Abstract
The mechanism of force enhancement during lengthening was investigated on single frog muscle fibres by using fast stretches to measure the rupture tension of the crossbridge ensemble. Fast stretches were applied to one end of the activated fibre and force responses were measured at the other. Sarcomere length was measured by a striation follower device. Fast stretching induced a linear increase of tension that reached a peak and fell before the end of the stretch indicating that a sudden increase of fibre compliance occurred due to forced crossbridge detachment induced by the fast loading. The peak tension (critical tension, Pc) and the sarcomere length needed to reach Pc (critical length, Lc) were measured at various tensions during the isometric tetanus rise and during force enhancement by slow lengthening. The data showed that Pc was proportional to the tension generated by the fibre under both isometric and slow lengthening conditions. However, for a given tension increase, Pc was 6.5 times greater during isometric than during lengthening conditions. Isometric critical length was 13.04 ± 0.17 nm per half-sarcomere (nm hs−1) independently of tension. During slow lengthening critical length fell as the force enhancement increased. For 90% enhancement, Lc reduced to 8.19 ± 0.039 nm hs−1. Assuming that the rupture force of the individual crossbridge is constant, these data indicate that the increase of crossbridge number during lengthening accounts for only 15.4% of the total force enhancement. The remaining 84.6% is accounted for by the increased mean strain of the crossbridges.
It is well known that slow stretching of an activated muscle fibre increases the tension developed well above the isometric force level (Katz, 1939; Sugi, 1972; Edman et al. 1978; Flitney & Hirst, 1978a,b; Julian & Morgan, 1981; Lombardi & Piazzesi, 1990; Stienen et al. 1992; Cavagna, 1993; Getz et al. 1998; Lee et al. 2001). This force enhancement is made up of two components: the first is velocity dependent and is established immediately after the start of stretch while the second is amplitude dependent but velocity independent (Edman & Tsuchiya, 1996; Bagni et al. 2002). Recent experiments suggested that the latter component is attributable to an activation-dependent ‘non-crossbridge stiffness’ (Bagni et al. 2002; Pinniger et al. 2006), very likely a calcium-dependent stiffening of titin molecule (Bagni et al. 2002, 2004; Labeit et al. 2003). The velocity-dependent component is instead active and it is attributable to the crossbridges.
Force enhancement during slow stretching has been studied by many investigators (Abbott & Aubert, 1952; Hill & Howarth, 1959; Sugi, 1972; Cavagna & Citterio, 1974; Edman et al. 1978, 1981; Julian & Morgan, 1979; Lombardi & Piazzesi, 1990), starting with the pioneering experiments of Katz (1939); however, its mechanism is still not completely understood. In principle, an increase in active force can be due to an increase of either crossbridge number or the mean crossbridge force (and extension), or both. Data in the literature on this subject are somewhat controversial. Based on fibre stiffness and T1 curve analysis (Ford et al. 1977), force enhancement was attributed either to an increase of crossbridge strain with a small increase in crossbridge number (Sugi & Tsuchiya, 1988; Lombardi & Piazzesi, 1990), or to a predominant increase of crossbridge number with little or no increment of crossbridge strain in both intact frog fibres (Linari et al. 2000) and mammalian skinned fibres (Linari et al. 2004). The present experiments were made to further investigate the mechanism of the active component of force enhancement using a technique introduced recently by our group to determine crossbridge properties (Bagni et al. 2005). The number of attached crossbridges was determined by measuring the force needed to forcibly detach the crossbridges ensemble (critical tension, Pc) by very fast stretches applied to activated fibres. The mean crossbridge extension (critical length, Lc) was calculated from measurements of the sarcomere elongation needed to reach the critical force. The advantage of this procedure to measure crossbridge number is that Pc, in contrast to stiffness measurements, is independent of the amount and the shape of the myofilament elasticity. Measurements were made during the rise of tetanic tension and during force enhancement induced by slow lengthening. The analysis of the results showed that both crossbridge number and mean crossbridge extension increased during slow lengthening, but crossbridge number increase contributed only 15.4% of the force enhancement while the remaining 84.6% was accounted for by increase of crossbridge extension.
