Abstract
Age-dependent changes in the architecture of the sinus node comprise an increasing ratio between fibroblasts and cardiomyocytes. This change is discussed as a potential mechanism for sinus node disease. The goal of this study was to determine the mechanism through which non-excitable cells influence the spontaneous activity of multicellular cardiomyocyte preparations. Cardiomyocyte monolayers (HL-1 cells) or embryonic stem cell-derived cardiomyocytes were used as two- and three-dimensional cardiac pacemaker models. Spontaneous activity and conduction velocity (θ) were monitored by field potential measurements with microelectrode arrays (MEAs). The influence of fibroblasts (WT-fibs) was determined in heterocellular cultures of different cardiomyocyte and fibroblast ratios. The relevance of heterocellular gap junctional coupling was evaluated by the use of fibroblasts deficient for the expression of Cx43 (Cx43−/−-fibs). The beating frequency and θ of heterocellular cultures depended negatively on the fibroblast concentration. Interspersion of fibroblasts in cardiomyocyte monolayers increased the coefficient of the interbeat interval variability. Whereas Cx43−/−-fibs decreased θ significantly less than WT-fibs, their effect on the beating frequency and the beat-to-beat variability seemed largely independent of their ability to establish intercellular coupling. These results suggest that electrically integrated, non-excitable cells modulate the excitability of cardiac pacemaker preparations by two distinct mechanisms, one dependent and the other independent of the heterocellular coupling established. Whereas heterocellular coupling enables the fibroblast to depolarize the cardiomyocytes or to act as a current sink, the mere physical separation of the cardiomyocytes by fibroblasts induces bradycardia through a reduction in frequency entrainment.
Non-muscle cells are the most populous cells in the heart, of which fibroblasts comprise a significant portion (45–75%) (Nag, 1980; Goldsmith et al. 2004). Under physiological conditions, fibroblasts are classically viewed as providing structural support for the heart by regulating the synthesis and degradation of extracellular matrix components such as collagen and fibronectin (Corda et al. 2000). However, under pathophysiological conditions such as myocyte death, inflammation, or hypertrophy, fibroblasts promote the remodelling of the myocardium that can result in a disproportionate accumulation of fibrillar collagen (Manabe et al. 2002). In the working myocardium, fibroblasts have traditionally been viewed as electrically isolated cells that indirectly increase arrhythmic activity by obstructing excitation spread. However, studies suggest that fibroblasts play a more active role in modulating the electrophysiology of the working myocardium (Camelliti et al. 2005; Kohl et al. 2005). Although fibroblasts are non-excitable cells, their high membrane resistance (Shibukawa et al. 2005), less negative resting potential (Rook et al. 1989; Rook et al. 1992), and mechano-sensitive currents (Kohl et al. 1994; Kamkin et al. 2002, 2005; Shibukawa et al. 2005) could influence the excitability of adjacent cardiomyocytes. In vitro, fibroblasts form functional gap junctions with cardiomyocytes via connexin 43 (Cx43) and Cx45 and bridge impulse propagation between cardiomyocyte strands separated by fibroblasts (Rook et al. 1989; Gaudesius et al. 2003; Miragoli et al. 2006). Further, it has been shown in heterocellular myocyte/fibroblast preparations that fibroblasts depolarize neighbouring cardiomyocytes, which dampens their excitability and reduces the conduction velocity (Miragoli et al. 2006). This influence of heterocellular interaction was exploited to design biological pacemakers or more generally, used to change the excitability of multicellular cardiomyocyte preparations in vitro and in vivo (Feld et al. 2002; Kizana et al. 2006).
In the sino-atrial node (SAN), where fibroblasts compose an even greater cell number and volume than in atrial or ventricular tissue (Goldsmith et al. 2004; Camelliti et al. 2005), they are thought not only to modulate impulse propagation, but also impulse generation. The percentage of fibroblasts is described to increase during ageing due to the loss of sinus nodal cells, which could be a potential contributor to ageing-induced bradycardia or sick sinus syndrome (Shiraishi et al. 1992; de Marneffe et al. 1995). This hypothesis is supported by in vitro experiments where intercellular coupling of neonatal cardiomyocytes with fibroblasts decreased the myocytes spontaneous beating frequency in cell pairs as well as in monolayers (Orita et al. 1993; Kamkin et al. 2002). This effect could be prevented by the use of fibroblasts deficient for the expression of Cx43 (Kizana et al. 2006). The mechanism of this heterocellular interaction could be of physiological significance since functional gap junction coupling between myocytes and fibroblasts was demonstrated in the SAN (Kohl et al. 1994, 1998, 2005).
Fibroblasts can mediate their effect in the SAN by (i) acting as a passive current sink, (ii) depolarizing the cardiomyocytes, and/or (iii) modulating the diastolic depolarization by stretch activated ion channels. In vivo, cell–cell communication in the SAN enables not only action potential (AP) propagation but also frequency entrainment between the SAN cardiomyocytes. Consequently, it has been shown that when SAN cells are dissociated their endogenous frequencies display significant variability (Jalife, 1984; Delmar et al. 1986). Increases of intercellular resistances between spontaneously active cardiomyocytes lead to complex patterns of spontaneous activity that derive from phase shifts that individual APs can induce in the diastolic depolarization of neighbouring cardiomyocytes (Michaels et al. 1986, 1987; Cai et al. 1993). Thus, it is reasonable to consider that fibroblasts not only modulate the absolute frequency of the cardiac pacemaker, but also influence the frequency entrainment between the cardiomyocytes and therefore the rhythmicity of the pacemaker itself (Verheijck et al. 1998).
In this study, we aimed to determine in vitro the mechanisms through which fibroblasts influence the spontaneous activity of multicellular cardiomyocyte cultures. The data suggest that fibroblasts modulate impulse generation by mechanisms both dependent and independent on intercellular coupling and that progressive interspersion of fibroblasts in the SAN can reproduce bradycardia and symptoms of sick sinus syndrome.
Methods
Cell culture of HL-1 cells, fibroblasts and mouse ES cells
HL-1 cells
HL-1 cells, a cell line derived from the murine atrial cardiomyocyte tumour lineage AT-1 was cultured in Claycomb medium (JRH Bioscience, Lenexa, KS, USA) supplemented with fetal bovine serum (FBS) (10%), l-glutamine (2 mm), and noradrenaline (0.1 mm) as previously described (Claycomb et al. 1998; White et al. 2004). For experiments, HL-1 cells (4 × 106 cells ml−1) were plated on microelectrode arrays (MEA) where they formed spontaneously active monolayers.
Fibroblasts
Fibroblasts were obtained from enzymatically isolated crude cellular fractions from neonate rat ventricle (Perez et al. 2005). Myocyte isolation was conducted in accordance to Institutional Animal Care and Use Committee guidelines (Loyola University Chicago) from 5-day-old Sprague–Dawley rat hearts as previously described (Perez et al. 2005). After decapitation of 1-day-old new born rat pups from one to two litters (∼10 pups per litter) hearts were quickly removed by thorachotomy and rinsed in normal Tyrode solution. Ventricles were trimmed free of atria and major blood vessels, minced and placed in digestion buffer containing 0.35 mg ml−1 collagenase (Worthington Biochemical Corp., Lakewood, NJ, USA; 37°C). After three sequential 20 min enzyme digestions, the released cells were filtered through a sterile 40 μm nylon mesh, washed and allowed to settle for 30 min in a Percol gradient to separate myocytes from non-myocytes. The obtained firbroblasts were cultured up to 10 passages in Dulbecco's modified Eagle's medium (DMEM) supplemented with FBS (10%; Invitrogen, Frederick, MD, USA). For fibroblasts deficient in the expression of Cx43 we used an immortalized embryonic fibroblast cell line generated from Cx43−/− mice (Cx43−/−-fibs) that was cultured as described (Martyn et al. 1997). To generate heterocellular cultures, fibroblasts were treated with mitomycin to prevent cell division and HL-1 cells and fibroblasts were mixed at the ratios indicated in the text before plating. All MEA measurements were performed in Hepes buffered Claycomb media.
Embryonic stem (ES) cells of the cell line CMV (Specialty Media; Phillipsburg, NJ, USA) were propagated in culture and stably transfected with a vector containing a neomycin resistance gene under the promoter of αMHC (Klug et al. 1996; Pasumarthi & Field, 2002; Kapur & Banach, 2007). The ES cells were differentiated as embryoid bodies (EBs) (Hescheler et al. 2002; Banach et al. 2003) and neomycin (350 μm) was added to the culture medium 1 day after spontaneously beating aggregates were observed (Kapur & Banach, 2007). After 5–6 days of selection, the beating cardiomyocyte aggregates were plated directly on MEAs or on a fibroblast monolayer that was already established on the MEA. Experiments were performed in Hepes buffered Iscove medium (Invitrogen, Frederick, MD, USA).
