Abstract
The Striatum is involved in the regulation of movements and motor skills. We have shown previously, that the osmolyte and neuromodulator taurine plays a role in striatal plasticity. We demonstrate now that hereditary taurine deficiency in taurine-transporter knock-out (TAUT KO) mice results in disinhibition of striatal network activity, which can be corrected by taurine supplementation. Modification of GABAA but not glycine receptors (taurine is a ligand for both receptor types) underlies this disinhibition. Whole-cell recordings from acutely isolated as well as cultured striatal neurons revealed a decreased agonist sensitivity of the GABAA receptor in TAUT KO neurons in the absence of changes in the maximal GABA-evoked current amplitude. The striatal GABA level in TAUT KO mice was unchanged. The amplitude enhancement of spontaneous IPSCs by zolpidem was stronger in TAUT KO than in wild-type (WT) animals. Tonic inhibition was absent in striatal neurons under control conditions but was detected after incubation with the GABA-transaminase inhibitor vigabatrin: bicuculline induced a larger shift of baseline current in WT as compared to TAUT KO neurons. Lack of taurine leads to reduced sensitivity of synaptic and extrasynaptic GABAA receptors and consequently to disinhibition. These findings help in understanding neuropathologies accompanied by the loss of endogenous taurine, for instance in hepatic encephalopathy.
Glutamatergic inputs from cortex and thalamus on GABAergic medium spiny neurons (MSN) of the striatum are modulated by the dopaminergic nigrostriatal projection. These principal neurons innervate the output structures of the basal ganglia, providing control of movement, storage of motor skill programmes and participation in cognitive activities (Graybiel, 1995). Corticostriatal synaptic plasticity represents the cellular basis for long-term regulatory control of basal ganglia.
Taurine has important physiological functions in cell volume regulation and intracellular calcium homeostasis (Huxtable, 1992). This major osmolyte and neuromodulator induces a long-term enhancement of corticostriatal synaptic transmission (LLETAU) (Sergeeva et al. 2003). In mammalian skeletal muscles it plays a role in the excitation–contraction coupling mechanism and taurine-deficient mice display a drastic decrease in overall exercise capacity in treadmill experiments (Warskulat et al. 2004). Taurine is an endogeneous ligand of GABAA and glycine receptors (Hussy et al. 1997; Sergeeva & Haas, 2001), exerting neuroprotection through these sites under osmotic/metabolic stress (Saransaari & Oja, 2000; O'Byrne & Tipton, 2000). At native glycine receptors the presence of the β-subunit determines potency and efficacy of taurine (Sergeeva & Haas, 2001). In heterologously expressed GABAA receptors composed of either α1β3 or α1β3γ2 subunits, taurine is a full or partial agonist, respectively, while both receptor types exhibit similar taurine sensitivities (Dominguez-Perrot et al. 1996).
Hepatic encephalopathy, a neuropsychiatric disorder associated with acute or chronic liver failure is caused by mild brain oedema and loss of endogeneous osmolytes (Haussinger et al. 1994, 2002). An increased GABAergic tone explains symptoms of HE (Ahboucha & Butterworth, 2004) and may involve GABAA receptor modifications by a number of factors such as allopregnanolone (Poulter et al. 1997; Ahboucha et al. 2006) and taurine (Butterworth, 1996). Principal neurons of the striatum are known to accumulate taurine in millimolar concentrations through the abundantly expressed taurine transporter (Clarke et al. 1983; Sergeeva et al. 2003). The lack of high-affinity taurine transport (TAUT) leads to taurine depletion in brain tissues: thus striatal, cortical, hippocampal, cerebellar and brain stem taurine levels represent 4.5, 2.4, 2.0, 3.0 and 9.7%, respectively, of corresponding WT levels (Sergeeva et al. 2003).
We demonstrate now that taurine deficiency causes a modification of neuronal GABAA receptors and weakens GABAergic inhibition within a striato-pallidal cell culture network.