Methods
Frogs (Rana esculenta) were killed by decapitation followed by destruction of the spinal cord, according to the procedure suggested by our local ethical committee. Single intact fibres, dissected from the tibialis anterior muscle (4–6 mm long, 60–120 μm diameter) were mounted by means of aluminium foil clips between the lever arms of a force transducer (natural frequency 40–60 kHz) and a fast electromagnetic motor (minimum stretch time 100 μs) in a thermostatically controlled chamber provided with a glass floor for both ordinary and laser light illumination. Stimuli of alternate polarity, 0.5 ms duration and 1.5 times threshold strength, were applied transversely to the fibre by means of platinum-plate electrodes at the minimum frequency necessary to obtain fused tetanic contractions. Sarcomere length was measured using the striation follower device (Huxley et al. 1981) in a fibre segment (1.2–2.5 mm long) selected for striation uniformity in a region as close as possible to the force transducer. This eliminated the effect of tendon compliance and reduced the effects of mechanical wave propagation on the sarcomere length measurements. Since fibres developing the maximum tetanic tension (P0) were quickly damaged by fast stretches, we performed only a few experiments at P0. Most of our data were obtained on the tetanus rise at a tension level between 0.2 and 0.6 P0 at which fibre damage was very much reduced. Lowering the tension was also important to reduce crossbridge strain non-uniformity (Colombini et al. 2007b). To determine the crossbridge rupture force, fast ramp test stretches (duration between 0.4 ms and 0.9 ms and 16–25 nm per half-sarcomere (nm hs−1) amplitude, corresponding to stretching velocity of 16.9 and 59.5 fibre lengths per second (l0 s−1)) were applied to one end of the activated fibre while force response was measured at the other end. Stretches were applied at given times during an isometric tetanus either alone or preceded by a slow lengthening which enhanced the tension. Experiments were made at 5°C at resting sarcomere length of about 2.1 μm, so that no change in effective overlap between myofilaments occurred during the experiments.
The linear phase of force increase induced by the fast stretch was usually preceded by a small and faster force rise (lasting less than 0.1 ms) occurring at the start of the stretch, whose amplitude was roughly proportional to the stretching speed. This phase probably arises from the passive properties of the fibre such as inertia or viscosity (Bagni et al. 1998) and for this reason the peak force was corrected for it. To do so, the slower linear part of the force rise was extrapolated back to the starting time of the stretch and the intercept on the ordinate was considered zero tension. Measurements were made after subtraction of the isometric force recorded with the same timing. This enabled correction for the tension rise due to the development of the tetanic tension. Critical tension was also corrected for the static tension (Bagni et al. 2002) due to the non-crossbridge stiffness of the fibre which accounts for the passive component of force enhancement by lengthening. This correction, however, was never greater than 5%. Passive (resting fibre) force response was negligible and no correction was made for it. Ramp stretching velocities ranged from 85 to 2.5 × 103 nm hs−1 s−1 (0.08–2.4 l0 s−1). Ringer solution had the following composition (mm): NaCl, 115; KCl, 2.5; CaCl2, 1.8; NaH2PO4, 0.85; Na2HPO4, 2.15. Force, fibre length and sarcomere length signals were measured with 10 μs time resolution with a digital oscilloscope (4094, Nicolet, USA) and transferred to a personal computer for further analysis.
Results
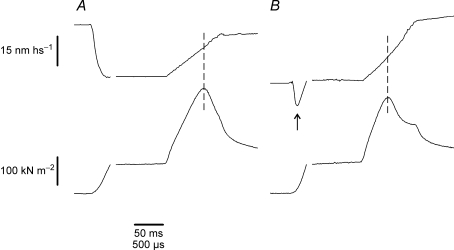
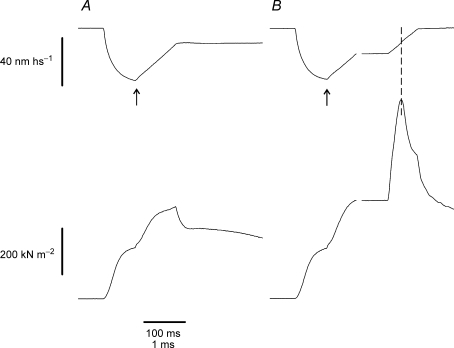
Figure 1A shows a typical force response to a fast stretch applied to a fibre activated under isometric conditions at tension of 0.5 P0 on the tetanus rise. With the exception of a transient initial phase, the tension rises almost linearly during the stretch reaching a peak Pc and then dropping quickly to a much lower level. This occurs in spite of the continuous stretching of the fibre, which means that the fall of force is caused by a sudden increase of fibre compliance due to forced crossbridge detachment consequent to force loading (Flitney & Hirst, 1978a; Bagni et al. 2005). Thus, Pc represents the rupture force of the crossbridge ensemble. Figure 1B shows that the shape of the force response is not affected by slow lengthening.
Figure 1. Force and sarcomere length responses induced by a fast stretch applied at tension of about 0.5 P0 on the tetanus rise (A) and during slow stretching (B).
