Abstract
In order to interpret the formation of receptive field surrounds in retinal neurons, a proton-mediated mechanism was proposed to mediate feedback from horizontal cells (HCs) to cone photoreceptors. To verify the idea that depolarized HCs release protons, we measured, by a fluorescence ratiometric technique, the pH of the immediate external surface (pHs) of HCs isolated from the carp or goldfish retina. When HCs stained by 5-hexadecanoylaminofluorescein, a pH-sensitive lipophilic dye, were depolarized by bath-application of kainate or high-K+ medium, pHs was lowered. The amount of pHs change was monotonically dependent on the degree of depolarization, as much as 0.21 ± 0.05 pH units by 100 mV depolarization (induced by 100 mm K+). Acidification was suppressed by 400 nm bafilomycin A1, a specific inhibitor of the vacuolar type H+ pump (V-ATPase), suggesting that depolarization released protons from HCs via the voltage-sensitive H+ pump. Immunocytochemical analysis, using an anti-V-ATPase antibody, revealed the existence of V-ATPase in dissociated HCs. These results support the hypothesis that the feedback from HCs to cones could be proton mediated.
The retina converts the visual image into neural signals and, at the initial stage, the individual photoreceptors work as individual pixels. However, this conversion is not simply pixel by pixel. Neurons in the initial part of the visual pathway, from retinal bipolar cells to lateral geniculate neurons, have concentric receptive fields with centre–surround antagonism (Kuffler, 1953). Such receptive field structure is believed to enhance the contrast of the image by lateral inhibition. It is generally agreed that the surround inhibition is due to horizontal cells (HCs) having an inhibitory feedback interaction with cone photoreceptors (Baylor et al. 1971). In the dark, HCs are maintained in a depolarized state by l-glutamate tonically released from cones. HCs have large receptive fields due to their electrical coupling. Thus, surround illumination induces substantial hyperpolarization in HCs and the size of the receptive fields matches with the receptive field surround of cones (Baylor et al. 1971; Verweij et al. 1996; Hirasawa & Kaneko, 2003). It has also been suggested that negative feedback from HCs contributes to the generation of colour opponency in the fish retina (Stell et al. 1975).
Originally, it was suggested that γ-aminobutyric acid (GABA) mediates the feedback signal from HCs to cones (Lam & Steinman, 1971; Tachibana & Kaneko, 1984). GABA release, however, was not the only mechanism since light and electron microscopic autoradiography showed that only H1 type HCs express glutamic acid decarboxylase (GAD), which catalyses conversion of glutamate to GABA (Marc et al. 1978). Tatsukawa et al. (2005) have shown that GABAergic input from HCs to cones is present but its contribution to negative feedback is weak and limited. It has also been demonstrated that picrotoxin, a GABA-receptor antagonist, could not completely suppress the cone receptive field surround (Hirasawa & Kaneko, 2003).
Novel mechanisms for the HC feedback have been proposed by Verweij et al. (1996) and by Hirasawa & Kaneko (2003). In both models, it is suggested that HC membrane depolarization suppresses the presynaptic L-type Ca2+ current (ICa) of cone photoreceptor terminals leading to suppression of the amount of transmitter released. Voltage-gated Ca2+ channels (VGCCs) of neurons are strongly pH sensitive in the physiological range (Tombaugh & Somjen, 1996). Acidification of extracellular medium reduces ICa and shifts the activation voltage to more positive potentials. pH-dependent suppression of ICa and a positive shift of the activation voltage have been observed also in the cone synaptic terminals (Barnes & Bui, 1991; Hirasawa & Kaneko, 2003). These changes could also suppress the transmitter release from cones. From ‘pH-clamp’ experiments on retinal slices of newt, Hirasawa & Kaneko (2003) suggested that depolarization of HCs would cause pH decrease in the cone synaptic cleft. During surround illumination, the synaptic cleft would be alkalinized, VGCCs would tonically be activated and transmitter release from cones would be enhanced. Accordingly, Hirasawa & Kaneko (2003) proposed that the negative feedback from HCs to cone terminals could be mediated by protons released from HCs by membrane depolarization. Vessey et al. (2005) supported this hypothesis by Ca2+ imaging studies of cone terminals of zebrafish retina.
The main aim of the present study was to examine whether the membrane potential of HCs can modify the pH of their immediate surroundings. The surface pH (pHs) of HCs dissociated from carp or goldfish retina was measured by a pH-sensitive lipophilic dye, 5-hexadecanoylaminofluorescein (HAF), which sticks to the external surface of cell membranes (Genz et al. 1999). We found that pHs was lowered during depolarization of HCs induced by bath-application of either kainate or high-K+ medium. We also found that the depolarization-induced pHs reduction was blocked by submicromolar concentration of bafilomycin A1, a specific inhibitor of the vacuolar type H+ pump (V-ATPase; Pappas & Ransom, 1993). Indeed, V-ATPase immunoreactivity was found in dissociated HCs. These results support the hypothesis that the feedback from HCs to cones could be mediated by regulation of proton concentration in the clefts of the invaginating synapses of the cone terminals. A preliminary account of the results was presented earlier in abstract form (Jouhou et al. 2006).
Methods
Horizontal cell isolation
Carp (Cyprinus carpio) of 150–600 g body weight or goldfish (Carassius auratus) of about 50 g body weight were decapitated and pithed, and the eye-balls were enucleated. The experimental procedures conformed to the Guidelines for the Care and Use of Laboratory Animals of The Physiological Society of Japan and of the University Animal Welfare Committee.
Fish were dissected after 15–20 min dark adaptation. An eye ball was hemisected and the retina was isolated. The retina was incubated in 6 units ml−1 of papain (PAPL, Worthington Biochemical Corp., Lakewood, NJ, USA) in Ringer solution and cells were dissociated by mechanical pipetting in a similar way to Kaneko & Tachibana (1986). Dissociated cells were allowed to anchor to the glass bottom of a culture dish (35 mm diameter, 2.5 ml volume) or to a coverslip coated with concanavalin A. Cells were superfused with Ringer solution (2 ml min−1) having the following composition (mm): 102 NaC1, 2.6 KC1, 28 NaHCO3, 1.0 MgCl2, 1.0 CaCl2 and 5.0 glucose. The 40 s delay due to solution change from the reservoir to the recording chamber was subtracted from the recording. The solution was saturated with a mixture of 95% O2 and 5% CO2 gas and maintained at pH 7.60. The pH values of Ringer solutions in the bottles containing different pharmacological agents were monitored continuously by pH meters (D-51, Horiba, Japan). When necessary, the pH of the solution was readjusted to within ±0.01 pH units by changing the bubbling speed of the 95% O2–5% CO2 gas mixture. All the recordings were done in the presence of 0.1 mm Co2+ in order to suppress Ca2+ spikes. In some experiments, a microchamber (0.02 ml volume) made of pieces of cover glass and a sheet of glass slide was used for quick change of superfusate (0.17 ml min−1) driven by a syringe pump (NE-1000, New Era Pump Systems, Wantagh, NY, USA). The delay due to the tubing (2.5 s) was subtracted from the recording.