Dye loading and coculture
To distinguish fibroblasts from HL-1 cardiomyocytes in heterocellular cultures, fibroblasts were loaded with DiO (8 μm, 20 min at 37°C; Molecular Probes) prior to coculture. The DiO signal on the MEA electrode field was imaged (excitation: 488 nm; emission: 520–560 nm) before electrical recordings and the percentage area of fibroblasts in the cocultures was quantified with Image J (W. S. Rasband, ImageJ, National Institutes of Health, Bethesda, MD, USA).
MEA recordings and analysis
Multielectrode arrays (MEAs; Multi Channel Systems, Reutlingen, Germany) were used for field potential recordings (FPs) from spontaneously active HL-1 monolayers and embryonic stem cell derived cardiomyocytes (ESdCs) (Banach et al. 2003; Halbach et al. 2003). The MEAs used in this study consisted of 60 electrodes with a diameter of Ø = 30 μm which had an interelectrode distance of 200 μm (integrated ground electrode). They were fibronectin coated (2.5 μg ml−1 dH2O) for 2 h prior to plating. Experiments were conducted at 37°C and data were stored on-line and analysed off-line with a customized toolbox programmed for MATLAB (The Mathworks, Natick, MA, USA; Egert et al. 2002; Halbach et al. 2003). Cultures were considered synchronized when all 60 electrodes of the MEA exhibited the same beating frequency. Activation time contour plots (Fig. 1C) revealed the direction of excitation spread in the cultures and the conduction velocity (θ) was obtained perpendicularly to the excitation wavefront.
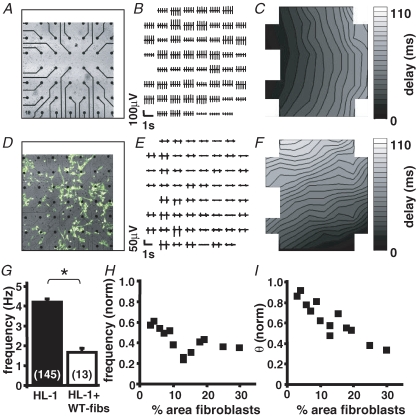
Figure 1. Spontaneous activity and excitation spread in homo- and heterocellular cardiomyocyte preparations:
Representative recordings of a homocellular HL-1 (A–C) and heterocellular (D–F) HL-1 + WT-fibs monolayer Transmission pictures (A and D) display a section of the electrode field and the location of the fibroblasts within the monolayer (white; areas in D). In homo- and heterocellular cultures only preparations were used where all 60 electrodes of the array exhibited synchronized activity (B and E). In both preparations the contour plots (C and F) exhibit continuous excitation spread; however, with a decreased velocity in the heterocellular preparation (homocellular: 2.0 cm s−1; heterocellular: 1.1 cm s−1). G, comparison of the beating frequency recorded in homo- (n = 145) and heterocellular (n = 13) monolayers of HL-1 cells and WT-fibs (*P < 0.05). The beating frequency (H) and conduction velocity (θ) (I) of heterocellular cultures were normalized to those of HL-1 monolayers; both parameter correlate negatively with the percentage area of fibroblasts on the MEA.
FRAP
Fluorescent recovery after photobleaching (FRAP) was used to compare the intercellular coupling between WT- and Cx43−/−-fibroblasts. Monolayers of either cell type were loaded with 2 μm calcein acetoxymethyl ester (calcein AM; Invitrogen, Eugene, OR, USA) in PBS (15 min, at room temperature). After washout, cells were incubated in Tyrode solution containing (mm): NaCl 140; KCl 5.4; CaCl2 2; MgCl2 1.5; glucose 10; Hepes 5 (pH adjusted to 7.38 with NaOH) for 20 min for dye de-esterification. On an inverted confocal microscope (Zeiss LSM 410) a calcein-loaded cell was photobleached by continuous illumination (2–8 s; 488 nm, argon ion laser) and FRAP was monitored every 10 s for up to 50 min. The emitted light was collected at 520–560 nm with a bandpass filter. To correct for global bleaching of the dye, FRAP of the photobleached cell was normalized to the average fluorescence change measured in 3 distant (> 200 μm) control cells.
Electrophysiological recordings
HL-1 or HL-1 + fibs cultures were grown to confluence on 25 mm glass coverslips. Patch electrodes (3–5 MΩ tip resistances; borosilicate glass (WPI)) were filled with a solution containing (mm): potassium aspartate 120; KCl 8; Na2ATP 5; MgCl2 1; Hepes 10, pH adjusted to 7.2 with KOH. Cultures were perfused at room temperature (22–23°C) with a physiological salt solution containing (mm): NaCl 140; KCl 5.4; CaCl2 1.8; MgCl2 1.2; glucose 10; Hepes 5, pH adjusted to 7.35 with NaOH. Upon obtaining the whole-cell configuration, spontaneous HL-1 action potentials (AP) were recorded with an Axon MultiClamp 700A in the current clamp mode. APs were sampled at 10 kHz and analysed off-line with custom programs written for MATLAB.
Noradrenaline, fibronectin and neomycin were purchased from Sigma Inc. (St Louis, MO, USA). Fluo-4/AM was from Invitrogen (Eugene, OR, USA). Antibodies Cx40 and Cx43 were purchased from Invitrogen (Eugene, OR, USA; ZMD.Z-JB1). The antibody against Cx45 was kindly provided by Dr Steinberg (Washington University, St Louis, MO, USA) (Johnson et al. 2002). Data sets were statistically evaluated using Student's paired t test. All data are presented as means ± standard error of the mean.
Results
Effect of fibroblast infiltration on cardiomyocyte monolayers
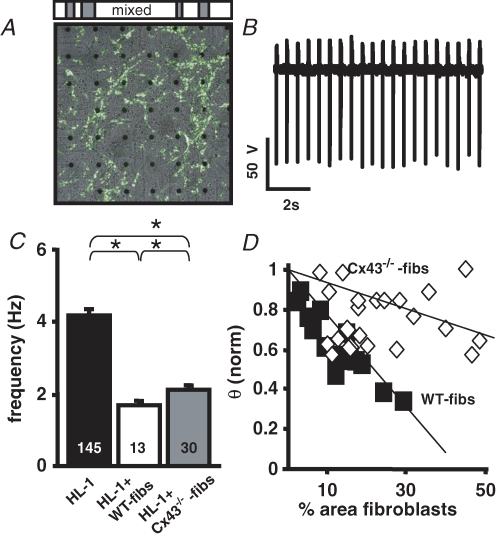
Pathophysiological or ageing related changes in the spontaneous rhythm of the primary cardiac pacemaker, the sino-atrial node (SAN), are often associated with an increase in the proportion of fibroblasts (Kamkin et al. 2002; Squires et al. 2005). To determine how non-excitable cells influence the spontaneous activity and excitation spread in multicellular cardiomyocyte preparations, we compared homocellular monolayers of HL-1 cells (Fig. 1A–C) with heterocellular monolayers consisting of HL-1 cells and mitotically inactivated fibroblasts (WT-fibs; Fig. 1D–F). One day after plating, MEA field potential recordings of homocellular HL-1 cell cultures showed synchronized negative spikes indicating that the cells are electrically coupled by gap junction channels (Fig. 1B). A mean frequency of 4.2 ± 0.1 Hz was measured in 145 independent MEA cultures (n = 145) with a conduction velocity (θ) of 2.2 ± 0.1 cm s−1. Since the monolayers exceeded the electrode grid, the origin of excitation itself was rarely located within the recording area. Heterocellular cultures of HL-1 cells + WT-fibs also developed synchronized activity within 1 day of culture (Fig. 1E); however, the beating frequency (1.7 ± 0.2 Hz; n = 13; Fig. 1G) and θ (1.1 cm s−1; Fig. 1F) were significantly decreased in comparison to homocellular cultures. Beating frequency and θ (Fig. 1H and I) correlated negatively with the percentage area of WT-fibs on the MEA, which indicates a dose-dependent influence of these unexcitable cells.