The stretch (17.5 nm hs−1 amplitude, 950 μs duration, corresponding to 17.54 l0 s−1) in B was applied at the same relative tension as in A but during slow lengthening (at 0.75 l0 s−1), which increased the tension by 20% with respect to the isometric tension. Upper traces: sarcomere length; lower traces: force. Note that the sarcomere elongation needed to reach the crossbridge rupture, is smaller in B than in A. Interruptions on the traces indicate the change from slow to fast time base. The arrow in B shows the start of slow ramp stretching. Vertical dashed line shows the critical tension and the corresponding critical length.
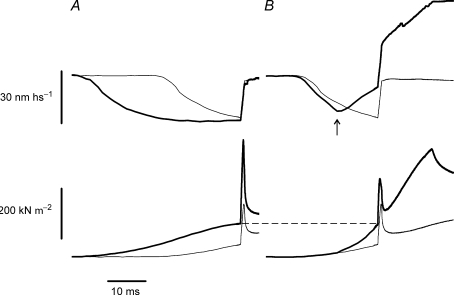
Fast stretches such as that in Fig. 1 were applied at various times after the start of stimulation on the tetanus rise, either alone or preceded by a slow lengthening, which enhanced the active force developed by the fibre with respect to isometric. Various degrees of force enhancement, up to more than 100%, were obtained by changing the stretching velocity. Figure 2 shows an example of the records obtained. In Fig. 2A the test stretch was applied at tension of 0.2 and 0.5 P0 under isometric conditions. In Fig. 2B the isometric response at 0.2 P0 shown in Fig. 2A is compared with the response obtained when the test stretch, applied at the same time, was preceded by a slow lengthening that enhanced the tension up to 0.5 P0, the same level as in the upper force record in Fig. 2A. Pc increased by 124% when the isometric tension changed from 0.2 to 0.5 P0 but increased by only 45% when the same force increase was induced by slow lengthening. Thus, in spite of the fast stretches being applied in both cases at the same tension, critical tension is much smaller during slow stretching compared to isometric conditions. This result suggests that force enhancement by slow lengthening is accompanied by a relatively small increase of crossbridge number.
Figure 2. Force and sarcomere length responses induced by fast stretches applied during the tetanus rise with and without slow lengthening.
Upper traces: sarcomere length; lower traces: force. A, force responses at two different isometric force levels (0.2 and 0.5 P0; thin and thick line, respectively); B, force responses at 0.2 P0, during isometric (thin line) and at 0.5 P0 during lengthening (thick line). Lengthening velocity, 1.65 l0 s−1. Force enhancement, 150%. In A the timing of stimulation is adjusted so that the isometric tension reaches 0.2 P0 or 0.5 P0 at the time of the fast stretch. In B the tension at 0.5 P0 is obtained by slow lengthening of the fibre before the fast stretch. The arrow on the sarcomere length trace in B shows the start of slow lengthening. Note that for the same tension, Pc, is much greater during the isometric rise than during slow lengthening.
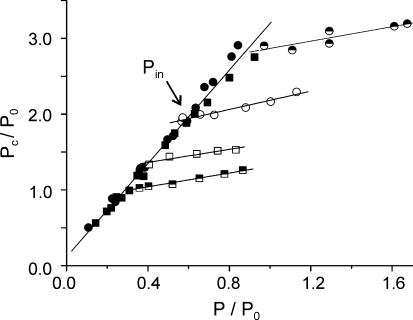
An example of the relationship between the tension developed by the fibre under isometric or lengthening conditions and critical tension is shown in Fig. 3. The isometric relation is compared with that obtained when fast stretches, applied at four different times on the rise, were preceded by slow conditioning stretches at various velocities. It can be seen that the P–Pc relation is linear in both cases; however, the slope is much greater under isometric conditions (filled symbols), than during forcible lengthening (open and half-filled symbols). Slope during lengthening was the same independently of the time at which the measurements were made. This made it possible to pool the results of all the fibres at all tensions by normalizing the tension developed by the fibre and the critical tension under isometric and lengthening conditions, for the force (Pin) corresponding to the intercept of the lines fitted to force data during lengthening with the line fitted to isometric data. The results were divided into classes of 0.25 P/Pin interval, class averaged and plotted in Fig. 4. The figure shows that for a given relative tension developed by the preparation, critical tension is much smaller under slow lengthening than during isometric conditions. The mean Pc/Pin ratios on the tetanus rise and during force enhancement obtained from the fittings, were 3.05 ± 0.09 and 0.47 ± 0.03, respectively (mean and s.e.m.). The slope of 3.05 corresponds to the direct proportionality between tension and crossbridge number, and therefore the slope of 0.47 during lengthening indicates that force enhancement by stretch is accompanied by a relatively small increment of crossbridge population.
Figure 3. Relative critical tension against relative tension under isometric (filled symbols) or during lengthening (open and half-filled symbols) in two fibres.