Recorded cells were identified as cone-driven HCs using criteria established previously (Kaneko & Stuart, 1984). The HCs had large smooth somata with several short and stout dendrites. Presumed rod-driven HCs (Kaneko & Yamada, 1972; Tsukamoto et al. 1986) were not used. As HCs isolated either from carp or goldfish retinae yielded indistinguishable results, we have presented the data from the two species taken together.
Ratiometric imaging method for pHs measurements
5-Hexadecanoylaminofluorescein (HAF; MW = 585.7; Molecular Probes, Eugene, OR, USA) (Genz et al. 1999), a pH-sensitive lipophilic fluorescent dye, was used for ratiometric imaging of surface pH (pHs) of dissociated HCs. The lipophilic long hydrocarbon chain of the HAF molecule sticks on or into the lipid plasma membrane whilst the pH-sensitive fluorescein moiety projects approximately 2 nm from the plasma membrane (estimated from the molecular size), making HAF sensitive to the immediate extracellular surface pH. A stock solution was made by dissolving 1 mg of HAF in 500 μl dimethyl sulfoxide (DMSO) and stored in a freezer. This was diluted in Ringer solution to 5 μm immediately before use (final concentration of DMSO was 0.01%). Dissociated cells were incubated in 5 μm HAF-containing Ringer solution for 20 min at 4°C in the dark. The fluorescence was measured after wash-out dye with dye-free Ringer for 10 min. Fluorescence images were captured by a cooled CCD camera (Retiga EXi, QImaging, Canada) mounted on a fluorescence microscope (BX50WI, Olympus, Japan) equipped with a × 40 long working distance water immersion objective lens (LUMPlanFl .40, Olympus). A pair of light stimuli from a mercury lamp (USH-102D, Ushio Electric, Japan), filtered at 490 or 440 nm (interference filters with 10 nm half-widths), were given alternately to excite the dye at 5 s interval using a rotary filter wheel (MAC2000, Ludl Electronic Products, Hawthorne, NY, USA). Thus, two sets of fluorescence images filtered by a dichroic mirror at a wavelength of 530 nm (XF2058, Chroma Technology Corp., Rockingham, VT, USA) were obtained every 5 s at a resolution of 464 × 347 pixels (binning at 3 × 3). The ratio of the 530 nm fluorescence emission intensity (representing the pHs value) for corresponding pixels of the two images in register (corresponding to 490 or 440 nm excitation) was calculated by using MetaFluor software (Universal Imaging, USA). Any slow drift in the time course of the ratiometric data was removed by high pass (below 0.0017 Hz) digital filtering. Measurements showing no reversibility of effect were abandoned.
Further experiment with higher time and spatial resolutions was done by confocal microscopy (TCS SP2 AOBS, Leica, Germany) to measure the fluorescence intensity of dissociated cells. Here, a microchamber (0.02 ml volume) was used and the confocal microscopy involved a shallow focal plane of 2–3 μm depth, utilizing a small pinhole with 2–3 μm diameter scanning parallel to the cover-glass. Extracellular surface pH (pHs) of dissociated HCs was determined by measuring HAF emission (bandwidth, 500–560 nm) excited by a single wavelength (488 nm).
Calibration of pH by ratiometric imaging methods
Absolute values of pHs were calibrated by measuring the fluorescence of stained dead cells in solutions of known pH values. Cells were killed with 1 μg ml−1 gramicidin D, a peptide ionophore, dissolved in DMSO and added to Ringer solution.
Measurement of membrane potential of HCs and pharmacology
The membrane voltages of HCs in various K+ concentrations were measured by the whole-cell version of the patch-clamp technique (current-clamp configuration) using a patch clamp amplifier (AxoPatch 2B, Axon Instruments, USA). The pipette solution contained (mm): 110 KCl, 1 MgCl2, 5 EGTA, 5 Hepes, 5 Mg-ATP and 1 Na-GTP. The pH of the pipette solution was adjusted to 7.20 by 1 n KOH. HCs were depolarized by bath-application of high-K+ Ringer solution, in which NaCl was replaced by equimolar KCl. The theoretical value of the membrane potential V in mV at 25°C was calculated by the Nernst equation, V = 59log10([K+]o/2.6), where [K+]o is the K+ concentration of the test solution in millimolar.
One milligram of bafilomycin A1 (MW = 622.8; LC Laboratories, USA), a specific inhibitor of the vacuolar type H+ pump (V-ATPase) (Pappas & Ransom, 1993), was dissolved in 100 μl DMSO and stored in a freezer at −20°C. It was diluted in Ringer solution immediately before use to the final concentration (the corresponding final concentration of DMSO was less than 0.002%). N-Ethyl-maleimide (NEM; MW = 125.1), another inhibitor of V-ATPase, was also used at a concentration of 0.5 mm. NEM is not as potent and specific as bafilomycin A1 since it can also inhibit the Na+/H+ exchanger, an amiloride-sensitive proton extruder (McKinney & Moran, 1995). All the chemicals were purchased from Sigma (St Louis, MO, USA), unless described otherwise.
Immunocytochemistry
Localization of V-ATPase was demonstrated by immunocytochemical staining. Isolated HCs were fixed with 99.5% ethanol for 5 min. Normal goat serum (005-000-121, Jackson ImmunoResearch, West Grove, PA, USA) diluted 10 times was used to pre-treat the cells to block background staining. Primary and secondary antibodies were dissolved in PBS (pH 7.2) containing 0.1% Triton X-100. The primary antibody was a rabbit polyclonal antibody against the C-subunit or B1/B2-subunits of V-ATPase (H-300 or H-180; Santa Cruz Biotechnology, Santa Cruz, CA, USA). It was diluted 100 times and applied for 12 h at room temperature. The secondary antibody was Cy2-conjugated anti-rabbit IgG developed in goat (111-225-003, Jackson ImmunoResearch) and was applied for 2 h in a refrigerator. The excitation wavelength of the chromophore was 490 nm. The images were observed by a fluorescence microscope (BX62, Olympus) with a mercury lamp (USH-102D).