Methods
Acutely isolated neurons were obtained from 6- to 11-week-old TAUT knockout (KO, n = 11) and wild-type (WT, n = 10) mice, while primary cultures were made from newborn TAUT KO (n = 30) and WT (n = 27) mice. The generation of TAUT-deficient mice as well as the genotyping protocol are described elsewhere (Heller-Stilb et al. 2002). Animal experiments were conducted according to local guidelines (Bezirksregierung Duesseldorf). In order to minimize the number of experimental animals and due to the reduced fertility of TAUT KO females, homozygous TAUT KO males were mated to heterozygous females, and individual newborn mice were used for the cultures and genotyped post mortem; cultures derived from heterozygotes were excluded from the study. Knock-out and wild-type mice (obtained from mating of wild-type animals) represented second generation littermates obtained from heterozygous pairs. Acutely isolated neurons from adult genotyped animals were obtained as follows: animals were decapitated, and brains were rapidly removed, placed into ice-cold Krebs–Ringer solution and cut to horizontal slices, 400 μm thick, using a Vibroslicer. The slice preparations included neostriatum and neocortex. After 2 h preincubation in a medium containing (mm) 124 NaCl, 3.7 KCl, 1.24 NaH2PO4, 1.3 MgSO4, 2.0 CaCl2, 26 NaHCO3 and 10 glucose, saturated with 95% O2–5% CO2, a single slice was transferred to a chamber filled with recording solution (mm): 150 NaCl, 3.7 KCl, 2.0 CaCl2, 2.0 MgCl2, 10 Hepes, 10 glucose, pH 7.4. Individual neurons were separated by vibrodissociation from the dorsal striatum and identified according to their size and shape as medium spiny neurons (MSN, soma size 10–15 μm, spherical or fusiform) or giant aspiny neurons (GAN, soma size more than 20 μm, round or polygonal) as previously described (Sergeeva & Haas, 2001). Whole-cell voltage-clamp recordings (hoding potential −70 mV) were done with an EPC-9 amplifier and TIDA for windows (HEKA, Lambrecht, Germany) at room temperature. The intracellular solution was composed of (mm): 140 CsCl, 2 MgCl2, 0.5 CaCl2, 5 EGTA, 2 ATP and 10 Hepes (pH 7.2 adjusted with CsOH). For the fast drug exposure and the construction of dose–response curves see Sergeeva et al. (2005). Modulators were applied simultaneously with GABA. Data are given as the mean ± s.e.m.
Spontaneous GABAergic IPSCs were recorded in the presence of 6-cyano-7-nitroquinoxaline-2,3,-dione (CNQX; 10 μm) and d(−)-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μm) and analysed with MiniAnalysis 4.2 (Synaptosoft, Leonia, NJ, USA). Peak amplitude, the 10–90% rise time, exponential decay time constant in a 100 ms window from the time of peak (τdec), area (pA × ms) and frequency of sIPSCs were calculated. All values were compared between control (before and after application) and presence of the modulator with the Kolmogorov–Smirnov two-sample test in every cell. Each of three testing periods lasted 60–90 s. The non-parametrical Mann–Whitney U test was used for comparison between two groups. The significance level was set at P < 0.05. All neuroactive substances were purchased from Tocris (Biozol, Eching, Germany) unless mentioned otherwise.
Multielectrode array (MEA) recordings were performed from primary dissociated cultures of striatum prepared from newborn mice according to the protocols previously described (Sergeeva et al. 2005). Briefly, animals were anaesthetized with isoflurane and decapitated, and coronal slices containing striatum and globus pallidus were cut in phosphate-buffered saline (PBS). Striata were dissected and triturated after trypsinization (20 min) and washed in nutrient medium consisting of fetal calf serum (10%), Eagle's minimal essential medium (89%), glucose (0.8%), glutamine (2 mm), insulin (0.1 U ml−1) and Hepes (10 mm). Dissociated cells were plated in a density of 1–2 × 105 cm−2 onto polyethylenimine-coated MEAs in a volume of 100 μl (multielectrode arrays, Multi Channel Systems, Reutlingen, Germany) and cultured in an incubator with 5% CO2–95% air and 98% relative humidity, at 35.5 ± 0.5°C. On the second day serum-free neurobasal medium containing supplement B27 (2%) was added to the final volume of 1 ml.
Extracellular potentials were recorded on MEAs with a square grid of 60 planar Ti/TiN-microelectrodes (30 μm diameter, 200 μm spacing) at 37°C. Signals from all 60 electrodes were simultaneously sampled at 25 kHz, visualized and stored using the standard software MCRack provided by Multi Channel Systems. Spike detection was performed offline using the software SpAnNer (RESULT Medizinische Analyseverfahren, Tönisvorst, Germany; for details see Otto et al. 2003). At the beginning of experiments the basal medium was replaced by a magnesium free Hepes-based recording solution (see above) and measurements were started after a 20 min adaptation phase. Every measurement comprised three recordings – control, test substance and washout (second control) – each 2 min long and separated by an intermediate period of 30 s. Recordings were made from MEAs if more than 10 channels were active. Each substance was applied to a MEA only once during one experimental day.