Fast stretches were applied at various times during the tetanus rise (isometric data) and at time when the isometric tension was 0.29 and 0.40 P0 (in one fibre, squares), and 0.57 and 0.83 P0 (in the other fibre, circles), preceded by slow lengthening at speeds between 0.08 and 2.4 l0 s−1, which induced various amount of force enhancement (lengthening data). The arrow shows the force (Pin) at the intercept between slow lengthening and isometric values for one set of data.
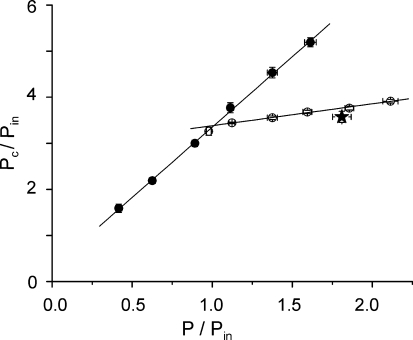
Figure 4. Normalized relations between critical tension and tension developed under isometric (filled symbols) and slow stretching conditions (open symbols).
Pooled data from 6 fibres. Each point represents the mean value ± s.e.m. for 0.25 P/Pin class averaging. The continuous lines fitted to the filled and open symbols represent the best fittings. The star represents the mean value ± s.e.m. from 5 experiments made at the tetanus plateau under condition of steady force enhancement (see later).
The results showed in Fig. 4 were obtained by applying the stretches at low tensions during the tetanus rise, a procedure which was necessary to avoid the damaging of the fibre by the stretch. Therefore, in contrast with previous experiments in the literature, these data were collected when tension was rising and before the force enhancement could reach a steady value. To verify if our results are comparable to data in the literature, fast stretches were also applied at tetanus plateau and during steady force enhancement. In five fibres it was possible to collect some data before fibre damaging. Records from one of these experiments, are shown in Fig. 5. It can be seen that the shape of the force response to the fast stretch is the same as during the tetanus rise. The critical tensions measured in these experiments were averaged and plotted as single point (star) on Fig. 4. The data lie very close to those obtained during the tetanus rise, showing that no significant difference exists between the mechanism of force enhancement on the tetanus rise and at plateau under steady state.
Figure 5. Force and sarcomere length responses to slow and fast stretches applied at tetanus plateau.
A, force response to slow stretching (0.29 l0 s−1) which enhances the force towards an almost steady value corresponding to 1.6 P0. B, force response to a fast stretch (29.75 l0 s−1) applied during the steady force enhancement. It can be seen that the shape of the force response is the same as during the tetanus rise. Pc during force enhancement is 1.95 times the tension developed at the time of the stretch. The average of the results obtained in all fibres at plateau is plotted in Fig. 4 as a single data point. The arrows show the start of slow stretching. The interruptions on the traces indicate the switch from slow to fast time base. Vertical dashed line shows the critical tension and the corresponding critical length.
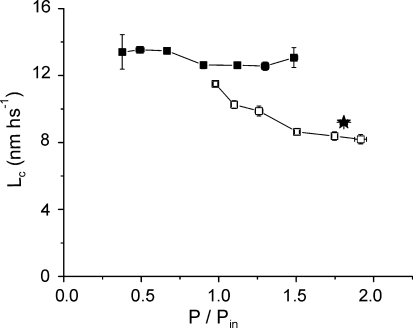
For the same fibres of Fig. 4 we also measured the sarcomere elongation Lc, needed to reach the critical tension. The class averaged data for all the fibres are reported in Fig. 6. It can be seen that Lc during the tetanus rise remained approximately constant, in agreement with previous results at higher temperature (Bagni et al. 2005). Its mean value was 13.04 ± 0.17 nm hs−1. In contrast, during force enhancement by slow stretching, Lc decreased progressively as force enhancement increased. At mean P/Pin ratio of 1.90, mean Lc fell to 8.19 ± 0.039 nm hs−1, 4.85 nm hs−1 below the isometric value. This means that, with respect to isometric conditions, crossbridges during force enhancement by lengthening require a shorter elongation to reach the rupture force. This suggests that slow stretching increases the mean crossbridge extension and force so that a smaller external extension is required to induce their rupture.
Figure 6. Critical length as a function of tension under isometric and slow lengthening conditions.
Pooled data from the same fibres as Fig. 4. Each point represents the mean ± s.e.m. for 0.25 P/Pin class averaging. Tensions are expressed relatively to Pin. Data on the isometric tetanus rise (filled symbols) show that Lc is independent of tension. On the contrary, during lengthening (open symbols), Lc decreases as the force enhancement increases. The averaged data, (obtained at plateau during steady force enhancement, represented with the star, show no significant difference from other data.