Data analyses
Data were analysed as means and standard errors of the means.
Results
Extracellular surface of HCs was acidified by membrane depolarization
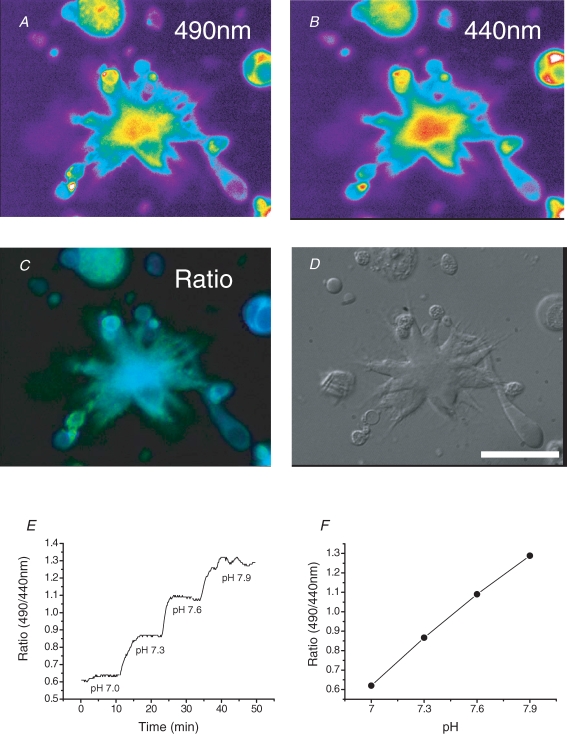
For the measurement of pHs, a corresponding pair of fluorescence images of HAF-stained cone-driven HCs excited by a pair of wavelengths (490 and 440 nm) were recorded at 530 nm (Fig. 1A and B). The ratio of the fluorescence in the two images was deduced as shown in Fig. 1C. Calibration curves of absolute pHs values (Fig. 1F) were obtained by measuring the fluorescence of stained dead cells in solutions of known pH values (Fig. 1E).
Figure 1. Fluorescence ratiometric measurement of extracellular surface pH (pHs) of isolated cone-driven horizontal cells (HCs) enzymatically dissociated from carp retina.
The pHs was measured by using a pH-sensitive lipophilic fluorescent dye, HAF (5-hexadecanoylaminofluorescein), which sticks to the external surface of the cell membrane. Scale bar, 30 μm. A and B, a pair of images of fluorescence emission of 530 nm excited by 490 nm and 440 nm, respectively, from a dissociated HC stained with 5 μm HAF. The HC placed on a glass-bottomed Petri dish was always perfused by Ringer solution kept at pH 7.60 with speed of 2 ml min−1. Red corresponds to higher emission in the pseudo-colour representation. C, the ratio image represents the ratio of A and B, corresponding to absolute value of pH. The yellowish colour shows alkaline pH and bluish colour acidic. D, a photograph of the same HC taken by differential interference microscopy. E, the ratio of fluorescence intensity was obtained from a stained dead cell serially perfused with Ringer solution having different known pH values. The ratio value at each time was obtained by the ratio of two images excited by a pair of wavelengths, each of which image was averaged within a circular area of about 20 μm diameter at the central part of soma. F, a pHs calibration curve in absolute values obtained by the measurement of ratio values shown in E. All the measurements throughout the paper were done in the presence of 0.1 mm Co2+ in order to suppress Ca2+ spikes.
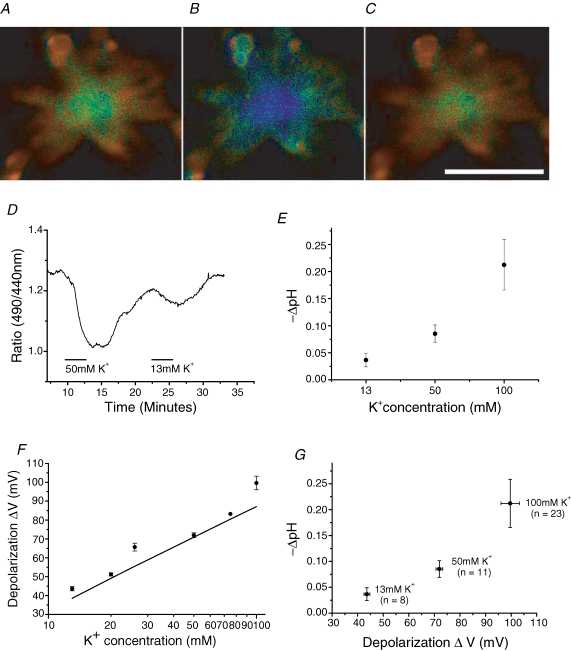
The effects of bath-application of high-K+ Ringer solution on pHs of HCs were investigated; a time series of pHs images of an isolated HC are shown in Fig. 2A–C. Superfusion of 100 mm K+-containing Ringer solution caused acidification of the extracellular cell surface by about 0.10 pH units, from pH 7.60 (shown by a bluish colour in Fig. 2B). Figure 2D shows the effects of 2 min applications of 50 and 13 mm K+ solutions. The effect of [K+]o on pHs was dose dependent. On average, the pHs change was −0.04 ± 0.01 pH units for 13 mm [K+]o, −0.09 ± 0.02 pH units for 50 mm [K+]o and −0.21 ± 0.05 pH units for 100 mm [K+]o (Fig. 2E). Figure 2E illustrates that the amount of pHs drop increased in a supralinear manner with [K+]o. When HCs were superfused with high K+, the membrane depolarization of HCs was proportional to log [K+]o (Fig. 2F). As demonstrated before (Kaneko & Shimazaki, 1975; Kaneko & Tachibana, 1985), the resting membrane potential of the fish HCs followed the Nernst equation for K+, like a K+ electrode (Fig. 2F). Figure 2G was obtained by combining the data of Fig. 2E and F for the three concentrations of K+ used (13, 50 and 100 mm) and illustrates that the pHs drop depended also in a supralinear manner on the magnitude of membrane depolarization of HCs. Thus, the degree of proton extrusion from HCs was found to be strongly voltage dependent. However, the high K+-induced pHs change became undetectable when HCs were superfused with a solution fortified for its pH buffering capacity (20 mm Hepes added to the control Ringer solution) (n = 3, not shown).