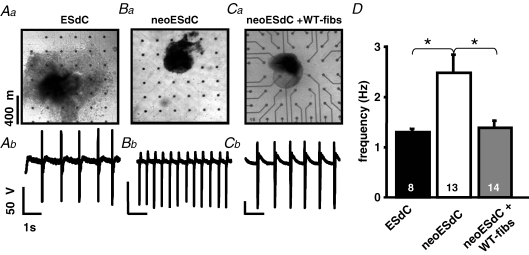
The use of a spontaneously active monolayer is a simplified experimental model of the more complex heterogeneous 3D-structure of the sinus node (Boyett et al. 2000). To determine if our results correlate with 3D-cardiomyocyte preparations, we used embryonic stem cell derived cardiomyocytes (ESdCs) that form spontaneously beating 3D-aggregates (Fig. 2Aa; Kehat et al. 2004; Xue et al. 2005). During differentiation ESdC aggregates are surrounded by and interspersed with a heterogeneous mixture of other cellular phenotypes (e.g. endothelial cells, fibroblasts and smooth muscle cells; Wobus & Boheler, 2005). When the ESdC was plated directly onto the MEA, it reached a stable beating frequency of 1.3 ± 0.1 Hz (n = 8; Fig. 2D) at day 14 of differentiation (Fig. 2Aa; Igelmund et al. 1999; Banach et al. 2003). Neomycin treated ESdCs (neoESdC), where non-excitable cells were removed, exhibited a decreased size (Fig. 2Ba) but remained spontaneously active (Pasumarthi & Field, 2002; Zandstra et al. 2003). The removal of the nonmyocytes from the neoESdC coincided with an increase in its beating frequency (2.5 ± 0.4 Hz; n = 13; Fig. 2Bb and D) compared to age matched non-treated ESdCs. When neoESdCs were plated onto fibroblast monolayers (neoESdCs + WT-fibs) established on MEAs (Fig. 2Ca), after 15 h of coculture, the increased beating frequency of neoESdCs was almost reversed to control (ESdC) levels (1.4 ± 0.2 Hz; n = 14; Fig. 2Cb and D). The results underline that surrounding and interspersed non-excitable cells reduce the beating frequency of 3D-pacemaker aggregates (Fig. 2D).
Figure 2. Non-myocytes attenuate the beating frequency of 3D-pacemaker aggregates.
Transmission light pictures of a heterogeneous ESdC aggregate (Aa), a neomycin treated ESdC aggregate (neoESdC) (Ba), and a neoESdC aggregate plated onto a monolayer of WT-fibs (Ca). Representative field potential recording from a single MEA electrode illustrates the beating frequency of the preparation (Ab–Cb). D, summarized data of the ESdC (black), neo-ESDC (white), and neoESdC + WT-fibs (grey) demonstrate that the ESdC beating frequency increases reversibly upon removal of non-myocytes (*P < 0.05).
HL-1 cells and fibroblasts establish heterocellular coupling
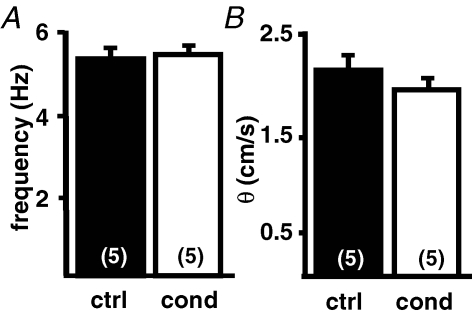
To determine if fibroblasts in the heterocellular cultures exert their influence via secretion of diffusible factors (Manabe et al. 2002), the culture medium of heterocellular cultures was collected and homocellular HL-1 monolayers were incubated in this conditioned medium. After 3 h of incubation, the analysis of the beating frequency (ctrl: 5.4 ± 0.3 versus conditioned: 5.5 ± 0.3; n = 5 each; Fig. 3A) and θ (ctrl: 2.1 ± 0.2 versus conditioned: 1.9 ± 0.1; n = 5 each; Fig. 3B) did not reveal any significant differences between the two culture conditions.
Figure 3. Conditioned media from fibroblasts has no influence on HL-1 cultures.
HL-1 cells were cultured in standard medium and medium conditioned from HL-1 + WT-fibs cocultures. The beating frequency (A) and conduction velocity (B) of HL-1 monolayers did not significantly change when cultured in conditioned medium.
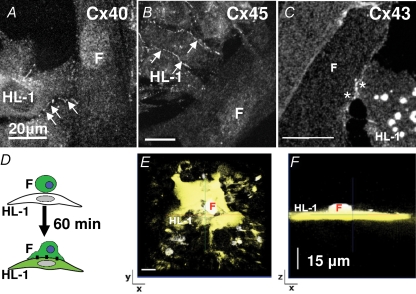
It has been demonstrated both in vitro and in vivo, that fibroblast can establish intercellular coupling with cardiomyocytes (Rook et al. 1992; Gaudesius et al. 2003; Camelliti et al. 2004). To determine the expression of gap junction channels in HL-1 + WT-fibs cocultures, cardiac specific connexins Cx40 (Fig. 4A), Cx45 (Fig. 4B) and Cx43 (Fig. 4C) were immunolabelled. Positive immunostaining for all three connexins was detected in the region of homocellular contact between HL-1 cells (Fig. 4A and B arrows); however, in the region of heterocellular contact between fibroblasts and HL-1 cells, punctuate fluorescent staining only revealed the presence of Cx43 (Fig. 4C; *). Detection of dye diffusion between calcein/AM loaded fibroblasts that were plated onto an HL-1 monolayer allowed us to further assess if positive immunostaining denoted functional gap junction channels (Fig. 4D). Figure 4E shows a confocal xy scan of an HL-1 monolayer 60 min after coculture was established. Part of the HL-1 monolayer exhibits dye loading and a z-stack image (Fig. 4F) visualizes a dye-loaded fibroblast on top of this area. Since no dye was present in the HL-1 cultures, the dye loaded fibroblast can be assumed to be the origin of dye diffusion. The experiment demonstrates that HL-1 cells establish functional intercellular coupling with cocultured fibroblasts most likely through Cx43 gap junction channels.
Figure 4. Heterocellular coupling between WT-fibs and HL-1 cells is established by Cx43.
Fluorescence images obtained from heterocellular cultures of HL-1 cells + WT-fibs (F). HL-1 cells exhibited characteristic punctuated fluorescent staining (arrows) at the sites of cell–cell interaction for Cx40 (A), Cx45 (B) and Cx43 (C). Only Cx43 immunostaining could be detected at the site of heterocellular contact (*). D, schematic illustration of the experimental protocol where calcein/AM loaded fibroblasts are plated on top of an HL-1 monolayer. E and F, xy- and xz-stack, respectively, of a representative experiment 60 min after coculture between HL-1 cells and calcein/AM loaded WT-fibs was established.
Cell-to-cell communication
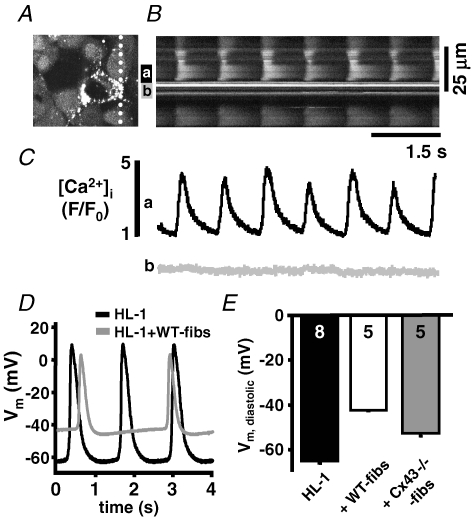
Cardiac fibroblasts are non-excitable cells, but have voltage-dependent and stretch activated cation channels (Kohl et al. 1994; Chilton et al. 2005; Shibukawa et al. 2005). To determine the role of stretch activated ion channels on the frequency decrease in heterocellular cultures, we measured the spontaneous activity and θ in cocultures in the presence of blockers of stretch activated ion channels. Neither streptomycin (100 U ml−1) (Belus & White, 2003; Kondratev & Gallitelli, 2003) nor butanedione monoxime (BDM, 7 mm), a myosin inhibitor that prevents contractions in neonatal monolayers (De Windt et al. 2001), could prevent the fibroblast induced decrease of the beating frequency (data not shown). We further evaluated the excitation induced change of the intracellular Ca2+ concentration ([Ca2+]i) in adjacent fibroblast and HL-1 cells. Figure 5 shows a 2D-confocal image (A) and line scan (B) through a spontaneously active HL-1 monolayer at a location of heterocellular contact (notice the linescan orientation marked with a dotted line; Fig. 5A). Spatially averaged plots of [Ca2+]i demonstrate the rhythmic appearance of Ca2+ transients in individual HL-1 cells (Fig. 5Ca) whereas [Ca2+]i in the adjacent fibroblast remained constant throughout the recording (Fig. 5Cb). The data do not support the hypothesis that the reduced beating frequency and conduction velocity in the heterocellular cultures are brought about by the activation of stretch activated Ca2+ channels in the fibroblasts.
Figure 5. Depolarization but not stretch activated Ca2+ influx modulates the beating frequency in heterocellular HL-1 cell monolayers.
