2:221–243. doi: 10.2174/1570159043476828. [DOI] [PubMed] [Google Scholar]
- Ballanyi K. Protective role of neuronal KATP channels in brain hypoxia. J Exp Biol. 2004b;207:3201–3212. doi: 10.1242/jeb.01106. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Kuwana S, Völker A, Morawietz G, Richter DW. Developmental changes in the hypoxia tolerance of the in vitro respiratory network of rats. Neurosci Lett. 1992;148:141–144. doi: 10.1016/0304-3940(92)90824-q. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Völker A, Richter DW. Anoxia induced functional inactivation of neonatal respiratory neurones in vitro. Neuroreport. 1994;6:165–168. doi: 10.1097/00001756-199412300-00042. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Völker A, Richter DW. Functional relevance of anaerobic metabolism in the isolated respiratory network of newborn rats. Pflugers Arch. 1996;432:741–748. doi: 10.1007/s004240050193. [DOI] [PubMed] [Google Scholar]
- Beato M, Nistri A. Interaction between disinhibited bursting and fictive locomotor patterns in the rat isolated spinal cord. J Neurophysiol. 1999;82:2029–2038. doi: 10.1152/jn.1999.82.5.2029. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Anticonvulsant adenosine A1 receptor-mediated adenosine action on neuronal networks in the brainstem-spinal cord of newborn rats. Neuroscience. 2000;96:359–371. doi: 10.1016/s0306-4522(99)00544-8. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem–spinal cord of neonatal rats. J Physiol. 1993;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayetanot F, Bodineau L, Frugiere A. 5-HT acting on 5-HT (1/2) receptors does not participate in the in vitro hypoxic respiratory depression. Neurosci Res. 2001;41:71–78. doi: 10.1016/s0168-0102(01)00266-8. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatonnet F, Dominguez del Toro E, Thoby-Brisson M, Champagnat J, Fortin G, Rijli FM, Thaeron-Antono C. From hindbrain segmentation to breathing after birth: developmental patterning in rhombomeres 3 and 4. Mol Neurobiol. 2003;28:277–294. doi: 10.1385/mn:28:3:277. [DOI] [PubMed] [Google Scholar]
- Dubayle D, Viala D. Localization of the spinal respiratory rhythm generator by an in vitro electrophysiological approach. Neuroreport. 1996;7:1175–1180. doi: 10.1097/00001756-199604260-00016. [DOI] [PubMed] [Google Scholar]
- Fazekas JF, Alexander FAD, Himwich HE. Tolerance of the newborn to anoxia. J Physiol. 1941;134:282–287. [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VK, Wong VH, Wang Y. Postnatal age influences the ability of rats to autoresuscitate from hypoxic-induced apnea. Am J Physiol Regul Integr Comp Physiol. 2000;279:R39–R46. doi: 10.1152/ajpregu.2000.279.1.R39. [DOI] [PubMed] [Google Scholar]
- Fregosi RF. Influence of hypoxia and carotid sinus nerve stimulation on abdominal muscle activities in the cat. J Appl Physiol. 1994;76:602–609. doi: 10.1152/jappl.1994.76.2.602. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Knuth SL, Ward DK, Bartlett D., Jr Hypoxia inhibits abdominal expiratory nerve activity. J Appl Physiol. 1987;63:211–220. doi: 10.1152/jappl.1987.63.1.211. [DOI] [PubMed] [Google Scholar]
- Fukuda Y. Respiratory neural activity responses to chemical stimuli in newborn rats: reversible transition from normal to ‘secondary’ rhythm during asphyxia and its implication for ‘respiratory like’ activity of isolated medullary preparation. Neurosci Res. 2000;38:407–417. doi: 10.1016/s0168-0102(00)00191-7. [DOI] [PubMed] [Google Scholar]
- Fung ML, Wang W, Darnall RA, St John WM. Characterization of ventilatory responses to hypoxia in neonatal rats. Respir Physiol. 1996;103:57–66. doi: 10.1016/0034-5687(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Gozal D, Torres JE, Gozal YM, Nuckton TJ. Characterization and developmental aspects of anoxia-induced gasping in the rat. Biol Neonate. 1996;70:280–288. doi: 10.1159/000244377. [DOI] [PubMed] [Google Scholar]
- Hübner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Iizuka M. GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat. J Physiol. 2003;551:617–633. doi: 10.1113/jphysiol.2003.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Jacquin TD, Borday V, Schneider-Maunoury S, Topilko P, Ghilini G, Kato F, Charnay P, Champagnat J. Reorganization of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron. 1996;17:747–758. doi: 10.1016/s0896-6273(00)80206-8. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Hayashi F, Tatsumi K, Kuriyama T, Fukuda Y. Inhibitory mechanisms in hypoxic respiratory depression studied in an in vitro preparation. Neurosci Res. 2000;38:281–288. doi: 10.1016/s0168-0102(00)00171-1. [DOI] [PubMed] [Google Scholar]
- Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol. 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol. 2003;70:347–361. doi: 10.1016/s0301-0082(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Kolenda H, Steffens H, Hagenah J, Schomburg ED. Different susceptibility of facilitatory and inhibitory spinal pathways to ischemia in the cat. Neurosci Res. 2003;47:357–366. doi: 10.1016/j.neures.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Dunin-Barkowski WL, Vidruk EH, Orem JM. Ventilatory response of the cat to hypoxia in sleep and wakefulness. J Appl Physiol. 2003;95:545–554. doi: 10.1152/japplphysiol.01051.2002. [DOI] [PubMed] [Google Scholar]