For immunohistochemistry cell cultures were grown on glass coverslips and fixed after 14–21 days in vitro for 5 min in 4% paraformaldehyde–0.3% glutaraldehyde followed after washing in PBS by 20 min incubation in 98% ethanolamine. Detection of the immunoreactivity was carried out via fluorescence-labelled secondary antibodies (for details see Sergeeva et al. 2005). The following antibodies were used at dilutions indicated: rabbit anti-GABA antibody and monoclonal anti-microtubule-associated protein 2 (MAP2) antibody (both from Sigma, Deisenhofen, Germany, 1 : 500); rabbit anti-taurine polyclonal antibody (WAK-Chemie Medical, Germany, 1 : 200) and rabbit anti-taurine transporter serum Tau11-A (DPC Biermann, Bad Nauheim, Germany, 1 : 500); Alexa Fluor 488-labelled goat anti-mouse IgG (1 : 500; Molecular Probes, Eugene, OR, USA) and Texas Red donkey anti-rabbit IgG (1 : 200, Dianova, Hamburg, Germany).
For determination of GABA levels, the dorsal striatum was dissected from coronal slices and frozen at −70°C. Frozen tissue was homogenized and weighed without thawing. Proteins were removed by incubation in 10% sulfosalicylic acid on ice for 1 h. After centrifugation lipids were extracted from the supernatant with dichloromethane. Plasma was added to an equal amount of 10% sulfosalicylic acid. GABA content was measured in a BioChrom20 amino acid analyser (Amersham-Pharmacia Biotech, Freiburg, Germany).
Results
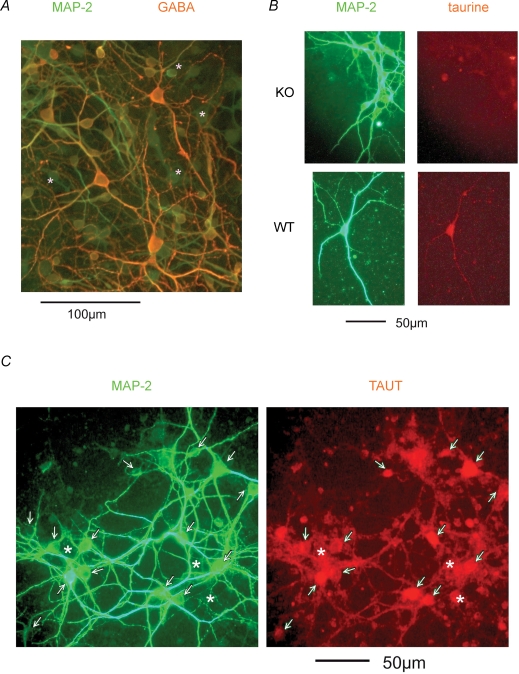
The vast majority of neurons in striato-pallidal primary cultures after 2 weeks in vitro were GABA-positive. In less than 5% of the cells no GABA staining was found (Fig. 1A). GABA-staining did not differ between WT and KO cultures. TAUT staining was strongly colocalized with MAP2 immunoreactivity. Neurons and glia were positive for taurine in WT cultures, while only some glial cells were weakly positive in TAUT KO cultures (Fig. 1B and C).
Figure 1. Immunohistochemical analysis of striatal cultures.
A, the majority of cultured neurons coexpress MAP2 and GABA. Asterisk marks GABA-immunonegative cells. B, neuronal taurine immunoreactivity is seen in WT, but not in TAUT KO, cultures. C, taurine transporter immunoreactivity is colocalized with the neuronal marker (marked with arrows), but also seen in glial cells (marked with asterisks).
Primary cultures grown on MEAs demonstrated, starting from days 5–7 in vitro, spontaneous activity, consisting of regular unsynchronized single spikes. This activity pattern was replaced during the third week in vitro by periods of synchronized activity followed by global silence periods. After establishment this ‘mature’ type of activity was maintained for up to 4 weeks followed thereafter by a decline of activity. Recordings were done between days 14 and 35 after plating. The amplitudes of extracellularly measured action potentials were 55.4 ± 9.8 μV (measured in 7 WT MEAs) and 52 ± 5.5 (in 8 TAUT KO MEAs, difference not significant).
Spontaneous activity in Mg2+-free solution was driven by NMDA and AMPA receptors: the NMDA receptor antagonist d-(−)-2-amino-5-phosphonopentanoic acid (d-AP5, 50 μm) reduced the spontaneous spike rate (SSR, number of spikes over all electrodes per minute) in TAUT KO (n = 9) and WT (n = 9) mice to 20% (P = 0.24). In the presence of d-AP5, burst duration and number of spikes per burst were reduced to the same extent in both animal groups: to 40% and 65%, respectively (pooled data). We noticed a significant difference in basal (control) burst duration (P = 0.03, 206 ± 29 ms in TAUT KO versus 128 ± 13 ms in WT cultures) and in the number of spikes per burst (P = 0.02, 10 ± 1 in KO versus 8 ± 1 in WT), but there was no difference in SSR (P = 0.2, 4826 ± 836 in KO versus 4472 ± 1097 in WT).