Figure 6 also shows (star) the average value of the critical length during force enhancement at tetanus plateau at the steady state. Similarly to Pc, Lc values at plateau lie very close to the data on the rise, confirming that no difference exists, regarding force enhancement, between these two conditions.
Discussion
The mechanism of force enhancement during slow stretching has been investigated by many authors over the years (Katz, 1939; Abbott & Aubert, 1952; Hill & Howarth, 1959; Sugi, 1972; Cavagna & Citterio, 1974; Edman et al. 1978, 1981; Flitney & Hirst, 1978a,b; Julian & Morgan, 1979, 1981; Lombardi & Piazzesi, 1990; Stienen et al. 1992; Cavagna, 1993; Getz et al. 1998; Lee et al. 2001); however, in spite of much effort, our understanding of the molecular process occurring during lengthening is still unclear. Contribution to force enhancement can be either active, from changes in crossbridges properties, or passive (changes in non-crossbridge elements). The passive force contribution to the measurements in this paper was corrected for (see Methods) and therefore our data refer exclusively to the crossbridge component.
In order to limit fibre damage, force enhancement in our experiments was induced by applying the slow conditioning stretches at low tensions during the tetanus rise and the measurements of Pc and Lc were consequently made before the force enhancement could attain the steady value. This procedure differs from all previous experiments in which slow conditioning stretches were usually applied at tetanus plateau and measurements were made during the force steady state that follows the attainment of the force peak (second phase of force enhancement; Getz et al. 1998). However, the results of the few experiments we made on the plateau with the classical protocol, showed no significant differences with those obtained on the tetanus rise (Figs 4 and 6).
In agreement with previous results at higher temperature (Bagni et al. 2005) we found that critical tension was directly proportional to the tension developed by the fibre during the tetanus rise, confirming that critical tension is proportional to crossbridge number. The Pc/Pin ratio on the tetanus rise was 3.05, a value greater than previously reported by our group (2.37; Bagni et al. 2005), but the difference is accounted for by the lower experimental temperature used here (5°C instead of 14°C). It is known in fact that lowering the temperature decreases the active tension without altering significantly the tension reached during the stretch (Piazzesi et al. 2003; our unpublished observations) and therefore increasing the Pc/Pin ratio.
Our results show that slow lengthening affects the crossbridge properties with respect to isometric conditions, in particular: (a) the rupture tension of the crossbridge ensemble, which was 3.05 times the tension developed under isometric conditions, dropped to 0.47 during force enhancement, and (b) the critical length, which was independent of force during the tetanus rise, decreased substantially with force during slow lengthening, falling from 13.04 nm hs−1 to 8.19 nm hs−1 from isometric to 90% force enhancement. These findings make clear that force enhancement by stretch is not due to a simple increase of crossbridge number as occurs with development of tension during the tetanus rise.
We showed previously that data obtained under many different experimental conditions such as isotonic shortening, ionic strength alteration, tetanus rise and tetanus relaxation were easily explained without assuming changes in individual crossbridge rupture force (Bagni et al. 2005; Colombini et al. 2007a). If this is true also for lengthening, changes in critical tension during force enhancement at various stretching speeds are attributable to changes in crossbridge number. As the ratio Pc/Pin of 3.05 found during the tetanic tension rise corresponds to the direct proportionality between tension and crossbridge number, the ratio of 0.47 during stretching shows that the increase of crossbridge number accounts for only 15.4% (0.47/3.05) of the force enhancement. Thus, most of the force enhancement during stretching (84.6%) is due to the increase of the mean strain of the crossbridge ensemble. Strained crossbridges develop a greater individual force and therefore, compared to isometric conditions, a smaller elongation is adequate to raise their force up to the rupture force. Hence critical length should diminish progressively with force enhancement. This is exactly what we found: increasing of force enhancement by slow stretching was accompanied by a parallel decrease of the critical length, in contrasts to isometric data. At 90% of force enhancement, critical length decreased by 4.85 nm hs−1, which means that, compared to isometric conditions, half-sarcomeres are strained by 4.85 nm hs−1 more. Does this strain increase derived from critical length measurements agree quantitatively with the force enhancement during stretching? To answer this question correctly it would be necessary to know the values of filament and crossbridge compliances and the shape of their stress–strain relationships. We know that filament compliance represents about 50% of the sarcomere compliance (Huxley et al. 1994; Wakabayashi et al. 1994) but the situation of the stress–strain relationships is less clear. For the case where both compliances are assumed to be Hookean, the following calculation can be used. During the tetanus rise stretching the half-sarcomeres by 13.04 nm hs−1 increments the isometric tension by 2.05 times. This means that the half-sarcomere strain at isometric tension (y0) is 13.04/2.05 = 6.36 nm hs−1. This strain is equally distributed between myofilaments and crossbridge compliances. If all the force enhancement were due to increase of half-sarcomere strain, at 90% of force enhancement, the strain would be 6.36 × 1.9 = 12.08 nm hs−1, 5.72 nm hs−1 greater than under isometric conditions. But our data show that 15.4% of force potentiation is due to crossbridge number increase, and therefore the strain increase necessary to account for 90% of potentiation reduces to 5.72 × 0.846 = 4.84 nm hs−1, very close to 4.85 nm hs−1 found experimentally. This value refers to the total half-sarcomere strain; the strain on the bridges will be one-half of that. It should be pointed out that this good agreement may be somewhat fortuitous, since it is not excluded that filament and crossbridge compliances are not linear (Higuchi et al. 1995; Bagni et al. 1999).