Figure 2. Acidification of extracellular surface of cone-driven HCs dissociated from goldfish retinae induced by membrane depolarization when the cells were perfused with high-K+ Ringer solution.
A–C, a series of time-dependent pHs changes of an HC measured by the ratiometric method (before, during 100 mm K+ application for 3 min and after wash-out, respectively). The pHs change is shown in amplified-representation of pseudo-colour. Bluish and yellowish colour corresponds to the lower and higher values of pHs, respectively. The pHs reduction at the centre of the soma was 0.10 pH units from pH 7.60. Scale bar, 30 μm. D, acidification responses of an HC observed by the ratiometric method at the extracellular surface serially induced by 2 min bath-applications of 50 and 13 mm KCl-containing Ringer solution, which could depolarize membrane potential of the cells. We could observe difference of response rise time, fast at 50 mm K+, slow at 13 mm K+. E, acidification of the HC extracellular surface measured by the ratiometric method was dependent on the concentration of KCl in the Ringer solution, being −0.04 ± 0.01 pH units for 13 mm [K+]o (n = 8), −0.09 ± 0.02 pH units for 50 mm [K+]o (n = 11) and −0.21 ± 0.05 pH units for 100 mm [K+]o (n = 23). F, membrane depolarization of the HCs was dependent on the concentration of KCl of the Ringer solution as measured by patch-clamp study in current-clamp mode in comparison with the control Ringer having 2.6 mm KCl. The straight line represents the Nernst equation for K+ as shown by V = (RT/F) × log10([K+]o/2.6), where [K+]o is the ionized K+ concentration in the superfusate, R is the gas constant, T is absolute temperature and F is the Faraday constant. G, high-K+-induced acidification of HCs was dependent on the amount of membrane depolarization, being 43.6 ± 1.2 mV in the solution containing 13 mm K+ (n = 6), 71.9 ± 1.3 mV in 50 mm K+ (n = 3) and 99.6 ± 3.6 mV in 100 mm K+ (n = 5) measured by patch-clamp method in current-clamp mode shown above. This graph was composed by combining E and F at 13, 50 and 100 mm KCl.
Time course and spatial extent of pHs changes
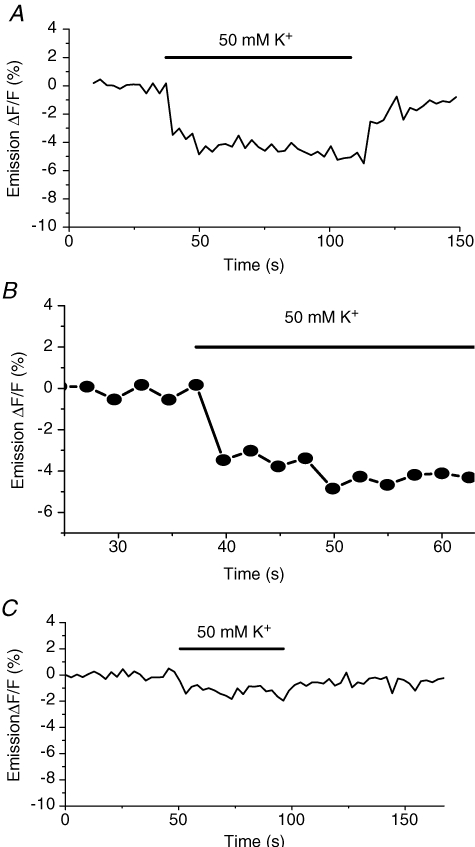
In order to observe pHs change of HCs induced by bath-application of high-K+ Ringer solution at a higher time and spatial resolution, we used a microchamber of 0.02 ml volume instead of a 35 mm culture dish. A confocal microscope with a single excitation wavelength at 488 nm enabled us to measure the pH change in a thin layer on the immediate extracellular surface of HCs, within a focal depth of 2–3 μm. This is a non-ratiometric method (Fig. 3A and B). The pHs clearly decreased, within the sampling interval of 2.5 s, at the onset of the application of high-K+ (Fig. 3A and B). When the focal plane of the confocal microscope was raised by 5.5 μm from the membrane surface, high-K+-induced pH change was not detectable (Fig. 3C).
Figure 3. Kinetics of pHs of a dissociated carp HC in a microchamber of 0.02 ml volume stimulated by bath-applications of 50 mm K+-containing Ringer solution.
The intensity of fluorescence from the HC surface was measured by non-ratiometric method by using a confocal microscope evoked by 488 nm and the change relative to the DC level of the fluorescence emission was shown in an arbitrary unit. Downward deflection shows acidification. Number of cells with similar results n = 5. A, in the application of high-K+ Ringer solution at the immediate cell surface, a large acidification response was observed immediately when switching to the high-K+ Ringer solution. B, onset of its acidification response to the high-K+ Ringer is shown in an expanded time scale. The fluorescence image was sampled at every 2.53 s. C, measurement of pHs measured from debris located at 5.5 μm above the cell surface. Note the smaller amount of acidifications.
Depolarization by kainate also induced acidification of HC surface
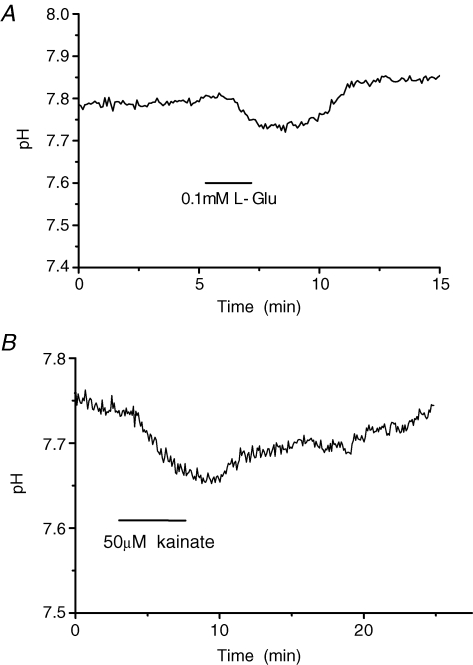
HCs are known to be depolarized by kainate, which activates ionotropic non-NMDA type glutamate receptors on HCs (Knapp & Dowling, 1987; Hirasawa et al. 2001a; Hirasawa & Kaneko, 2003) and can mimic the situation in darkness. Bath-application of l-glutamate (100 μm) or kainate (50 μm) induced a depolarization of isolated HCs by more than 50 mV and a decrease of their pHs by about 0.10 pH units (Fig. 4A and B, respectively).