The AMPA receptor antagonist CNQX reduced SSR to the same extent (P = 0.104) in KO (to 64.1 ± 7.6%, n = 12) and WT (to 78.8 ± 8.8%, n = 9) cultures and enhanced by 50% the burst duration (P = 0.72) and number of spikes per burst (P = 0.39).
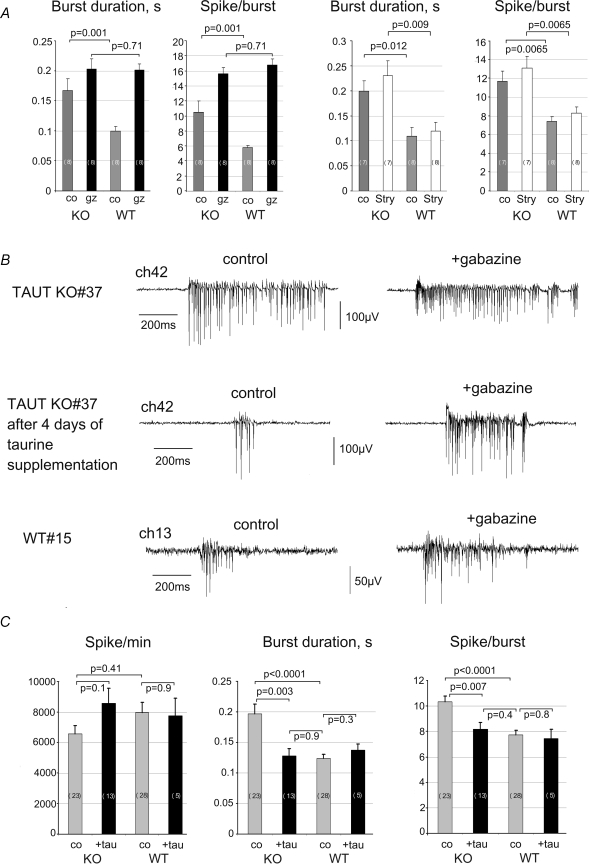
The GABAA receptor antagonist gabazine (10 μm, SR 95531) increased the burst duration by 98 ± 23% in WT (n = 8) versus 16 ± 18% in KO (n = 8) over control (P = 0.018) and the number of spikes per burst by 195 ± 45% in WT versus 49 ± 16% in KO over control (P = 0.01). While control burst duration and number of spikes per burst differed between KO and WT, no difference was observed under gabazine (Fig. 2A). SSR did not differ (P = 0.19) between WT and KO MEAs (8761 ± 1425 and 11129 ± 768).
Figure 2. Striatal network activity measurements with Multi Electrode Arrays (MEA) reveal a deficit in GABAA receptor mediated inhibition in TAUT KO cultures.
A, averaged activity parameters show the difference in control (co) medium and the similarity in the presence of gabazine (gz) between TAUT KO and WT cultures. In contrast, in the presence of strychnine (stry) these differences are preserved. B, example of spontaneous burst activity of striatal neurons recorded from channel 42 after 2 weeks in culture from MEA no. 37 (upper traces). The activity patterns recorded from the same channel after treatment with taurine (tau, 1 mm, middle traces) became similar to those seen in an age-matched WT culture (lower traces). C, summary of values for the activity parameters (Spike/burst: number of spikes per burst; Spike/min: number of spikes per minute) under basal conditions in four groups: KO and WT after 4 days of taurine supplementation (black bars) and without treatment (grey bars). Number of MEAs tested is given on corresponding bars.
In contrast to gabazine, the antagonist at glycine receptors, strychnine (0.5 μm, Fig. 2, Sigma/RBI, Deisenhofen, Germany), and the antagonist at GABAB receptors, CGP32345 (100 μm, 4 WT and 4 KO MEAs tested), did not significantly influence parameters of network activity. Thus, the differences between TAUT KO and WT cultures in burst duration and number of spikes per burst were attributed to diminished GABAA receptor mediated inhibition in the former animal group.
Taurine (1 mm for 2 min) reversibly inhibited SSR to 84.12 ± 7.5% (n = 8, KO) and to 77 ± 6.9% (n = 12, WT) of control level (P = 0.42). In TAUT KO MEAs incubated 4 days with taurine (1 mm) subsequent recordings in taurine-free medium revealed a significant decrease in burst duration and in the number of spikes per burst compared to untreated MEAs (Fig. 2C and D), while treatment with 0.1 mm taurine did not change the activity parameters in TAUT KO cultures. The same treatments had no effects on WT cultures. Thus, long-term taurine supplementation was able to restore deficient GABAergic inhibition in TAUT KO cultures.