It has been suggested that most of the increase of tension during slow stretching is due to crossbridges attached in a pre-power-stroke state which are extended into a region of high force by the stretch (Getz et al. 1998; Pinniger et al. 2006). This mechanism would account for the high force production and for the low ATP hydrolysis rate in stretched fibres (Abbott & Aubert, 1951). The presence of pre-power-stroke bridges would influence both Pc and Lc according to their number and properties. However, their contribution to our data remains unclear as Pc and Lc represent the average values of all the different crossbridge states. It is interesting to point out that the loading of the crossbridges by the stretch increases their detachment rate (Colombini et al. 2007b) and may lead to detachment before they could go trough the normal cycling, thus explaining the low ATP consumption during stretching.
The hypothesis that force enhancement is mostly due to the increase of crossbridge extension is in agreement with previous data and models of force enhancement (Lombardi & Piazzesi, 1990; Getz et al. 1998; Pinniger et al. 2006) but disagrees with more recent data on frog and mammalian fibres suggesting that force enhancement is mainly, if not exclusively, due to increase of crossbridge number with no significant changes in crossbridge extension (Linari et al. 2000, 2004). The reason of this discrepancy is not clear. Our conclusions are based on the assumption that individual crossbridge rupture force is the same under isometric and lengthening conditions. If this is true, the rupture force of the crossbridge ensemble, which is proportional to number of crossbridges and individual rupture force, is a direct measure of changes of crossbridge number. In principle, however, it is possible that the rupture force of the individual bridge decreases during lengthening with respect to isometric. In this case, critical tension would be affected not only by the crossbridge number but also by changes of crossbridge rupture force. If the force enhancement is due exclusively to the increase of crossbridge number, the drop of the Pc/Pin ratio from the isometric value of 3.05 to 0.47 during slow stretching must be due to a corresponding decrease of the rupture force of the individual bridge which compensates the opposite effect of the increased crossbridge population on Pc. Smaller force enhancements, obtained with smaller lengthening velocities and associated with a smaller increase of crossbridge number, would require a smaller drop of the rupture force. Thus to explain our findings with the hypothesis that force enhancement is mostly due to the increase of crossbridges population, it is necessary to assume that the individual rupture force of the actomyosin bond is continuously variable decreasing progressively as the force enhancement increases. The rupture force of a chemical bond depends on the potential energy landscape and corresponds to the slope of the bond energy profile at inflection point between the energy minimum and the transition state. Although we cannot exclude an influence of slow stretching on the bond properties, it seems unlikely that the potential energy profile could change smoothly with stretching speed and exactly as necessary to justify the experimental data.
The hypothesis that force enhancement is mainly due to a crossbridge number increase was based mainly on the comparison of fibre stiffness (a putative measure of crossbridge number) at isometric plateau and during force enhancement. Calculation of fraction of attached bridges from stiffness data depends on filament and crossbridge elasticity and it is generally assumed that both elasticities are Hookean. This assumption, however, has not been firmly proven experimentally and actually some data in the literature suggest that actin filament compliance may be nonlinear (Higuchi et al. 1995). A nonlinearity seems to be present also on myosin filament elasticity. A recent X-ray diffraction study (Huxley et al. 2006) in fact, reported that myosin compliance, calculated from spacing changes of M3 and M6 reflections during isotonic shortening, was dependent on tension. This is an important point since compliance nonlinearity can affect significantly the calculation of fraction of attached crossbridges (Bagni et al. 1999).