- Macefield G, Nail B. Phrenic and external intercostal motoneuron activity during progressive asphyxia. J Appl Physiol. 1987;63:1413–1420. doi: 10.1152/jappl.1987.63.4.1413. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Beato M, Nistri A. Alternating rhythmic activity induced by dorsal root stimulation in the neonatal rat spinal cord in vitro. J Physiol. 2001;530:105–112. doi: 10.1111/j.1469-7793.2001.0105m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Essif E, Fregosi RF. Effect of hypoxia on abdominal motor unit activities in spontaneously breathing cats. J Appl Physiol. 1996;81:2428–2435. doi: 10.1152/jappl.1996.81.6.2428. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurons. Prog Neurobiol. 1991;37:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Okada Y, Kawai A, Mückenhoff K, Scheid P. Role of the pons in hypoxic respiratory depression in the neonatal rat. Respir Physiol. 1998;111:55–63. doi: 10.1016/s0034-5687(97)00105-9. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Dong X, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Hypoxia-induced changes in neuronal network properties. Mol Neurobiol. 2005;32:251–283. doi: 10.1385/MN:32:3:251. [DOI] [PubMed] [Google Scholar]
- Potts JT, Rybak IA, Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci. 2005;25:1965–1978. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Folkow LP, Blix AS. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu Rev Physiol. 2007;69:113–143. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Wilken B, Richter DW. The hypoxic response of neurones within the in vitro mammalian respiratory network. J Physiol. 1998;507:571–582. doi: 10.1111/j.1469-7793.1998.571bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. J Physiol. 1991;443:231–256. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Secchia L, Bornes TD, Palathinkal DM, Ballanyi K. Dependence on extracellular Ca2+/K+ antagonism of inspiratory centre rhythms in slices and en bloc preparations of newborn rat brainstem. J Physiol. 2007;584:489–508. doi: 10.1113/jphysiol.2007.142760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle WA, Witte TA. Survival of the respiratory (gasping) mechanisms in young animals subjected to anoxia. Proc Soc Exp Biol New York. 1941;47:495–497. [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Solomon IC. Glutamate neurotransmission is not required for, but may modulate, hypoxic sensitivity of pre-Botzinger complex in vivo. J Neurophysiol. 2005;93:1278–1284. doi: 10.1152/jn.00932.2004. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, O'Neal MH., 3rd CO2/H+ chemoreception in the cat pre-Bötzinger complex in vivo. J Appl Physiol. 2000;88:1996–2007. doi: 10.1152/jappl.2000.88.6.1996. [DOI] [PubMed] [Google Scholar]
- Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol. 2003;36:113–122. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- St John WM. Neurogenesis, control, and functional significance of gasping. J Appl Physiol. 1990;68:1305–1315. doi: 10.1152/jappl.1990.68.4.1305. [DOI] [PubMed] [Google Scholar]
- St John WM, Zhou D, Fregosi RF. Expiratory neural activities in gasping. J Appl Physiol. 1989;66:223–231. doi: 10.1152/jappl.1989.66.1.223. [DOI] [PubMed] [Google Scholar]
- Taccola G, Marchetti C, Nistri A. Modulation of rhythmic patterns and cumulative depolarization by group I metabotropic glutamate receptors in the neonatal rat spinal cord in vitro. Eur J Neurosci. 2004;19:533–541. doi: 10.1111/j.0953-816x.2003.03148.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Tanaka H, Oki J. Development of spinal motoneurons in rats after a neonatal hypoxic insult. Pediatr Neurol. 1999;21:715–720. doi: 10.1016/s0887-8994(99)00080-6. [DOI] [PubMed] [Google Scholar]
-
Voipio J, Ballanyi K. Interstitial
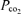 and pH, and their role as chemostimulants in the isolated respiratory network of neonatal rats. J Physiol. 1997;499:527–542. doi: 10.1113/jphysiol.1997.sp021946. [DOI] [PMC free article] [PubMed] [Google Scholar]
and pH, and their role as chemostimulants in the isolated respiratory network of neonatal rats. J Physiol. 1997;499:527–542. doi: 10.1113/jphysiol.1997.sp021946. [DOI] [PMC free article] [PubMed] [Google Scholar] - Voituron N, Frugiere A, Champagnat J, Bodineau L. Hypoxia-sensing properties of the newborn rat ventral medullary surface in vitro. J Physiol. 2006;577:55–68. doi: 10.1113/jphysiol.2006.111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völker A, Ballanyi K, Richter DW. Anoxic disturbance of the isolated respiratory network of neonatal rats. Exp Brain Res. 1995;103:9–19. doi: 10.1007/BF00241960. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in the premature infant-current concepts. Prev Med. 1994;23:638–645. doi: 10.1006/pmed.1994.1106. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Chersa T, Whelan PJ. Tissue PO2 and the effects of hypoxia on the generation of locomotor-like activity in the in vitro spinal cord of the neonatal mouse. Neuroscience. 2003;117:183–196. doi: 10.1016/s0306-4522(02)00831-x. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Vasilakos K, Remmers JE. Phylogeny of vertebrate respiratory rhythm generators: The Oscillator Homology Hypothesis. Respir Physiol Neurobiol. 2006;154:47–60. doi: 10.1016/j.resp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Yasuma F, Kimoff RJ, Kozar LF, England SJ, Bradley TD, Phillipson EA. Abdominal muscle activation by respiratory stimuli in conscious dogs. J Appl Physiol. 1993;74:16–23. doi: 10.1152/jappl.1993.74.1.16. [DOI] [PubMed] [Google Scholar]
- Zaporozhets E, Cowley KC, Schmidt BJ. Propriospinal neurons contribute to bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol. 2006;572:443–458. doi: 10.1113/jphysiol.2005.102376. [DOI] [PMC free article] [PubMed] [Google Scholar]