The striatal GABA level was not modified in TAUT KO mice: GABA concentration, measured as μmol (g wet weight)−1, represented 2.19 ± 0.36 (n = 4) versus 2.15 ± 0.29 (n = 4) in striatum of TAUT KO versus WT mice (P = 0.71). Therefore GABAA receptor modification in TAUT KO cultures was primarily dependent on taurine, not on GABA level.
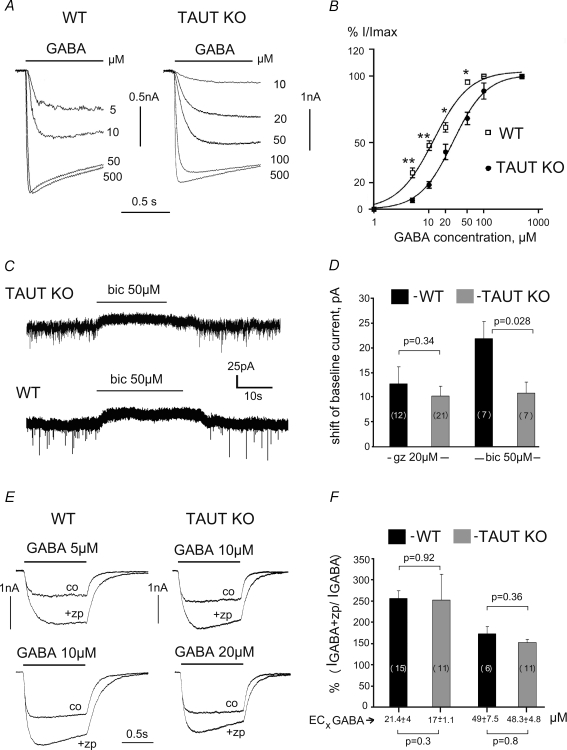
Whole-cell patch-clamp recordings demonstrated that all neurons cultured (n = 17) or acutely isolated (n = 48) from dorsal striatum responded to GABA. Gabazine (10 μm) abolished these responses (n = 8), indicating activation of GABAA receptors. In acutely isolated neurons maximal GABA (0.5 mm)-evoked currents did not differ in amplitude in the two animal groups either between medium spiny neurons (MSN) (0.85 ± 0.19 nA, n = 18, WT and 0.73 ± 0.17 nA, n = 15, KO, P = 0.66) or cholinergic giant aspiny neurons (GAN) (2.23 ± 0.8 nA, n = 7, WT and 1.68 ± 069 nA, n = 4, KO, P = 0.63). Both neuronal types (MSN and GAN) displayed higher EC50 values for GABA in TAUT KO mice in comparison with WT (Fig. 3A and B). As there was no significant difference in EC50 between MSN and GAN we pooled the data. EC50 values were 12.7 ± 1.7 μm (n = 18) for the WT and 27.22 ± 2.0 μm (n = 14) for the TAUT KO (P < 0.01). No difference between the slope functions of GABA dose–response curves was detected (nh = 1.32 ± 0.32 versus 1.42 ± 0.11 in WT and KO, respectively, P = 0.5).
Figure 3. GABA-evoked whole-cell responses demonstrate decreased sensitivity to GABA in TAUT KO compared to WT GABAARs.
A, recordings from acutely isolated striatal neurons. B, dose–response curves summarize data obtained from 18 WT and 14 TAUT KO neurons. *P < 0.05, **P < 0.01. C, bicuculline (bic) causes a shift in the baseline current in striatal cultures after 2 days incubation with vigabatrin (100 μm). D, averaged data from such experiments with bicuculline or gabazine (gz). E, zolpidem (zp) modulation of GABA-evoked currents (co: control response). GABA was applied at 2 concentrations: near EC20 and near EC50 (exact averaged values are given below in F). Number of tested neurons is given on corresponding bars.
Cultured neurons from WT mice were more sensitive to GABA compared to TAUT KO mice (corresponding EC50 vlaues were 8.0 ± 0.47 μm (n = 7) and 22.6 ± 1.8 μm (n = 6), P = 0.046). Neither slope functions of dose–response curves (nh = 1.1 ± 0.04 versus 1.0 ± 0.04 in WT and KO, respectively, P = 0.32) nor maximal GABA-evoked currents (1.42 ± 0.3 nA in WT(n = 10) and 1.2 ± 0.43 nA in TAUT KO (n = 7), P = 0.4) were different.