Confocal 2D-image (A) and line scan (B) of a fluo-4/AM loaded heterocellular culture of HL-1 cells + WT-fibs. C, spatially averaged [Ca2+]i recorded from an HL-1 cell (a) and the adjacent WT-fib (b) indicate rhythmic Ca2+ transients in the HL-1 cell are not accompanied by changes of [Ca2+]i in the WT-fib. D, current clamp AP recordings from HL-1 cells in homo- (black) and hetero- (grey) cellular monolayers. E, HL-1 cells' Vm was depolarized in heterocellular monolayers, but depolarization was attenuated in HL-1 + Cx43−/− fibs cultures. The fibroblast density within the monolayer cultures was between 13% and 22%.
It has been proposed that fibroblasts modulate θ due to their depolarizing influence on cardiomyocytes and the subsequent inactivation of voltage-dependent Na+ channels (INa) (Miragoli et al. 2006). To determine the influence of fibroblasts on HL-1 resting potential (Vm), we recorded HL-1 cells' Vm in heterocellular HL-1 + WT-fibs monolayers (Fig. 5D and E). The data revealed that Vm was significantly depolarized in heterocellular cultures (HL-1: 65.2 ± 2.3 mV, n = 8; versus HL-1 + WT-fibs: − 42.2 ± 0.5 mV, n = 5) and the AP upstroke velocity was reduced (dV/dt: HL-1: 1.6 ± 0.11 V s−1; HL-1 + WT-fibs: 1.28 ± 0.05 V s−1). In the representative examples shown in Fig. 5D, the beating frequency of the heterocellular culture was significantly reduced, which supports the hypothesis that a fibroblast mediated depolarization of the HL-1 cells is one reason for the changed excitability in heterocellular cultures; however, an additional effect of the fibroblasts as a current sink cannot be excluded.
Cx43−/−-fibroblasts
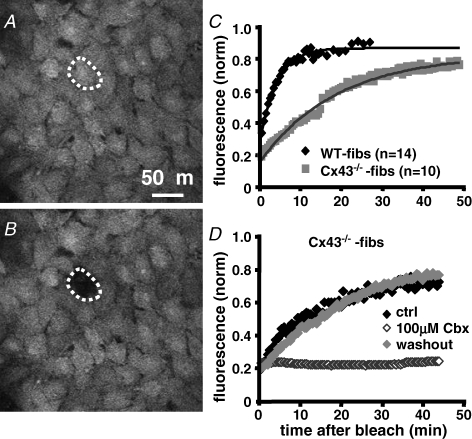
To determine if the fibroblasts' influence on the spontaneous activity and θ can be prevented by the suppression of gap junction channel formation, we conducted the MEA coculture experiments using a fibroblast cell line deficient for the expression of Cx43 (Cx43−/−-fibs; Martyn et al. 1997; Kizana et al. 2005). We evaluated the difference of intercellular coupling established between WT-or Cx43−/−-fibs by FRAP measurements in calcein AM loaded monolayers (Fig. 6A and B). The fluorescence in both cell types recovered after bleaching; however, the time constant of recovery was significantly increased in Cx43−/−-fibs (Fig. 6C; WT-fibs: 246 ± 14 s, n = 14; Cx43−/−-fibs: 1140 ± 50 s, n = 10). Addition of the gap junction inhibitor carbenoxolone (100 μm) to the bath solution prevented FRAP in both preparations (Fig. 6D) confirming that dye recovery was mediated by intercellular diffusion. The remaining fluorescence recovery in Cx43−/−-fibs could be enabled by Cx45 whose expression at low levels was demonstrated in these cells (Martyn et al. 1997); however, expression of other connexin isoforms cannot be entirely ruled out.
Figure 6. Cx43−/−-fibs exhibit significantly reduced intercellular coupling.
Fibroblast monolayers loaded with the gap junction permeant dye calcein/AM before (A) and after (B) photobleaching of a single cell within the monolayer. C, the time-dependent fluorescence recovery after photobleaching (FRAP) was recorded for 30 and 50 min, respectively, in WT- (♦; n = 14) and Cx43−/−-fibs (;▪ n = 10). The time constant of recovery was significantly lower in WT-fibs (τ = 246 ± 14 s) in comparison to Cx43−/−-fibs (t = 1140 ± 50 s), an indicator for the significantly reduced intercellular coupling in Cx43−/−-fibs. D, FRAP can be reversibly inhibited by incubation of the Cx43−/−-fibs monolayers with the gap junction inhibitor carbenoxolone (100 μm).
To determine the mechanism through which fibroblasts influence the spontaneous activity of cardiomyocyte preparations when heterocellular coupling is attenuated, the excitability parameters (HL-1 cells' Vm, frequency, and θ) were measured in coculture experiments with Cx43−/−-fibs (Figs 5 and 7). MEA recordings of heterocellular HL-1 cells + Cx43−/−-fibs cultures revealed that the beating frequency was significantly increased (Fig. 7A and B; 2.1 ± 0.1 Hz; n = 30) in comparison to heterocellular HL-1 + WT-fibs cultures (1.7 ± 0.2 Hz; n = 13; Fig. 7C). However, the attenuation of heterocellular coupling did not allow the beating frequency to recover to control levels of homocellular HL-1 monolayers (4.2 ± 0.9 Hz; n = 145). A different pattern evolved for θ (Fig. 7D). Even when Cx43−/−-fibs covered almost 50% of the MEA area, θ of the heterocellular cultures was only slightly attenuated (Fig. 7D). HL-1 cells in HL-1 + Cx43−/−-fibs heterocellular cultures had a Vm that was significantly less positive (− 52.6 ± 1.6 mV; n = 4) than those recorded in WT-fibs heterocellular cultures although no significant difference in the WT- and Cx43−/−-fibs Vm could be detected (WT: 27 ± 3.8 mV, n = 5; Cx43−/−: −22.5 ± 5 mV; n = 5). Likewise, the AP upstroke velocity in Cx43−/−-fibs heterocellular cultures was comparable to control conditions (1.7 ± 0.21 V s−1). The data indicate that the intercellular coupling between HL-1 cells and Cx43−/−-fibs is significantly attenuated in comparison to WT-fibs. However, while this attenuation can almost compensate the fibroblast induced change in θ, it cannot rescue the fibroblast induced reduction of the spontaneous activity.
Figure 7. Cx43−/−-fibs have an attenuated effect on the conduction velocity but not on the beating frequency.
A, transmission picture of the MEA electrode field and the location of Cx43−/−-fibs within the HL-1 monolayer (white area). B, representative FP from an electrode in the HL-1 + Cx43−/−-fibs coculture. Cx43−/−-fibs induced modulation of HL-1 monolayer beating frequency (C), and θ (D).
Fibroblasts as obstacles within the cardiomyocyte preparation
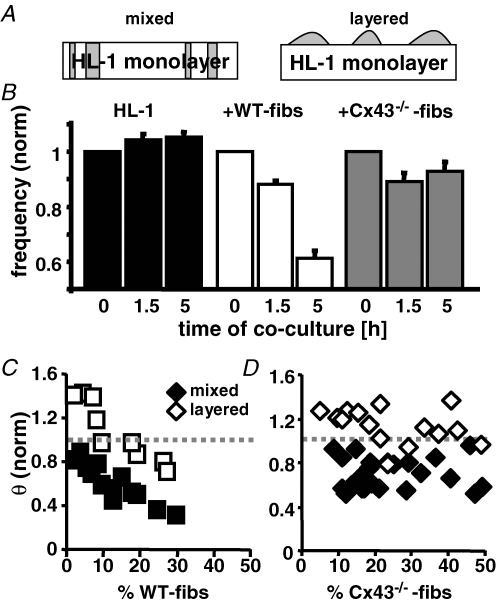
In our fibroblast and HL-1 cell cocultures, fibroblasts are interspersed in the monolayer and physically separate HL-1 cells from one another. To distinguish the influence that fibroblasts exert by establishing intercellular coupling with HL-1 cells from that as an unexcitable obstacle, we plated WT-and Cx43−/−-fibs on top of already established HL-1 monolayers (‘layered’ culture; (Kizana et al. 2006; Miragoli et al. 2006). Figure 8A shows a sketch of the cell distribution in ‘mixed’ and ‘layered’ culture conditions. Frequency and θ of layered cultures were recorded before and during the first 5 h after coculture was established (Fig. 8B). After 1.5 h the normalized beating frequency in the layered-culture preparations was slightly reduced in comparison to control conditions (WT-fibs: 0.88 ± 0.04, n = 10; Cx43−/−-fibs: 0.89 ± 0.04, n = 16); however, whereas in the WT-fibs layered-cultures the normalized frequency progressively declined over the next 3.5 h (0.61 ± 0.03; n = 10), it remained constant in the Cx43−/−-fibs cocultures (0.93 ± 0.04; n = 16). The effect of the fibroblasts on θ after 5 h in the layered versus the mixed cultures was attenuated at all fibroblast densities introduced (Fig. 8C and D). WT-fibs heterocellular cultures now exhibited a biphasic change of θ, with an increase or supranormal θ at low WT-fibs densities (Miragoli et al. 2006). In Cx43−/−-fibs ‘layered’ cultures, an increase in θ could be observed compared to Cx43−/−-fibs mixed cultures. Overall the effect of WT-fibs on the spontaneous activity and θ in layered and mixed cocultures is comparable although it is attenuated in the former. However, in Cx43−/−-fibs preparations there was a difference between mixed and layered culture conditions. Only in the layered HL-1 + Cx43−/−-fibs cultures were HL-1 cells able to maintain their beating frequency. The data imply two independent mechanisms by which fibroblasts modulate the spontaneous activity of cardiac pacemaker preparations, one regulated by intercellular coupling and a second brought about by the physical separation of the cardiomyocytes and independent of the intercellular coupling established.