Figure 4. Bath-application of 100 μML-glutamate- (A) and 50 μm kainite- (B) containing Ringer solution acidified the extracellular membrane surface of dissociated HCs of carp retinae as measured by the ratiometric method.
Number of cells with similar results: A, n = 3, and B, n = 10.
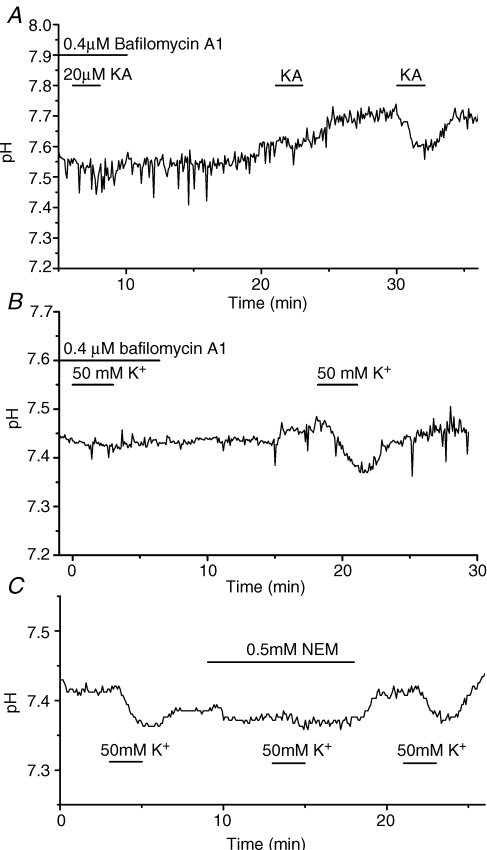
Bafilomycin A1 suppressed the kainate- or high K+-induced acidification
The decrease of pHs of HCs induced by bath-application of 20 μm kainate was reversibly suppressed by 0.4 μm bafilomycin A1, a spacific inhibitor of the vacuolar type H+ pump (VATPase) (Fig. 5A). Recovery from the suppression required prolonged washing for up to 20 min. High-K+-induced acidification was also suppressed reversibly by 0.4 μm bafilomycin A1 (Fig. 5B). The effect of bafilomycin A1 was evident at a concentration as low as 0.2 μm and was maximum at 1 μm. High-K+-induced pHs decrease was also suppressed reversibly by the presence of 0.5 mm N-ethylmaleimide (NEM), another inhibitor of the V-ATPase (Fig. 5C).
Figure 5. H+ pump inhibitors, bafilomycin A1 and N-ethylmaleimide (NEM), blocked acidification of extracellular surface of goldfish HCs induced by bath-application of either 20 μm kainate or 50 mm K+ as observed by the ratiometric method.
A, kainate-induced acidification was blocked in the presence of 0.4 μm bafilomycin A1, a specific inhibitor of the H+ pump, and partially recovered after 10 min wash-out and then fully recovered after 20 min wash-out by the control Ringer solution (n = 4). B, 0.4 μm bafilomycin A1 suppressed 50 mm K+-induced acidification of the cells, which recovered after 13 min wash-out (n = 4). C, 0.5 mm NEM, an inhibitor of the H+ pump, also almost suppressed the 50 mm K+-induced acidification response (middle), which recovered to the original amplitude after 5 min wash-out (n = 4). (Note that the pH of NEM-containing Ringer in this figure was not well adjusted and was 0.02 pH units lower than the control Ringer.)
Other possible mechanisms for high K+-induced acidification of HC surface
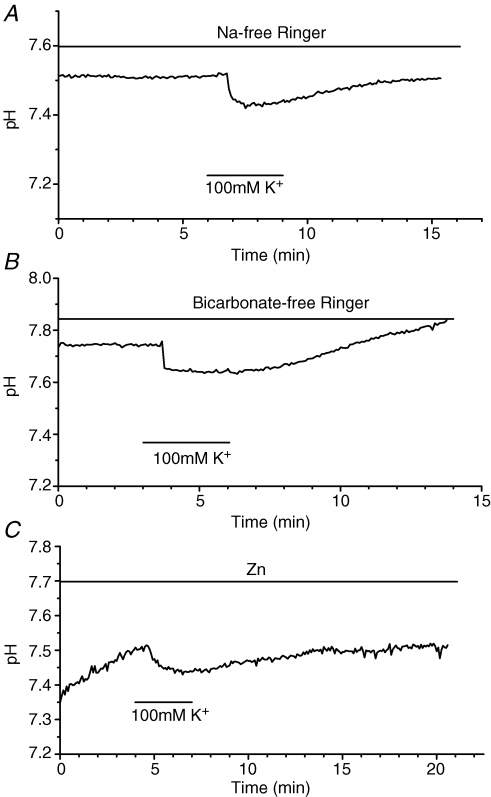
High-K+-induced extracellular acidification was seen in the presence of 0.1 mm Co2+, a general channel blocker of VGCCs, or when Ca2+ in the Ringer solution was completely replaced by Mg2+ (not shown; n = 3). These results suggest that a Ca2+ channel or Ca2+ pump had no significant involvement in the acidification of the extracellular surface of HCs. The high-K+-induced extracellular acidification was unaffected also by Na+-free Ringer solution (Na+ replaced by choline+; Fig. 6A), by HCO3−-free Ringer solution (HCO3− replaced by 2 mm Hepes and NaCl; Fig. 6B) or by 0.2 mm ZnCl2-containing Ringer solution (a blocker of H+ channels; Kapus et al. 1993; Kuno et al. 1997) (Fig. 6C). These observations suggested that Na+-dependent transporters, such as the Na+/HCO3− cotransporter, Na+/H+ exchanger, and H+ channels were unlikely to have a significant role in the high-K+ induced extracellular acidification of isolated HCs.
Figure 6. High-K+-induced acidification of carp HC surface was not blocked by deletion of Na+ or by HCO3− in the perfusates or by the presence of Zn+, a proton channel blocker.
A, the acidification response was not suppressed in the absence of Na+ in the Ringer solution (NaCl being replaced by choline chloride and NaHCO3 replaced by KHCO3) (n = 4). B, the acidification response was not suppressed in the absence of bicarbonate in the Ringer (28 mm NaHCO3 being replaced by 2 mm Hepes and 26 mm NaCl). (n = 3). C, the acidification was not blocked by 0.2 mm ZnCl2-containing Ringer (n = 4). These data suggested that the existence of Na+/HCO3− cotransporters Na+/H+ exchanger and H+ channels in the HC membrane were not essential to the acidification response.