Our data indicate no difference in GABAA receptor numbers in striatal neurons of TAUT KO versus WT mice, measured in terms of maximal current amplitudes. Thus a different subunit composition or phosphorylation status rather than a quantitative difference in GABAA receptors in TAUT KO versus WT cultures was responsible for the decreased GABAergic inhibition.
GABAA receptors of high sensitivity to agonist may be involved in tonic (extrasynaptic) inhibition (Farrant & Nusser, 2005). Under control conditions none of six cells tested displayed a shift of baseline current in response to the GABAA receptor antagonists gabazine (20 μm) and bicuculline (50 μm). However, after 2–5 days incubation of the cultures with the irreversible GABA-transaminase inhibitor vigabatrin (100 μm, see Wu et al. 2003) such a shift was observed in the majority of the cells (76%). In contrast to experiments with gabazine, where no significant difference between amplitudes of ‘tonic current’ was seen between KO and WT neurons, a larger current amplitude was detected with bicuculline in WT cells (Fig. 3C and D). The difference in the action of the two antagonists may be explained by the prevalent expression of the GABAA receptor α2 subunit in the striatum (Korpi et al. 1996).
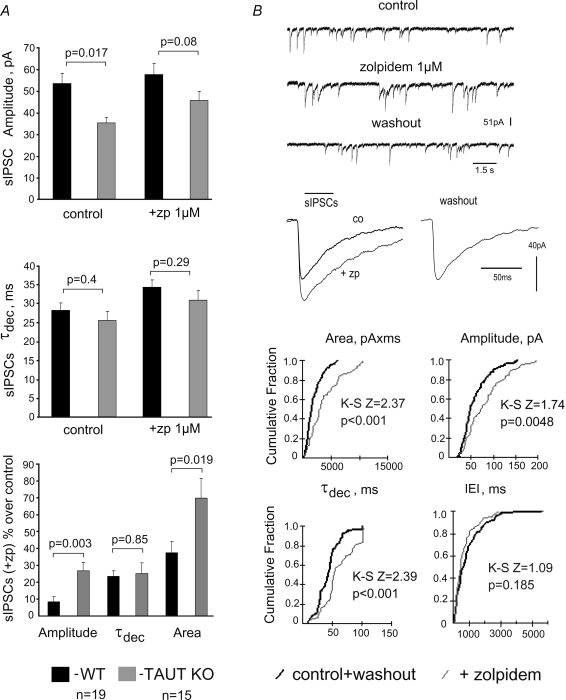
Zolpidem (1 μm) enhanced submaximal whole-cell GABA-evoked responses in TAUT KO and WT neurons to the same extent (Fig. 3E and F). Averaged amplitudes and areas, but not τdec of sIPSCs were enhanced by zolpidem more markedly in TAUT KO than in WT neurons (Fig. 4). Significant changes in sIPSC amplitudes were detected with the Kolmogorov–Smirnov test (example is shown in Fig. 4B) in 10 out of 19 neurons in KO versus 1 out of 16 WT neurons (Fisher exact probability test, P = 0.005). The frequency of sIPSCs was not different between TAUT KO (n = 19) versus WT (n = 16) neurons either under zolpidem (0.87 ± 0.2 Hz versus 0.8 ± 0.12 Hz, P = 0.8) or in control (0.57 ± 0.1 Hz versus 0.5 ± 0.07 Hz, P = 0.36), indicating that presynaptic release of GABA was not affected under taurine deficiency. The larger sIPSC amplitude potentiation by zolpidem in TAUT KO mice can thus be explained by decreased sensitivity to GABA of postsynaptic GABAA receptors.
Figure 4. Analysis of spontaneous IPSCs (sIPSCs) in cultured striatal neurons.
A, averaged values for amplitudes and kinetics of sIPSCs and their percentage change from control under zolpidem (lower bar graph). B, recordings of sIPSCs and their analysis in an individual TAUT KO medium spiny neuron. Significance of parameter changes was probed with the Kolmogorov–Smirnov 2-sample test (K-S Z and P values are given on corresponding cumulative fraction histograms). Average traces of all recorded sIPSCs in control (n = 39), under zolpidem (n = 32) and upon washout (n = 53) are given in the middle.
Discussion
We demonstrate that disinhibition of striato-pallidal cell culture network activity in TAUT KO mice results from reduced agonist sensitivity of synaptic and extrasynaptic GABAA receptors and can be rescued by taurine supplementation. Our findings shed light on the role of taurine in the control of GABAergic inhibition as well as its involvement in the regulation of movement.