Figure 8. The fibroblasts' ability to modulate cardiomyocytes spontaneous activity depends on the culture conditions.
A, schematic representation of 2 coculture conditions tested. In the mixed culture, fibroblasts (grey) are interspersed between the HL-1 cells whereas in the layered culture they reside on top of the HL-1 monolayer. B, frequency of cardiomyocyte monolayers 0 h, 1.5 h and 5 h after ‘layered’ coculture with WT- (n = 10) or Cx43−/−-fibs (n = 16) was established. Control cultures consisting of only HL-1 monolayers were recorded at the same time intervals (n = 8). Conduction velocity of mixed (filled symbols) and layered (open symbols) HL-1 + WT-fibs (C) or + Cx43−/−-fibs (D) cocultures are plotted against the fibroblast density on the MEA.
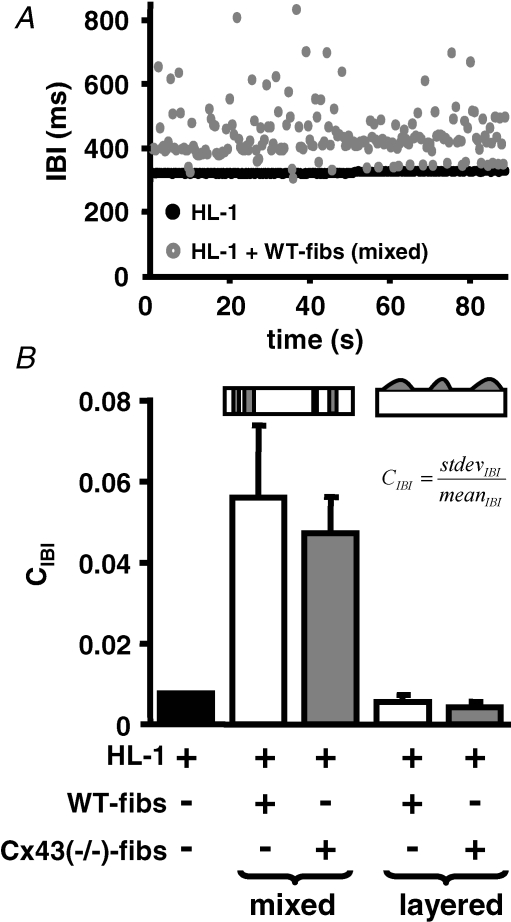
Influence of intercellular coupling on frequency synchronization
It has been proposed that low resistance intercellular coupling within cardiomyocyte aggregates, or a reduction in the number of interacting cardiomyocytes can result in an overall reduction of the beating frequency, as well as an increase in the coefficient of variation of the interbeat interval (IBI) (Clay & DeHaan, 1979; Ypey et al. 1979; Kojima et al. 2005, 2006). A comparison of the IBI variability (quantified as the coefficient of IBI: CIBI = IBIstdev/IBImean) of mixed and layered heterocellular cultures of HL-1 cells + WT-fibs or HL-1 cells + Cx43−/−-fibs revealed that CIBI was significantly increased in mixed heterocellular cultures (mixed cultures: HL-1 + WT-fibs: 0.056 ± 0.017, n = 13; HL-1 + Cx43−/−-fibs: 0.047 ± 0.009, n = 30; HL-1: 0.008 ± 0.0001, n = 78; Fig. 9B). Figure 9A shows the IBI of an HL-1 culture and an HL-1 + WT-fibs mixed coculture with 16.3% fibroblast density. The increased dispersion of the IBI in the mixed culture is perceivable. The changes in the IBI in this case did not correlate with noticeable changes in the origin of excitation. In layered cultures, although the same proportion of fibroblasts were cocultured, the CIBI did not significantly vary from that of homocellular HL-1 monolayers (layered cultures: WT-fibs: 0.006 ± 0.002, n = 10; Cx43−/−-fibs: 0.005 ± 0.0003, n = 16; Fig. 9B), which is consistent with the lack of Cx43−/−-fibs influence on the spontaneous activity in these cultures.
Figure 9. Fibroblast interspersion increases the CIBI in cardiomyocyte monolayers.
A, presentation of the IBI as a function of time for HL-1 monolayer (black) and HL-1 + WT-fibs ‘mixed’ cocultures illustrates the increased dispersion of the IBI in the latter culture. B, the CIBI of ‘mixed’ HL-1 + WT- (white) or Cx43−/−-fibs (grey) cocultures is significantly increased in comparison to control HL-1 monolayers (black), whereas ‘layered’ cocultures of WT- (white) or Cx43−/−-fibs (grey) were comparable to control preparations. No significant difference in the CIBI between WT-and Cx43−/−-fibs cocultures was determined in either culture condition.
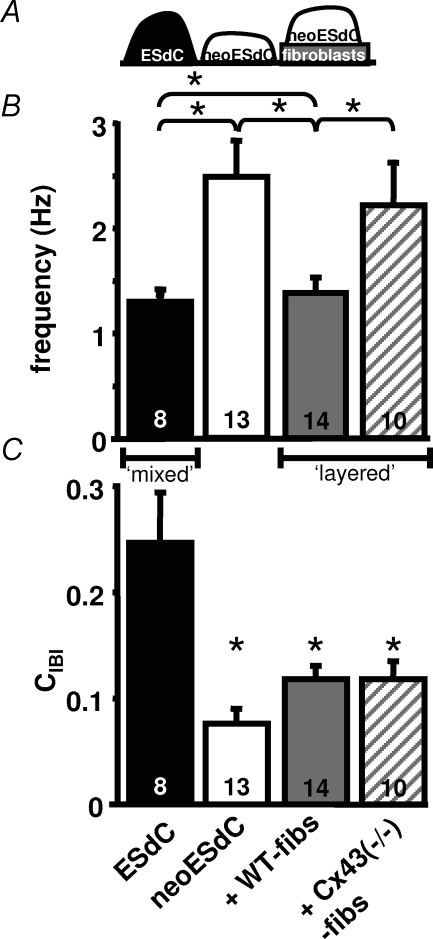
To determine if the hypothesis that fibroblasts modulate spontaneous activity by separating cardiomyocytes holds true for 3D-cardiomyocyte preparations, we conducted experiments with neo-ESdCs (see Fig. 2). As described, removal of non-cardiomyocytes resulted in a significant increase in the neo-ESdCs beating frequency, which could be restored to control conditions by plating the neo-ESdCs onto WT-fibs monolayers (Fig. 10B; ESdC: 1.3 ± 0.1 Hz, n = 8; neo-ESdC + WT-fibs: 1.39 ± 0.15 Hz, n = 14). That this reversal depended on the formation of heterocellular coupling between cardiomyocytes and fibroblasts is supported by the inability of Cx43−/−-fibs monolayers to restore the beating frequency of neo-ESdCs (Fig. 10B; neo-ESdC + Cx43−/−-fibs: 2.21 ± 0.41 Hz; n = 10; neoESdCs: 2.49 ± 0.36 Hz, n = 13). Analysis of the CIBI in the respective cultures revealed (Fig. 10C) a significant decrease in CIBI when non-excitable cells were removed from the ESdC aggregate by neomycin treatment (ESdC: 0.24 ± 0.04; n = 8; neo-ESdC: 0.074 ± 0.01; n = 13). This mechanism is comparable to the removal of interspersed or ‘mixed’ fibroblasts from a cardiomyocyte monolayer. The decrease in CIBI was not reversed by layering the neo-ESdC on WT- or Cx43−/−-fibs (neoESdC + WT-fibs: 0.12 ± 0.01; n = 8; neo-ESdC + Cx43−/−-fibs: 0.12 ± 0.01; n = 10) a mechanism comparable to establishing a layered culture (Fig. 8). The results are therefore in agreement with the data obtained from the cardiomyocyte monolayers and support the hypothesis that fibroblasts modulate the spontaneous activity of cardiac preparations not only by depolarizing the cells or acting as a current sink but also by separating spontaneously active cells and reducing their frequency entrainment.