The force enhancement mechanism has been investigated also by using the X-ray diffraction technique (Amemiya et al. 1988; Linari et al. 2000). Both meridional M3 (I) and equatorial 1,1 intensities (I11) are strongly reduced during slow lengthening while an intensity of 1,0 reflection (I10) is almost unaltered. These results are in contrast with the hypothesis of an increasing number of crossbridges during lengthening. The new crossbridge mass added at a precise spacing along the myosin filament and to the 1,1 plane, should in fact increases significantly both I intensity and I11/I10 ratio. To explain this contradiction it has been assumed that lengthening induces disorder of the regular myosin head distribution at 14.3 nm along the myosin filament, decreasing IM3 below the isometric value (Linari et al. 2000). Similarly the reduction of the I11/I10 ratio has been attributed to disorder of the myofilament lattice caused by forcible lengthening (Amemiya et al. 1988).
In summary, our data indicate that force enhancement during stretching is mostly due to the increase of crossbridge mean strain accompanied by a small crossbridge number increase. Lengthening of active muscles is a physiological condition occurring frequently in animals and man during normal activity and it characterized by high force production with low ATP consumption. In spite of the low energy required, exercises involving stretching of active muscles (eccentric exercises) are better suited than isometric exercises to enhance muscle characteristics (LaStayo et al. 2000). Thus, in addition to the importance for crossbridge models of muscle contraction, the knowledge of the molecular mechanism underlying the force enhancement during slow stretching may be also important in designing exercise protocols which maximize the enhancement of muscle performances.
Acknowledgments
This study was supported by the Università di Firenze, Ministero della Ricerca Scientifica (PRIN) and Ente Cassa di Risparmio di Firenze 2004.1671.
References
- Abbott BC, Aubert XM. Changes in energy of a muscle during very slow stretch. Proc R Soc Lond B Biol Sci. 1951;139:104–107. doi: 10.1098/rspb.1951.0049. [DOI] [PubMed] [Google Scholar]
- Abbott BC, Aubert XM. The force exerted by active striated muscle during and after change of length. J Physiol. 1952;117:77–86. [PMC free article] [PubMed] [Google Scholar]
- Amemiya Y, Iwamoto H, Kobayashi T, Sugi H, Tanaka H, Wakabayashi K. Time-resolved X-ray diffraction studies on the effect of slow length changes on tetanized frog skeletal muscle. J Physiol. 1988;407:231–241. doi: 10.1113/jphysiol.1988.sp017412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni MA, Cecchi G, Cecchini E, Colombini B, Colomo F. Force responses to fast ramp stretches in stimulated frog skeletal muscle fibres. J Muscle Res Cell Motil. 1998;19:33–42. doi: 10.1023/a:1005348209816. [DOI] [PubMed] [Google Scholar]
- Bagni MA, Cecchi G, Colombini B. Crossbridges properties investigated by fast ramp stretching of activated frog muscle fibres. J Physiol. 2005;565:261–268. doi: 10.1113/jphysiol.2005.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni MA, Cecchi G, Colombini B, Colomo F. Sarcomere tension-stiffness relation during the tetanus rise in single frog muscle fibres. J Muscle Res Cell Motil. 1999;20:469–476. doi: 10.1023/a:1005582324129. [DOI] [PubMed] [Google Scholar]
- Bagni MA, Cecchi G, Colombini B, Colomo F. A non-crossbridge stiffness in activated frog muscle fibers. Biophys J. 2002;82:3118–3127. doi: 10.1016/S0006-3495(02)75653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni MA, Colombini B, Geiger P, Berlinguer Palmini R, Cecchi G. Non-crossbridge calcium-dependent stiffness in frog muscle fibers. Am J Physiol Cell Physiol. 2004;286:1353–1357. doi: 10.1152/ajpcell.00493.2003. [DOI] [PubMed] [Google Scholar]
- Cavagna GA. Effect of temperature and velocity of stretching on stress relaxation of contracting frog muscle fibres. J Physiol. 1993;462:161–173. doi: 10.1113/jphysiol.1993.sp019549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Citterio G. Effect of stretching on the elastic characteristics and the contractile component of frog striated muscle. J Physiol. 1974;239:1–14. doi: 10.1113/jphysiol.1974.sp010552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini B, Bagni MA, Cecchi G, Griffiths PJ. Effects of solution tonicity on crossbridge properties and myosin lever arm disposition in intact frog muscle fibres. J Physiol. 2007a;578:337–346. doi: 10.1113/jphysiol.2006.117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini B, Bagni MA, Romano G, Cecchi G. Characterization of actomyosin bond properties in intact skeletal muscle by force spectroscopy. Proc Natl Acad Sci U S A. 2007b;104:9284–9289. doi: 10.1073/pnas.0611070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Elzinga G, Noble MIM. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol. 1978;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Elzinga G, Noble MIM. Critical sarcomere extension required to recruit a decaying component of extra force during stretch in tetanic contractions of frog skeletal muscle fibers. J Gen Physiol. 1981;78:365–382. doi: 10.1085/jgp.78.