Previous studies have demonstrated that continuous exposure of neurons to GABA induces a series of poorly understood regulatory mechanisms, which alter the number and function of GABAA receptors (Hablitz et al. 1989; Kim et al. 1993). For the first time we describe here a role of the endogeneous ligand taurine for GABAA receptor functions. The concentration of taurine used for the long-term treatment of cultures corresponds to the EC25 for activation of glycine receptors in striatal cholinergic neurons and represents the threshold for GABAA receptor activation (Sergeeva & Haas, 2001). Accordingly, spontaneous firing of cultured neurons was only slightly inhibited by taurine. We assume that long-term exposure to extracellular (not intracellular) taurine is necessary for the correction of TAUT KO network activity. The failure of taurine supplementation to modify the activity of WT cultures may be related to the release of endogeneous taurine from cultured neurons at this or even higher concentrations under physiological conditions. The zwitterionic nature of taurine prevents its passage through lipid bilayers and allows the Na+-dependent taurine transport system to build up concentration gradients between cellular and extracellular spaces. However, at 10 mm, taurine can be taken up by the GABA transporters (Sergeeva et al. 2003).
The α1-subunit preferring benzodiazepine-site agonist zolpidem is used to probe the degree of receptor occupancy at synapses (Perrais & Ropert, 1999): sIPSC amplitudes at synapses operating with maximal GABA concentration are not potentiated in contrast to synapses with a submaximal concentration of GABA. Alternatively, a lower receptor sensitivity to GABA at maintained agonist concentration and receptor density may also enable the zolpidem-induced potentiation of sIPSC amplitude, seen in TAUT KO neurons.
Which kind of GABAA receptor modification may underlie the reduction of its sensitivity to GABA? We assume that a change in receptor expression and protein synthesis must be involved as several days of incubation with taurine were necessary to ‘normalize’ the phenotype of TAUT KO cultures. Mechanisms of such modification besides GABAA receptor expression may include changes in the receptor phosphorylation or receptor clustering.
What is the behavioural consequence of striatal disinhibition in TAUT KO mice? It was shown previously that injection of bicuculline into rat ventral striatum results in locomotor activation (Wong et al. 1991). On the other hand TAUT KO mice suffer from functionally impaired skeletal muscles, move less and show an 80% decrease in overall exercise capacity in treadmill experiments compared to the WT mice (Warskulat et al. 2004). Therefore, taurine is involved in the organization of movement at central and peripheral levels. Postural tremor in patients with hepatic encephalopathy is attributed to functional alterations in the motor cortex–basal ganglia–thalamo-cortical loops (Timmermann et al. 2004). Loss of endogeneous osmolytes, such as taurine, in the pathogenesis of hepatic encephalopathy (Haussinger et al. 1994, 2002) may therefore be related to basal ganglia dysfunction (Weissenborn & Kolbe, 1998) through the taurine-dependent modification of GABAA receptors. Taurine may offer a therapeutic potential for motor and cognitive disorders through the rescue of both GABAergic inhibition (present paper) and hyperammonaemia-induced failure of synaptic plasticity (Chepkova et al. 2006).
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft, SFB 575 ‘Experimental Hepatology’ and a Heisenberg Grant to O.A.S. We are grateful to Dr I. Mönnighoff for her help with measurements of taurine and GABA levels in tissue samples.
References
- Ahboucha S, Butterworth RF. Pathophysiology of hepatic encephalopathy: a new look at GABA from the molecular standpoint. Metab Brain Dis. 2004;19:331–343. doi: 10.1023/b:mebr.0000043979.58915.41. [DOI] [PubMed] [Google Scholar]
- Ahboucha S, Pomier-Layrargues G, Mamer O, Butterworth RF. Increased levels of pregnenolone and its neuroactive metabolite allopregnanolone in autopsied brain tissue from cirrhotic patients who died in hepatic coma. Neurochem Int. 2006;49:372–378. doi: 10.1016/j.neuint.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. Taurine in hepatic encephalopathy. Adv Exp Med Biol. 1996;403:601–606. doi: 10.1007/978-1-4899-0182-8_66. [DOI] [PubMed] [Google Scholar]