Figure 10. Lack of Cx43 in fibroblast monolayers prevents beating frequency recovery of neo-ESdCs.
A, schematic drawing of cell distribution of ESdCs, neoESdCs and neoESdCs + WT-fibs on the MEAs. B, the beating frequency of ESdCs (black), neo-ESdCs (white), and neo-ESdCs in coculture with either WT- (grey) or Cx43−/−-fibs (striped) shows that nonmyocytes attenuate ESdCs spontaneous activity depending on the intercellular coupling established in the layered cocultures. C, the CIBI variability in the same cultures decreased significantly with the removal of non-myocytes and did not recover when neo-ESdCs and fibroblast monolayer are layered independent of their intercellular coupling.
Discussion
In this study we present experimental evidence that fibroblasts induce a decrease in the beating frequency and an increase of the interbeat-interval variability in cardiac pacemaker preparations. The fibroblasts act via two additive mechanisms, (i) depolarization of the cardiomyocytes and (ii) the physical separation of neighbouring cardiomyocytes. While intercellular coupling is a prerequisite for the depolarizing effect of the fibroblast and their function as a current sink, an increase in the heterocellular coupling resistance did not prevent the reduced frequency entrainment brought about by the physical separation of the cardiomyocytes. Our data held true for 3D-pacemaker preparations, and therefore present a potential mechanism by which increased fibroblast content can lead to ageing-dependent sinus node diseases.
Monolayers as models for spontaneous activity
HL-1 cells express cardiac specific proteins (Claycomb et al. 1998) and voltage-dependent ion channels that are comparable to those of atrial muscle cells (Claycomb et al. 1998; Sartiani et al. 2002; Xia et al. 2004). The hyperpolarization activated unselective cation channel If was described to contribute to their spontaneous activity (Sartiani et al. 2002). As supported by our immunostainings, intercellular coupling in HL-1 cells is established by the cardiac specific gap junction proteins Cx40, Cx43 and Cx45 (White et al. 2004). There is no doubt that a spontaneously active monolayer is a simplification of physiological pacemakers like the sino-atrial node; nevertheless monolayers have been used previously as pacemaker models and were shown to follow the power-law behaviour of heart rate variability. This indicates that they reproduce distinct variables of the cardiac pacemaker (Kucera et al. 2000; Ponard et al. 2007).
As demonstrated in vitro and in numerical models, cardiomyocyte monolayers develop transiently active spiral waves (Bub et al. 1998; Panfilov, 2002; ten Tusscher & Panfilov, 2003). Their appearance increases in monolayers of decreased density and decreased intercellular coupling (Panfilov, 2002; Bub et al. 2005). In silico experiments suggest that non-excitable cells in these cultures stabilize the spirals and decrease their conduction velocity (ten Tusscher & Panfilov, 2003). In our monolayer cultures the origin of excitation is not identified, and a reduction in the conduction velocity would result in a decreased rotational speed and apparent ‘beating frequency’. To exclude the development of spiral waves, we plated the HL-1 cells at a constant high density and performed control experiments on MEAs with increased interelectrode distances (500 μm; 3.5 mm). None of the results indicated that the spontaneous activity of the HL-1 monolayers was based on self sustained spiral waves. Furthermore, the stable beating frequency is similar to monolayers of neonatal rat ventricular myocytes with focal spontaneous activity (Panfilov, 2002; Bub et al. 2005; Ponard et al. 2007).
Depolarizing influence of fibroblasts on cardiomyocytes
Similar to Kamkin and colleagues, the increase in fibroblast density correlated with the attenuation of spontaneous activity (Orita et al. 1993; Kamkin et al. 2003). The observed decrease in the conduction velocity was also comparable to the changes previously described for these conditions (Panfilov, 2002; Miragoli et al. 2006). Fibroblasts can exert their influence on cardiomyocytes by establishing intercellular coupling via gap junction channels or by acting as unexcitable obstacles within the preparation. In our preparation, as in fibroblast–neonatal cardiomyocyte pairs (Rook et al. 1989), Cx43 is the dominating connexin isoform. But the presence of additional connexin isoforms, e.g. Cx45, cannot be ruled out (Martyn et al. 1997; Miragoli et al. 2006). However, the attenuated effect of Cx43−/−-fibs on the Vm and θ of HL-1 cells (Figs 5E and 7D) indicates that heterocellular coupling is one prerequisite for the fibroblasts' modulatory influence (Kizana et al. 2006).
By establishing heterocellular coupling, fibroblasts could enhance cardiomyocyte diastolic depolarization by mechanically induced activation of cation channels (Kohl et al. 1994; Kohl & Noble, 1996; Camelliti et al. 2005). An increased sensitivity of these channels in the infarcted myocardium was correlated with bradyarrhythmia (Kamkin et al. 2002; Kamkin et al. 2003). Our data do not support the presence of stretch induced cation conductance in our model (Fig. 5A and B). But the amount of stretch experienced by fibroblasts is likely to be less in monolayer cultures than in the whole heart as can also be seen in the line scan (Fig. 5B) by the lack of distortion at the HL-1/fibroblast site of interaction during contraction.
A decrease in spontaneous activity and conduction velocity can further be explained by the depolarizing effect that the fibroblasts exert on the cardiomyocytes (Fig. 5D and E). Miragoli et al. (2006) demonstrated that the membrane potential of neonatal rat cardiomyocytes correlates negatively with an increased density of cocultured myofibroblasts. The depolarization of the HL-1 cells' Vm during coculture with WT-fibs (Fig. 5D and E) supports the hypothesis that depolarization activated Na+ current contributes to the cells' spontaneous activity similar to subsidiary pacemaker cells of the cat atrium (Rubenstein & Lipsius, 1989). Inactivation or block of INa consequently prolongs the diastolic depolarization and reduces the spontaneous activity as described for sinus node function in transgenic mice deficient for the expression of SCN5A (Lei et al. 2005).
Reducing intercellular coupling by the use of Cx43−/−-fibs consequently almost abolished the fibroblasts' effect on Vm and θ. However, their negative chronotropic effect on the HL-1 spontaneous activity is maintained, supporting the hypothesis that the fibroblasts influence is not exclusively mediated by intercellular coupling.
It is well accepted that dissociated cardiomyocytes re-establish intercellular coupling in vitro in cell pairs, monolayers, and 3D aggregates. The intercellular coupling goes along with a synchronization of the cells' spontaneous activity where the mutual entrainment of the cells determines the overall beating frequency (DeHaan & Hirakow, 1972; Ypey et al. 1979; Jongsma et al. 1983). In parallel to the development of intercellular coupling, changes in the CIBI are described. The CIBI is increased in single cells in comparison to larger cell aggregates, correlates with the intercellular resistance established between the interacting cells, and decreases with increasing numbers of interacting cells (Clay & DeHaan, 1979; Ypey et al. 1979; Kojima et al. 2005, 2006). Our experimental results revealed an increase in CIBI for cultures where fibroblasts are interspersed between the cardiomyocytes independent of the expression of Cx43 (Fig. 9B). The increase in CIBI could indicate that interspersing fibroblasts reduce the mutual entrainment of the cardiomyocytes most likely by decreasing the number of cell–cell interactions within a given area. Interestingly even fibroblasts that express Cx43 cannot maintain the mutual entrainment seen in homocellular cardiomyocyte cultures. The situation seems comparable to the preparation of sinus nodal strips, where two spontaneously active areas of the strip are separated by a sucrose gap and phase shifts are induced through electrotonic coupling (Jalife, 1984). Similarly, cardiomyocyte cell pairs and aggregates that start to develop intercellular coupling pass through a phase of increased CIBI. At this point, the coupling resistance between the prior independent preparations is still high and frequency entrainment is not yet established between them (Clay & DeHaan, 1979; Ypey et al. 1979; Verheijck et al. 1998). Translated to our culture it could mean that cardiomyocytes separated by a fibroblast fail to establish mutual entrainment and phase shifts increase the CIBI. Since AP propagation over fibroblast barriers has been demonstrated, it can be hypothesized that the fibroblasts act as a high pass filter that transmits the rapid upstroke of the AP but not the slow diastolic depolarization during the excitation cycle.