Localization of V-ATPase
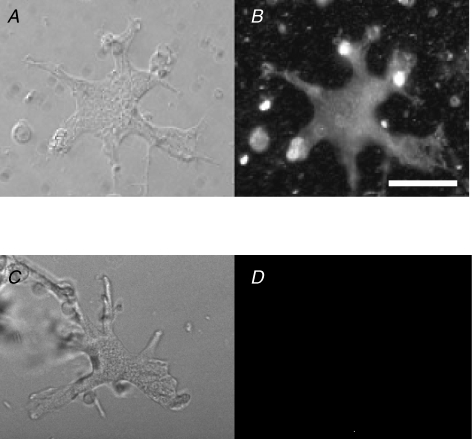
We investigated the existence of V-ATPase by immunocytochemical staining of dissociated HCs. Immunoreactivity against B1/B2 subunits of V-ATPase bound with Cy2-conjugated secondary fluorescent antibody is shown in Fig. 7B. The control image without the primary antibody is shown for comparison in Fig. 7D. Corresponding differential interference microscopic images are shown in Fig. 7A and C, respectively. Immunoreactivity against the C subunit of V-ATPase was similar (data not shown). It was concluded that V-ATPase protein was expressed in isolated HCs.
Figure 7. Localization of V-ATPase in dissociated HCs of goldfish retinae observed by immunocytochemical staining of anti-subunits B1/B2 of V-ATPase.
The secondary fluorescent antibody was Cy2 (green fluorescent dye)-conjugated anti-rabbit IgG. Scale bar, 30 μm. A, the differential interference microscopic image of a dissociated HC. B, the fluorescence microscopic image of the same cell showing immunoreactivity against V-ATPase (n = 10). C, the differential interference microscopic image of another dissociated HC. D, the control images of the cell in C observed without primary antibody but with the secondary fluorescent antibody (n = 4).
Discussion
Extracellular membrane surface pH of HCs, different from bulk solution pH
Use of 5-hexadecanoylaminofluorescein (HAF), a pH-sensitive fluorescent dye, made it possible to measure pHs of the extracellular plasma membrane of HCs at the distance within 2 nm due to the pH-sensitive fluorescein moiety of HAF bound to a long hydrocarbon chain linked to the membrane. In contrast, it was not easy to measure extracellular pH change at the plasma membrane surface of HCs when we used pH-sensitive microelectrodes. It was also hard to detect pHs changes of HCs at a distance more than 6 μm from the cell surface stained with HAF when we used a confocal microscope having a shallow focal depth of 2–3 μm. The difficulty could be due to rapid diffusion of protons from the membrane surface. Thus, pHs was dependent on the distance from the cell surface. There are two possibilities for the difference between pHs and the pH of the bulk solution (pHo). First, pHs could be under the control of active pH homeostasis possibly involving a ‘standing H+ flux’, as proposed by Molina et al. (2004). Second, protons near the surface of HCs may not be very mobile at least within 2 nm distance from the cell surface where the pH sensitive moiety of lipophilic dye attached.
Depolarization-induced extracellular acidification of HCs
By using HAF, we demonstrated that the immediate surrounding of isolated HCs became acidic when membrane potential was depolarized. High K+-induced depolarization brought about a decrease in pHs, which was not detected in the presence of 20 mm Hepes, i.e. when the pH buffering capacity of the medium was enhanced. This indicated that the reduction of the fluorescence emission ratio was due to the pHs reduction rather than any passive effect of membrane voltage change. It is probable that the pHs change we observed in isolated HCs was caused by membrane depolarization that is primarily dependent on K+ concentration controlled by anomalous rectifier K+ channels, following the Nernst equation (Kaneko & Shimazaki, 1975; Kaneko & Tachibana, 1985).
Kainate, an agonist of non-N-methyl-d-aspartate (NMDA) type glutamate receptors, also induced similar pHs reduction in isolated HCs. Therefore, it is not K+, but depolarization of the membrane that brings about the pHs decrease. Importantly, HCs in the retina are depolarized in the dark by the activation of non-NMDA-type glutamate-receptor channels (Lu et al. 1998; Hirasawa et al. 2001a,b). Thus, HCs in the retina would cause acidification of their immediate extracellular membrane surface in the dark when HCs themselves are kept depolarized. During light-evoked hyperpolarization, the reverse process is likely to occur. Indeed, Yamamoto et al. (1992) reported alkalinization of extracellular space of retina by about 0.2 pH units by light-adaptation in isolated retinae.
Extracellular pH of invaginating synaptic cleft within cone pedicles
It is likely that the cleft of the invaginating synapse of the cone terminal is the site most strongly affected by protons extruded from HCs, since the cleft has a narrow and tortuous structure limiting free diffusion of small molecules. DeVries (2001) estimated the magnitude of pH change by glutamate release from cones in the dark to be −0.2 pH units. If we assume the dimension of the synaptic cleft to be 5 μm × 5 μm × 20 nm, for cone pedicles of fish, the corresponding volume would be around 500 attolitre (500 × 10−18 l, 0.5 μm3) and the number of free protons in the cleft would be 12 at pH 7.4. Acidification by 0.2 pH units would correspond to ∼7 free protons (58% increase). The volume of the synaptic cleft in mammals is estimated to be as small as 3 attolitre (Raviola & Gilula, 1975) or 210 attolitres (Rao-Mirotznik et al. 1995). Therefore, the proton extrusion from HCs could induce a surprisingly large pH decrease due to the narrow space of the invaginating synapses. Moreover, a much greater number of free protons secreted into the cleft would be buffered by the huge number of bicarbonate ions before pHs can be measured. Our results of pH changes are probably an underestimate, compared with the pH changes within the invaginating synaptic clefts, since a major fraction of the protons would diffuse into the ‘infinite’ pool of buffered saline used to purfuse the isolated HC preparation.
Time course of HC surface acidification
The latency of the high-K+-induced acidification response measured was around 1 min when we used the culture dish perfusion system. The latency became immeasurable, less than 2.5 s sampling interval, when we used the microchamber perfusion system. In order to relate the membrane voltage to pH changes more directly and to measure the time course of pH changes more precisely, it would be necessary to measure the pHs of voltage-clamped HCs. We attempted this type of experiment many times, but were unsuccessful. Perhaps, attachment of HAF to the surface membrane of HCs prevented effective giga-seal formation and/or HAF-stained HCs became very fragile.