- Chepkova AN, Sergeeva OA, Haas HL. Taurine rescues hippocampal long-term potentiation from ammonia-induced impairment. Neurobiol Dis. 2006;23:512–521. doi: 10.1016/j.nbd.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, Smith AD, Bolam JP. Uptake of [3H]taurine into medium-size neurons and into identified striatonigral neurons in the rat neostriatum. Brain Res. 1983;289:342–348. doi: 10.1016/0006-8993(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Dominguez-Perrot C, Feltz P, Poulter MO. Recombinant GABAA receptor desensitization: the role of the gamma 2 subunit and its physiological significance. J Physiol. 1996;497:145–159. doi: 10.1113/jphysiol.1996.sp021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Trends Neurosci. 1995;18:60–62. [PubMed] [Google Scholar]
- Hablitz JJ, Tehrani MH, Barnes EM., Jr Chronic exposure of developing cortical neurons to GABA down-regulates GABA/benzodiazepine receptors and GABA-gated chloride currents. Brain Res. 1989;501:332–338. doi: 10.1016/0006-8993(89)90650-1. [DOI] [PubMed] [Google Scholar]
- Haussinger D, Schliess F, Kircheis G. Pathogenesis of hepatic encephalopathy. J Gastroenterol Hepatol. 2002;17(Suppl. 3):S256–S259. doi: 10.1046/j.1440-1746.17.s3.10.x. [DOI] [PubMed] [Google Scholar]
- Haussinger D, vom Laubenberger JDS, Ernst T, Bayer S, Langer M, Gerok W, Hennig J. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology. 1994;107:1475–1480. doi: 10.1016/0016-5085(94)90552-5. [DOI] [PubMed] [Google Scholar]
- Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Haussinger D. Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J. 2002;16:231–233. doi: 10.1096/fj.01-0691fje. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol. 1997;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Kim HY, Sapp DW, Olsen RW, Tobin AJ. GABA alters GABAA receptor mRNAs and increases ligand binding. J Neurochem. 1993;61:2334–2337. doi: 10.1111/j.1471-4159.1993.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Seeburg PH, Luddens H. Modulation of GABAA receptor tert-[35S]butylbicyclophosphorothionate binding by antagonists: relationship to patterns of subunit expression. J Neurochem. 1996;66:2179–2187. doi: 10.1046/j.1471-4159.1996.66052179.x. [DOI] [PubMed] [Google Scholar]
- O'Byrne MB, Tipton KF. Taurine-induced attenuation of MPP+ neurotoxicity in vitro: a possible role for the GABAA subclass of GABA receptors. J Neurochem. 2000;74:2087–2093. doi: 10.1046/j.1471-4159.2000.0742087.x. [DOI] [PubMed] [Google Scholar]
- Otto F, Gortz P, Fleischer W, Siebler M. Cryopreserved rat cortical cells develop functional neuronal networks on microelectrode arrays. J Neurosci Methods. 2003;128:173–181. doi: 10.1016/s0165-0270(03)00186-9. [DOI] [PubMed] [Google Scholar]
- Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter MO, Ohannesian L, Larmet Y, Feltz P. Evidence that GABAA receptor subunit mRNA expression during development is regulated by GABAA receptor stimulation. J Neurochem. 1997;68:631–639. doi: 10.1046/j.1471-4159.1997.68020631.x. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Taurine and neural cell damage. Amino Acids. 2000;19:509–526. doi: 10.1007/s007260070003. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Andreeva N, Garret M, Scherer A, Haas HL. Pharmacological properties of GABAA receptors in rat hypothalamic neurons expressing the ɛ-subunit. J Neurosci. 2005;25:88–95. doi: 10.1523/JNEUROSCI.3209-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva OA, Chepkova AN, Doreulee N, Eriksson KS, Poelchen W, Monnighoff I, Heller-Stilb B, Warskulat U, Haussinger D, Haas HL. Taurine-induced long-lasting enhancement of synaptic transmission in mice: role of transporters. J Physiol. 2003;550:911–919. doi: 10.1113/jphysiol.2003.045864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva OA, Haas HL. Expression and function of glycine receptors in striatal cholinergic interneurons from rat and mouse. Neuroscience. 2001;104:1043–1055. doi: 10.1016/s0306-4522(01)00130-0. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Butz M, Kircheis G, Haussinger D, Schnitzler A. Pathological oscillatory coupling within the human motor system in different tremor syndromes as revealed by magnetoencephalography. Neurol Clin Neurophysiol. 2004;2004:26. [PubMed] [Google Scholar]
- Warskulat U, Flogel U, Jacoby C, Hartwig HG, Thewissen M, Merx MW, Molojavyi A, Heller-Stilb B, Schrader J, Haussinger D. Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J. 2004;18:577–579. doi: 10.1096/fj.03-0496fje. [DOI] [PubMed] [Google Scholar]
- Weissenborn K, Kolbe H. The basal ganglia and portal-systemic encephalopathy. Metab Brain Dis. 1998;13:261–272. doi: 10.1023/a:1020628607245. [DOI] [PubMed] [Google Scholar]
- Wong LS, Eshel G, Dreher J, Ong J, Jackson DM. Role of dopamine and GABA in the control of motor activity elicited from the rat nucleus accumbens. Pharmacol Biochem Behav. 1991;38:829–835. doi: 10.1016/0091-3057(91)90250-6. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol. 2003;89:2021–2034. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]