Relevance of fibroblasts for sinus nodal function
In our cocultures the percentage of fibroblasts ranged between 0 and 50%. Since fibroblasts comprise 45–75% of the SAN total volume, the amount of fibroblast integrated in the heterocellular cultures seems comparable to physiological conditions. Although we do not know the exact composition and fibroblast content of the ESdC aggregates, their 48% reduction in beating frequency after neomycin treatment would correlate with 10–20% fibroblast content if compared to monolayer culture. However, the effect of the fibroblasts might be attenuated in a 3D environment where cells establish contact with a larger number of cells than in the monolayer. In the SAN, intercellular coupling between fibroblasts and cardiomyocytes is established by Cx45 (Camelliti et al. 2004). The intercellular resistance in these heterocellular pairs is not known, but expected to be low due to the small single-channel conductance of Cx45. As a result, our experiments with the high resistance Cx43−/−-fibs might more truly represent the physiological conditions. In that case, the interspersion would be the main mechanism by which fibroblasts influence the beating frequency and the CIBI. In contrast to our preparations, a fibroblast induced depolarization of human SAN cells would mainly be expected to modify the early diastolic depolarization through ICa,T and If rather than INa, which is not described to play a role in these human SAN cells. In our experiments we have not specifically addressed the fibroblasts' role as a current sink in cardiac pacemaker preparations; however, this mechanism and the fibroblast induced depolarization depend on the establishment of intercellular coupling. Therefore, the use of Cx43−/−-fibs would affect it in a similar way as the HL-1 cells' Vm (Fig. 5D and E). In humans, a decrease in heart rate variability correlates with an increased incidence of ventricular fibrillation or sudden cardiac death as well as increased age (Kucera et al. 2000; Ponard et al. 2007). This according to our results would not correlate with the ageing related increase in fibroblast content of the SAN. However, additional ageing-dependent changes in cardiomyocyte number, intercellular coupling, and SAN innervations might further influence this parameter.
Conclusion
Our data show that fibroblasts induce a concentration-dependent reduction in the beating frequency of pacemaker-like cardiomyocyte aggregates. This in part can be explained by the cardiomyocytes' depolarization and change in excitability after establishing gap junctional coupling with the fibroblasts. However, another component of fibroblast infiltration is the increase in the coefficient of interbeat interval variability, which cannot be compensated by the intercellular coupling between fibroblasts and cardiomyocytes tested. We propose, therefore, that the change in beating frequency is mainly induced by a reduction of frequency entrainment between individual myocytes. Future experiments are needed to determine if changes of IBI variability and beating frequency correlate with fibroblast infiltration in vivo or if heart rate variability under physiological conditions depends on the presence of non-excitable cells.
Acknowledgments
The authors would like to thank Prof L. J. Field (Krannert Institute of Cardiology, Indiana University School of Medicine, Indianapolis) for providing the αMHC-neomycin construct and Prof A. F. Lau (Cancer Research Center of Hawaii, J. A. Burns School of Medicine, University of Hawaii) for sharing the Cx43−/−-fibs. This work was supported by grants from the American Heart Association (AHA 0610110Z to J.F.; AHA 0655656Z to R.M.A.; AHA 0330393Z to K.B.), NIH R01 HL89617-01 to K.B. and the Potts Foundation, Loyola University, Chicago (RFC 11086) to K.B.
References
- Banach K, Halbach MD, Hu P, Hescheler J, Egert U. Development of electrical activity in cardiac myocyte aggregates derived from mouse embryonic stem cells. Am J Physiol Heart Circ Physiol. 2003;284:H2114–H2123. doi: 10.1152/ajpheart.01106.2001. [DOI] [PubMed] [Google Scholar]
- Belus A, White E. Streptomycin and intracellular calcium modulate the response of single guinea-pig ventricular myocytes to axial stretch. J Physiol. 2003;546:501–509. doi: 10.1113/jphysiol.2002.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Bub G, Glass L, Publicover NG, Shrier A. Bursting calcium rotors in cultured cardiac myocyte monolayers. Proc Natl Acad Sci U S A. 1998;95:10283–10287. doi: 10.1073/pnas.95.17.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub G, Shrier A, Glass L. Global organization of dynamics in oscillatory heterogeneous excitable media. Phys Rev Lett. 2005;94:028105. doi: 10.1103/PhysRevLett.94.028105. [DOI] [PubMed] [Google Scholar]
- Cai D, Lai YC, Winslow RL. Complex dynamics in coupled cardiac pacemaker cells. Phys Rev Lett. 1993;71:2501–2504. doi: 10.1103/PhysRevLett.71.2501. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, Maccannell KA, Imaizumi Y, Clark RB, Dixon IM, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H2931–H2939. doi: 10.1152/ajpheart.01220.2004. [DOI] [PubMed] [Google Scholar]
- Clay JR, DeHaan RL. Fluctuations in interbeat interval in rhythmic heart-cell clusters. Role of membrane voltage noise. Biophys J. 1979;28:377–389. doi: 10.1016/S0006-3495(79)85187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda S, Samuel JL, Rappaport L. Extracellular matrix and growth factors during heart growth. Heart Fail Rev. 2000;5:119–130. doi: 10.1023/A:1009806403194. [DOI] [PubMed] [Google Scholar]
- de Marneffe M, Gregoire JM, Waterschoot P, Kestemont MP. The sinus node and the autonomic nervous system in normals and in sick sinus patients. Acta Cardiol. 1995;50:291–308. [PubMed] [Google Scholar]
- De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, Glascock BJ, Kimball TF, del Monte F, Hajjar RJ, Molkentin JD. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2001;98:3322–3327. doi: 10.1073/pnas.031371998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaan RL, Hirakow R. Synchronizatin of pulsation rates in isolated cardiac myocytes. Exp Cell Res. 1972;70:214–220. doi: 10.1016/0014-4827(72)90199-1. [DOI] [PubMed] [Google Scholar]
- Delmar M, Jalife J, Michaels DC. Effects of changes in excitability and intercellular coupling on synchronization in the rabbit sino-atrial node. J Physiol. 1986;370:127–150. doi: 10.1113/jphysiol.1986.sp015926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egert U, Knott T, Schwarz C, Nawrot M, Brandt A, Rotter S, Diesmann M. MEA-Tools: an open source toolbox for the analysis of multi-electrode data with MATLAB. J Neurosci Methods. 2002;117:33–42. doi: 10.1016/s0165-0270(02)00045-6. [DOI] [PubMed] [Google Scholar]
- Feld Y, Melamed-Frank M, Kehat I, Tal D, Marom S, Gepstein L. Electrophysiological modulation of cardiomyocytic tissue by transfected fibroblasts expressing potassium channels: a novel strategy to manipulate excitability. Circulation. 2002;105:522–529. doi: 10.1161/hc0402.102661. [DOI] [PubMed] [Google Scholar]
- Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- Halbach M, Egert U, Hescheler J, Banach K. Estimation of action potential changes from field potential recordings in multicellular mouse cardiac myocyte cultures. Cell Physiol Biochem. 2003;13:271–284. doi: 10.1159/000074542. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Wartenberg M, Fleischmann BK, Banach K, Acker H, Sauer H. Embryonic stem cells as a model for the physiological analysis of the cardiovascular system. Methods Mol Biol. 2002;185:169–187. doi: 10.1385/1-59259-241-4:169. [DOI] [PubMed] [Google Scholar]
- Igelmund P, Fleischmann BK, Fischer IR, Soest J, Gryshchenko O, Bohm-Pinger MM, Sauer H, Liu Q, Hescheler J. Action potential propagation failures in long-term recordings from embryonic stem cell-derived cardiomyocytes in tissue culture. Pflugers Arch. 1999;437:669–679. doi: 10.1007/s004240050831. [DOI] [PubMed] [Google Scholar]
- Jalife J. Mutual entrainment and electrical coupling as mechanisms for synchronous firing of rabbit sino-atrial pace-maker cells. J Physiol. 1984;356:221–243. doi: 10.1113/jphysiol.1984.sp015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CM, Kanter EM, Green KG, Laing JG, Betsuyaku T, Beyer EC, Steinberg TH, Saffitz JE, Yamada KA. Redistribution of connexin45 in gap junctions of connexin43-deficient hearts. Cardiovasc Res. 2002;53:921–935. doi: 10.1016/s0008-6363(01)00522-3. [DOI] [PubMed] [Google Scholar]