Involvement of vacuolar-type H+ pump in surface acidification of HCs
Acidification of extracellular surface by kainate or high-K+ was suppressed by 0.4 μm bafilomycin A1, a specific inhibitor of the vacuolar type H+ pump (V-ATPase; Pappas & Ransom, 1993), in a dose-dependent manner. This result suggested that the proton extrusion mechanism was primarily due to voltage-dependent outward H+ pumping enhanced by membrane depolarization. However, it is possible that minor contributions from Na+-dependent transporters, such as the Na+/HCO3− cotransporter or Na+/H+ exchanger, or the H+ channel, also occurred. More work is required to elucidate these possibilities.
Schredelseker & Pelster (2004) have shown the existence of V-ATPase in early stage zebrafish retina by investigating the expression of isoforms of V-ATPase (B1 and B2 subunits). Consistent with our pharmacology, immunocytochemical studies of dissociated HCs confirmed the existence of V-ATPase in the HC. Although the light microscopic imaging could not reveal the precise cellular location of V-ATPase molecules, it is unlikely to be intracellular and not related to synaptic vesicles, which have not been found in fish HCs (Stell, 1967). Furthermore, Schwartz (1987) has shown that release of GABA from fish HCs is driven by GABA transporters and not by exocytosis. Thus, it seems simpler to assume that the present immunocytochemical imaging of isolated HCs represents V-ATPase molecules on the HC plasma membrane, consistent with the physiological measurements revealing acidification of the immediate extracellular space due to H+ extrusion induced by membrane depolarization.
These HCs may contribute to pH regulation within the invaginating synaptic cleft. V-ATPase located in plasma membrane is known as an electrogenic H+ pump, driven by ATP hydrolysis and pumping H+ outward, thereby inducing extracellular acidification. When ATP hydrolysis is at a low level, based on the thermodynamics of the H+ pump (Bevensee & Boron, 1998), V-ATPase could mediate efflux or influx of protons depending on whether the membrane potential is depolarizing or hyperpolarizing, respectively, thereby down- or up-regulating transmitter release via ICa at the synaptic terminals. Accordingly, the voltage-dependent H+ pump can influence synaptic interaction within the neural circuitry.
A comparison with the positive feedback hypothesis due to proton influx mechanism in HCs
Using self-referencing H+-selective electrodes, previous work showed a reduction in the extracellular proton concentration by decreasing standing H+ efflux upon glutamate-induced depolarization of dissociated rod-driven HCs in skate retina (Molina et al. 2004) and cone-driven HCs in catfish retina (Kreitzer et al. 2007). Dixon et al. (1993) described that perfusion of glutamate onto catfish HCs also brought about intracellular acidification probably mediated via metabotropic glutamate receptors. Molina et al. (2004) showed immunocytochemically localization of Ca2+–H+-ATPase (Ca2+ pump) on the plasma membrane of rod-driven HCs, suggesting the involvement of a Ca2+ pump in proton influx. Kreitzer et al. (2007) also suggested pharmacologically the involvement of the Ca2+ pump in the cone-driven HCs and suggested that depolarization-induced alkalinization at the synaptic clefts could lead to resensitization of cone synapses and enhancement of glutamate release. These findings would appear to be different from the effects of glutamate described here. However, due to the proposed Ca2+–H+-ATPase, working in both rod- and cone-driven HCs, depolarization-induced extracellular alkalinization during darkness at the photoreceptor synaptic clefts would potentiate transmitter release from the photoreceptors via enhancement of ICa. Such a mechanism would facilitate positive feedback upon photoreceptors as suggested by Molina et al. (2004) and Kreitzer et al. (2007). In contrast, in a comparable study, Hosoi et al. (2005) revealed by voltage-clamp studies of cones and rods in retinal slices of newt that inhibitory action of exocytosed protons released in darkness or by depolarizing-clamp voltages was suppressed by strong pH buffering (40 mm Hepes). Hosoi et al. (2005) also found that the protons inhibited ICa in cone but not in rod terminals, leading to a decrease in glutamate release from cones but not from rods. These results are consistent with our negative feedback hypothesis. The difference in the H+ transporting mechanism might, however, be due to species difference.
Protons as an intercellular messenger mediating the negative feedback signal from HCs to cones
Our present findings support the hypothesis that negative feedback from HCs to cones is mediated by protons in the synaptic cleft of the invaginating cone terminal synapse (Hirasawa & Kaneko, 2003). This hypothesis was also supported by the abolition of the feedback signal under dual voltage-clamp of HCs and cones in the ‘pH-clamp’ condition using Hepes (Cadetti & Thoreson, 2006). Thus, HC membrane depolarization would inhibit the cone transmitter release by suppressing the cone synaptic ICa via the increase of the proton concentration in the synaptic cleft. The strength of the negative feedback signal from HCs to cones would depend on the magnitude of HC membrane depolarization. Accordingly, H+ efflux can be induced depending on the membrane potential of the cell, as well as the difference between intra- and extracellular pH and the concentration of ATP. In conclusion, our results, taken together, support the notion that cone-driven cyprinid HCs could involve protons as a messenger for negative feedback upon cone photoreceptor terminals and thus modulate spatial and temporal contrast in visual perception.
Acknowledgments
We are thankful to Dr M. B. A. Djamgoz, Imperial College London, UK for carefully editing our language and for kind suggestions on our manuscript. We would like to thank to Dr M. Kurokawa and Mr S. Ito, Department of Biological Sciences, Tokyo Metropolitan University for advice in the initial stage of immunocytochemistry. We thank Ms R. Ikuta and Ms N. Tokonami for technical help. This study was supported by JSPS Grant nos. 14380378 and 18500313 to A.K. and No.17657051 to M.Y.