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KAP, Tsuchiya T. Strain of passive elements during force enhancement by stretch in frog muscle fibres. J Physiol. 1996;490:191–205. doi: 10.1113/jphysiol.1996.sp021135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney FW, Hirst DG. Cross-bridge detachment and sarcomere ‘give’ during stretch of active frog's muscle. J Physiol. 1978a;276:449–465. doi: 10.1113/jphysiol.1978.sp012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney FW, Hirst DG. Filament sliding and energy absorbed by the crossbridges in active muscle subjected to cyclical length changes. J Physiol. 1978b;276:467–479. doi: 10.1113/jphysiol.1978.sp012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford LE, Huxley AF, Simmons RM. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977;269:441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz EB, Cooke R, Lehman SL. Phase transition in force during ramp stretches of skeletal muscle. Biophys J. 1998;75:2971–2983. doi: 10.1016/S0006-3495(98)77738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H, Yanagida T, Goldman Y. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J. 1995;69:1000–1010. doi: 10.1016/S0006-3495(95)79975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV, Howarth JV. The reversal of chemical reactions in contracting muscle during an applied stretch. Proc R Soc Lond B Biol Sci. 1959;151:169–193. [Google Scholar]
- Huxley AF, Lombardi V, Peachey LD. A system for fast recording of longitudinal displacement of a striated muscle fibre. J Physiol. 1981;317:12–13. [Google Scholar]
- Huxley HE, Reconditi M, Stewart A, Irving T. X-ray interference studies of crossbridge action in muscle contraction: evidence from muscles during steady shortening. J Mol Biol. 2006;363:762–772. doi: 10.1016/j.jmb.2006.08.055. [DOI] [PubMed] [Google Scholar]
- Huxley HE, Stewart A, Sosa H, Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J. 1994;67:2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian FJ, Morgan DL. The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J Physiol. 1979;293:379–392. doi: 10.1113/jphysiol.1979.sp012895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian FJ, Morgan DL. Variation of muscle stiffness with tension during tension transients and constant velocity shortening in the frog. J Physiol. 1981;319:193–203. doi: 10.1113/jphysiol.1981.sp013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci U S A. 2003;100:13716–13721. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaStayo PC, Pierotti DJ, Pifer J, Hoppeler H, Lindstedt SL. Eccentric ergometry: increases in locomotor muscle size and strength at low training intensities. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1282–R1288. doi: 10.1152/ajpregu.2000.278.5.R1282. [DOI] [PubMed] [Google Scholar]
- Lee HD, Herzog W, Leonard T. Effects of cyclic changes in muscle length on force production in in-situ cat soleus. J Biomech. 2001;34:979–987. doi: 10.1016/s0021-9290(01)00077-x. [DOI] [PubMed] [Google Scholar]
- Linari M, Bottinelli R, Pellegrino MA, Reconditi M, Reggiani C, Lombardi V. The mechanism of the force response to stretch in human skinned muscle fibres with different myosin isoforms. J Physiol. 2004;554:335–352. doi: 10.1113/jphysiol.2003.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M, Lucii L, Reconditi M, Vanicelli Casoni ME, Amenitsch H, Bernstorff S, Piazzesi G, Lombardi V. A combined mechanical and X-ray diffraction study of stretch potentiation in single frog muscle fibres. J Physiol. 2000;526:589–596. doi: 10.1111/j.1469-7793.2000.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V, Piazzesi G. The contractile response during steady lengthening of stimulated frog muscle fibres. J Physiol. 1990;431:141–171. doi: 10.1113/jphysiol.1990.sp018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzesi G, Reconditi M, Koubassova N, Decostre V, Linari M, Lucii L, Lombardi V. Temperature dependence of the force-generating process in single fibres from frog skeletal muscle. J Physiol. 2003;549:93–106. doi: 10.1113/jphysiol.2002.038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinniger GJ, Ranatunga KW, Offer GW. Crossbridge and non-crossbridge contributions to tension in lengthening rat muscle: force-induced reversal of the power stroke. J Physiol. 2006;573:627–643. doi: 10.1113/jphysiol.2005.095448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJM, Versteeg PGA, Papp Z, Elzinga G. Mechanical properties of skinned rabbit psoas and soleus muscle fibres during lengthening: effects of phosphate and Ca2+ J Physiol. 1992;451:503–523. doi: 10.1113/jphysiol.1992.sp019176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H. Tension changes during and after stretch in frog muscle fibres. J Physiol. 1972;225:237–253. doi: 10.1113/jphysiol.1972.sp009935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H, Tsuchiya T. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J Physiol. 1988;407:215–229. doi: 10.1113/jphysiol.1988.sp017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y, Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994;67:2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]