- Jongsma HJ, Tsjernina L, de Bruijne J. The establishment of regular beating in populations of pacemaker heart cells. A study with tissue-cultured rat heart cells. J Mol Cell Cardiol. 1983;15:123–133. doi: 10.1016/0022-2828(83)90288-2. [DOI] [PubMed] [Google Scholar]
- Kamkin A, Kiseleva I, Isenberg G, Wagner KD, Gunther J, Theres H, Scholz H. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Prog Biophys Mol Biol. 2003;82:111–120. doi: 10.1016/s0079-6107(03)00009-9. [DOI] [PubMed] [Google Scholar]
- Kamkin A, Kiseleva I, Lozinsky I, Scholz H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res Cardiol. 2005;100:337–345. doi: 10.1007/s00395-005-0529-4. [DOI] [PubMed] [Google Scholar]
- Kamkin A, Kiseleva I, Wagner KD, Pylaev A, Leiterer KP, Theres H, Scholz H, Gunther J, Isenberg G. A possible role for atrial fibroblasts in postinfarction bradycardia. Am J Physiol Heart Circ Physiol. 2002;282:H842–H849. doi: 10.1152/ajpheart.00240.2001. [DOI] [PubMed] [Google Scholar]
- Kapur N, Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J Physiol. 2007;581:1113–1127. doi: 10.1113/jphysiol.2006.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Kizana E, Ginn SL, Allen DG, Ross DL, Alexander IE. Fibroblasts can be genetically modified to produce excitable cells capable of electrical coupling. Circulation. 2005;111:394–398. doi: 10.1161/01.CIR.0000153812.64956.EF. [DOI] [PubMed] [Google Scholar]
- Kizana E, Ginn SL, Smyth CM, Boyd A, Thomas SP, Allen DG, Ross DL, Alexander IE. Fibroblasts modulate cardiomyocyte excitability: implications for cardiac gene therapy. Gene Ther. 2006;13:1611–1615. doi: 10.1038/sj.gt.3302813. [DOI] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl P, Camelliti P, Burton FL, Smith GL. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol. 2005;38(Suppl.):45–50. doi: 10.1016/j.jelectrocard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- Kohl P, Day K, Noble D. Cellular mechanisms of cardiac mechano-electric feedback in a mathematical model. Can J Cardiol. 1998;14:111–119. [PubMed] [Google Scholar]
- Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol. 1994;79:943–956. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- Kohl P, Noble D. Mechanosensitive connective tissue: potential influence on heart rhythm. Cardiovasc Res. 1996;32:62–68. [PubMed] [Google Scholar]
- Kojima K, Kaneko T, Yasuda K. Stability of beating frequency in cardiac myocytes by their community effect measured by agarose microchamber chip. J Nanobiotechnol. 2005;3:4. doi: 10.1186/1477-3155-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Kaneko T, Yasuda K. Role of the community effect of cardiomyocyte in the entrainment and reestablishment of stable beating rhythms. Biochem Biophys Res Commun. 2006;351:209–215. doi: 10.1016/j.bbrc.2006.10.037. [DOI] [PubMed] [Google Scholar]
- Kondratev D, Gallitelli MF. Increments in the concentrations of sodium and calcium in cell compartments of stretched mouse ventricular myocytes. Cell Calcium. 2003;34:193–203. doi: 10.1016/s0143-4160(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Kucera JP, Heuschkel MO, Renaud P, Rohr S. Power-law behavior of beat-rate variability in monolayer cultures of neonatal rat ventricular myocytes. Circ Res. 2000;86:1140–1145. doi: 10.1161/01.res.86.11.1140. [DOI] [PubMed] [Google Scholar]
- Lei M, Goddard C, Liu J, Leoni AL, Royer A, Fung SS, Xiao G, Ma A, Zhang H, Charpentier F, Vandenberg JI, Colledge WH, Grace AA, Huang CL. Sinus node dysfunction following targeted disruption of the murine cardiac sodium channel gene Scn5a. J Physiol. 2005;567:387–400. doi: 10.1113/jphysiol.2005.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- Martyn KD, Kurata WE, Warn-Cramer BJ, Burt JM, TenBroek E, Lau AF. Immortalized connexin43 knockout cell lines display a subset of biological properties associated with the transformed phenotype. Cell Growth Differ. 1997;8:1015–1027. [PubMed] [Google Scholar]
- Michaels DC, Matyas EP, Jalife J. Dynamic interactions and mutual synchronization of sinoatrial node pacemaker cells. A mathematical model. Circ Res. 1986;58:706–720. doi: 10.1161/01.res.58.5.706. [DOI] [PubMed] [Google Scholar]
- Michaels DC, Matyas EP, Jalife J. Mechanisms of sinoatrial pacemaker synchronization: a new hypothesis. Circ Res. 1987;61:704–714. doi: 10.1161/01.res.61.5.704. [DOI] [PubMed] [Google Scholar]
- Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- Orita H, Fukasawa M, Hirooka S, Fukui K, Kohi M, Washio M, Sasaki H. Protection of cardiac myocytes from hypothermic injury by cardiac fibroblasts isolated from neonatal rat ventricle. J Surg Res. 1993;55:654–658. doi: 10.1006/jsre.1993.1199. [DOI] [PubMed] [Google Scholar]
- Panfilov AV. Spiral breakup in an array of coupled cells: the role of the intercellular conductance. Phys Rev Lett. 2002;88:118101. doi: 10.1103/PhysRevLett.88.118101. [DOI] [PubMed] [Google Scholar]
- Pasumarthi KB, Field LJ. Cardiomyocyte enrichment in differentiating ES cell cultures: strategies and applications. Methods Mol Biol. 2002;185:157–168. doi: 10.1385/1-59259-241-4:157. [DOI] [PubMed] [Google Scholar]
- Perez CG, Copello JA, Li Y, Karko KL, Gomez L, Ramos-Franco J, Fill M, Escobar AL, Mejia-Alvarez R. Ryanodine receptor function in newborn rat heart. Am J Physiol Heart Circ Physiol. 2005;288:H2527–H2540. doi: 10.1152/ajpheart.00188.2004. [DOI] [PubMed] [Google Scholar]
- Ponard JG, Kondratyev AA, Kucera JP. Mechanisms of intrinsic beating variability in cardiac cell cultures and model pacemaker networks. Biophys J. 2007;92:3734–3752. doi: 10.1529/biophysj.106.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook MB, Jongsma HJ, de Jonge B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflugers Arch. 1989;414:95–98. doi: 10.1007/BF00585633. [DOI] [PubMed] [Google Scholar]
- Rook MB, van Ginneken AC, de Jonge B, el Aoumari A, Gros D, Jongsma HJ. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol Cell Physiol. 1992;263:C959–977. doi: 10.1152/ajpcell.1992.263.5.C959. [DOI] [PubMed] [Google Scholar]
- Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989;64:648–657. doi: 10.1161/01.res.64.4.648. [DOI] [PubMed] [Google Scholar]
- Sartiani L, Bochet P, Cerbai E, Mugelli A, Fischmeister R. Functional expression of the hyperpolarization-activated, non-selective cation current If in immortalized HL-1 cardiomyocytes. J Physiol. 2002;545:81–92. doi: 10.1113/jphysiol.2002.021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibukawa Y, Chilton EL, Maccannell KA, Clark RB, Giles WR. K+ currents activated by depolarization in cardiac fibroblasts. Biophys J. 2005;88:3924–3935. doi: 10.1529/biophysj.104.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi I, Takamatsu T, Minamikawa T, Onouchi Z, Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation. 1992;85:2176–2184. doi: 10.1161/01.cir.85.6.2176. [DOI] [PubMed] [Google Scholar]
- Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, Mains IM, Mingoia JT, Flack EC, Lindsey ML. Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005;39:699–707. doi: 10.1016/j.yjmcc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- ten Tusscher KH, Panfilov AV. Influence of nonexcitable cells on spiral breakup in two-dimensional and three-dimensional excitable media. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:062902. doi: 10.1103/PhysRevE.68.062902. [DOI] [PubMed] [Google Scholar]
- Verheijck EE, Wilders R, Joyner RW, Golod DA, Kumar R, Jongsma HJ, Bouman LN, van Ginneken AC. Pacemaker synchronization of electrically coupled rabbit sinoatrial node cells. J Gen Physiol. 1998;111:95–112. doi: 10.1085/jgp.111.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–H829. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- Xia M, Salata JJ, Figueroa DJ, Lawlor AM, Liang HA, Liu Y, Connolly TM. Functional expression of L- and T-type Ca2+ channels in murine HL-1 cells. J Mol Cell Cardiol. 2004;36:111–119. doi: 10.1016/j.yjmcc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- Ypey DL, Clapham DE, DeHaan RL. Development of electrical coupling and action potential synchrony between paired aggregates of embryonic heart cells. J Membr Biol. 1979;51:75–96. doi: 10.1007/BF01869344. [DOI] [PubMed] [Google Scholar]
- Zandstra PW, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, Pasumarthi KB, Field LJ. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767–778. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]