References
- Barnes S, Bui Q. Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties. J Neurosci. 1991;11:4015–4023. doi: 10.1523/JNEUROSCI.11-12-04015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MG, O'Bryan PM. Receptive fields of cones in the retina of turtle. J Physiol. 1971;214:265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Boron WF. The thermodynamics and physiology of cellular pH regulation. In: Kaila K, Ransom BR, editors. pH and Brain Function. New York: Wiley-Liss, Inc.; 1998. pp. 173–194. [Google Scholar]
- Cadetti L, Thoreson WB. Feedback effects of horizontal cell membrane potential on cone calcium currents studied with simultaneous recordings. J Neurophysiol. 2006;95:1992–1995. doi: 10.1152/jn.01042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K, Copenhagen DR. l-Glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron. 1993;11:267–277. doi: 10.1016/0896-6273(93)90183-r. [DOI] [PubMed] [Google Scholar]
- Genz AK, von Engelhardt W, Busche R. Maintenance and regulation of the pH microclimate at the luminal surface of the distal colon of guinea-pig. J Physiol. 1999;517:507–519. doi: 10.1111/j.1469-7793.1999.0507t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Shiells RA, Yamada M. Analysis of spontaneous EPSCs in retinal horizontal cells of the carp. Neurosci Res. 2001a;40:47–86. doi: 10.1016/s0168-0102(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Shiells RA, Yamada M. Blocking AMPA receptor desensitization prolongs spontaneous EPSC decay times and depolarizes H1 horizontal cells in carp retinal slices. Neurosci Res. 2001b;40:217–225. doi: 10.1016/s0168-0102(01)00229-2. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Arai I, Tachibana M. Group III metabotropic glutamate receptors and exocytosed protons inhibit L-type calcium currents in cones but not in rods. J Neurosci. 2005;25:4062–4072. doi: 10.1523/JNEUROSCI.2735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouhou H, Yamamoto K, Homma A, Hara M, Kaneko A, Yamada M. Depolarization of isolated horizontal cells acidifies their immediate surrounding by activating V-ATPase. J Physiol Sci. 2006;56(Suppl.):S91. doi: 10.1113/jphysiol.2007.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Shimazaki H. Effects of external ions on the synaptic transmission from photorecptors to horizontal cells in the carp retina. J Physiol. 1975;252:509–522. doi: 10.1113/jphysiol.1975.sp011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Stuart AE. Coupling between horizontal cells in the carp retina revealed by diffusion of Lucifer yellow. Neurosci Lett. 1984;47:1–7. doi: 10.1016/0304-3940(84)90377-x. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. Effects of l-glutamate on the anomalous rectifier potassium current in horizontal cells of Carassius auratus retina. J Physiol. 1985;358:169–182. doi: 10.1113/jphysiol.1985.sp015546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Tachibana M. Effects of γ-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol. 1986;373:443–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Yamada M. S-potentials in the dark-adapted retina of the carp. J Physiol. 1972;227:261–273. doi: 10.1113/jphysiol.1972.sp010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapus A, Romanek R, Qu AY, Rotstein O, Grinstein S. A pH-sensitive and voltage-dependent proton conductance in the plasma membrane of macrophages. J Gen Physiol. 1993;102:729–760. doi: 10.1085/jgp.102.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AG, Dowling JE. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature. 1987;325:437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Kreitzer MA, Collis LP, Molina AJA, Smith PJS, Malchow RP. Modulation of extracellular proton fluxes from retinal horizontal cells of the catfish by depolarization and glutamate. J Gen Physiol. 2007;130:169–182. doi: 10.1085/jgp.200709737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kuno M, Kawawaki J, Nakamura F. A highly temperature-sensitive proton current in mouse bone marrow-derived mast cells. J Gen Physiol. 1997;109:731–740. doi: 10.1085/jgp.109.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DM, Steinman L. The up-take of [3H]-aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971;68:2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Shen Y, Yang XL. Desensitization of AMPA receptors on horizontal cells isolated from crucian carp retina. Neurosci Res. 1998;31:123–135. doi: 10.1016/s0168-0102(98)00031-5. [DOI] [PubMed] [Google Scholar]
- McKinney LC, Moran A. Regulation of intracellular pH in J774 murine macrophage cells: H+ extrusion processes. Am J Physiol Cell Physiol. 1995;268:C210–C217. doi: 10.1152/ajpcell.1995.268.1.C210. [DOI] [PubMed] [Google Scholar]
- Marc RE, Stell WK, Bok D, Lam DMK. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978;182:221–246. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Molina AJ, Verzi MP, Birnbaum AD, Yamoah EN, Hammar K, Smith PJ, Malchow RP. Neurotransmitter modulation of extracellular H+ fluxes from isolated retinal horizontal cells. J Physiol. 2004;560:639–657. doi: 10.1113/jphysiol.2004.065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CA, Ransom BR. A depolarization-stimulated, bafilomycin-inhibitable H+ pump in hippocampal astrocytes. Glia. 1993;9:280–291. doi: 10.1002/glia.440090406. [DOI] [PubMed] [Google Scholar]
- Rao-Mirotznik R, Harkins AB, Buchsbaum G, Sterling P. Mammalian rod terminal: architechture of a binary synapse. Neuron. 1995;14:561–569. doi: 10.1016/0896-6273(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Intramembrane organaization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol. 1975;65:192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker J, Pelster B. Isoforms vatB1 and vatB2 of the vacuolar type ATPase subunit B are differentially expressed in embryos of the zebrafish (Danio rerio) Dev Dynamics. 2004;230:569–575. doi: 10.1002/dvdy.20074. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release γ-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Stell WK. The structure and relationships of horizontal cells and photoreceptor-bipolar synaptic complexes in goldfish retina. Am J Anat. 1967;121:401–424. doi: 10.1002/aja.1001210213. [DOI] [PubMed] [Google Scholar]
- Stell WK, Lightfoot DO, Wheeler TG, Leeper HF. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science. 1975;190:989–990. doi: 10.1126/science.1188380. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. γ-Aminobutyric acid acts at axon terminals of turtle photoreceptors: difference in sensitivity among cell types. Proc Natl Acad Sci U S A. 1984;81:7961–7964. doi: 10.1073/pnas.81.24.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, Kaneda M. GABA mediated component in the feedback response of turtle retinal cones. Vis Neurosci. 2005;22:317–324. doi: 10.1017/S0952523805223076. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ current in isolated rat CA1 neurons. J Physiol. 1996;493:719–732. doi: 10.1113/jphysiol.1996.sp021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Yamada M, Kaneko A. Morphological and physiological studies of rod-driven horizontal cells with special reference to the question of whether they have axons and axon terminals. J Comp Neurol. 1986;255:305–316. doi: 10.1002/cne.902550213. [DOI] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde M, Baldridge W, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;20:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto F, Borgula GA, Steinberg RH. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp Eye Res. 1992;54:689–697. doi: 10.1016/0014-4835(92)90023-l. [DOI] [PubMed] [Google Scholar]