Abstract
There is now strong evidence that an endothelium-derived hyperpolarizing factor (EDHF), other than nitric oxide (NO) or prostaglandin (PG), exists for dilating arteries and arterioles. In vitro studies on isolated vessels pointed out a role for EDHF as a back-up mechanism when the NO pathway is impaired, but there was a lack of in vivo studies showing a functional role for EDHF. Ageing has pronounced effects on vascular function and particularly on endothelium-dependent relaxation, providing a novel situation in which to assess the contributions of EDHF. The purpose of the present study was thus to determine if, in vivo, there was a functional role for EDHF as a back-up mechanism in the cutaneous microcirculation in the ageing process. We investigated in vivo the contribution of each endothelial factor (NO, PG and EDHF) in the cutaneous vasodilatation induced by iontophoretic delivery of acetylcholine and local pressure application in young adult (6–7 months) and old (22–25 months) mice, using pharmacological inhibitors. The cutaneous vasodilator responses induced by acetylcholine and local pressure application were dependent upon NO and PG pathways in young adult mice, whereas they were EDHF-dependent in old mice. EDHF appears to serve as a back-up mechanism when ageing reaches pathological states in terms of the ability for NO and PG to relax cutaneous microvessels, allowing for persistent cutaneous vasodilatator responses in old mice. However, as a back-up mechanism, EDHF did not completely restore cutaneous vasodilatation, since endothelial responses were reduced in old mice compared to young adult mice.
Age-related vascular alterations that can explain the increase in cardiovascular risk with ageing are strongly correlated with endothelial dysfunction in humans (Algotsson et al. 1995; Rossi et al. 2002; Tao et al. 2004) and animals (Muller-Delp et al. 2002; Woodman et al. 2003). The mechanisms for the detrimental effects of age on endothelium-dependent vasodilatation are not totally clear, since the contribution of each endothelial factor in vasodilatation changes with age (Matz & Andriantsitohaina, 2003). Previous studies have reported lower levels of nitric oxide (NO) and prostaglandins (PG) in advanced age (Holowatz et al. 2005). Studies on isolated vessels pointed out that endothelium-derived hyperpolarizing factor (EDHF) may serve as a back-up mechanism when NO bioavailability is reduced (McCulloch et al. 1997; Nishikawa et al. 2000; Katusic, 2002). However a full understanding of the concept that EDHF may serve as an important compensatory mechanism when NO availability is reduced requires exploration at the level of the whole organism, since the role of EDHF in vascular physiology has been established mainly from studies of isolated vessels. The purpose of the present study was thus to determine the in vivo functional role of EDHF as a back-up mechanism in the cutaneous microcirculation in the ageing process.
In the present study, we studied in vivo endothelium-mediated vasodilatation induced by iontophoretic delivery of acetylcholine (ACh). Indeed ACh is the most commonly used pharmacological agent to interact with the endothelium and mediate its endothelium-dependent vasodilatation via a muscarinic receptor on the endothelial surface. This leads to a rise in intracellular calcium concentration and increases the synthesis and release of endothelial factors.
The neurovascular control of the cutaneous microcirculation also includes a vasodilator response to local pressure application (Fromy et al. 1998). This increase in cutaneous blood flow delays the occurrence of tissue ischaemia due to applied pressure, thus protecting the skin against pressure. The development of this pressure-induced vasodilatation (PIV) depends on the activation by pressure of sensory C fibres (Fromy et al. 1998, 2000), leading to the release of neurotransmitters that act at the endothelial level to stimulate the synthesis and release of endothelial factors (Fromy et al. 1998, 2000) inducing smooth muscle relaxation. Since neurovascular interaction is crucial for PIV, it represents a good end-point measurement to study the functional role of EDHF in a novel and integrative way in young adult and old mice. However, in addition to age-related changes in vessels, ageing markedly influences several morphological and functional features of the peripheral nervous system, increasing the susceptibility to peripheral neuropathy (Verdu et al. 2000; Melcangi et al. 2003; Di Iorio et al. 2006). For example, capsaicin-sensitive nerve function is impaired with advanced age in vivo in rat skin (Munce & Kenney, 2003). However, neuropathy in mice is less severe, even in long-lived mice species, than in old rats (Robertson et al. 1993). In the present study, we used mice without evidence of an alteration of small or large nerve fibre function, showing the absence of peripheral neuropathy, allowing focus on endothelial changes occurring with the ageing process.
Although the exact identity of EDHF remains elusive, candidates including potassium ions and epoxyeicosatrienoic acids have been proposed (Edwards et al. 1998; Fisslthaler et al. 1999). There is a general consensus that EDHF-mediated effects are resistant to the effects of NO synthase (NOS) or cyclooxygenase (COX) inhibitors, but are highly sensitive to the combination of the potassium channel inhibitors apamin and charybdotoxin (APA + ChTX) (Edwards & Weston, 2001; Busse et al. 2002; Campbell & Gauthier, 2002), although each toxin injected alone had little or no effect (Petersson et al. 1997; Fitzgerald et al. 2007). To determine the in vivo functional role of EDHF as a back-up mechanism in the cutaneous microcirculation in the ageing process, we investigated the contribution of endothelial factors (NO, PG and EDHF) in the cutaneous vasodilatation induced by iontophoretic delivery of ACh and local pressure application (PIV) in young adult and old mice, using pharmacological inhibitors.
Methods
Animal instrumentation
Experiments were performed in male C57BL/6 mice weighing 25–30 g provided by Janvier Laboratory (Le Genest-St-Isle, France). Procedures for the maintenance and use of the experimental mice were carried out in accordance with the principles of French legislation and the experiments were approved by the ethics committee for animal experimentation of the University of Angers, France. Animals were divided into two groups: young adult mice (6–7 months) and old mice (22–25 months). Before experimentation, animals were acclimatized for 1 week in a regulated environment with a constant temperature of 24°C.
Two days prior to the microvascular experiments, the hair was removed from the skin overlying the cranium (for PIV experiments) and the backs (for iontophoretic experiments) with a depilatory lotion to provide hairless areas for skin blood flow measurements, local pressure application and iontophoretic drug delivery. There was no evidence of harm or pain caused by the depilation. On the day of the microvascular experiment, animals were anaesthetized with thiopental (65 mg (kg body weight)−1, i.p.). The corneal reflex was tested before and after each experiment and found to be absent. The anaesthetized mice were placed in an incubator (MMS, Chelles, France) to maintain a stable skin temperature (35.0 ± 0.5°C), which was monitored with a skin thermocouple. Mice were placed in the prone position and the head was fixed on a frame followed by a 20 min resting period to allow for stabilization of blood pressure and skin temperature. The systolic arterial blood pressure (ABP) was measured using a non-invasive tail cuff system (RTBP 2000, Kent Scientific, Litchfield, CT, USA). At the end of each experiment, animals were killed by an overdose of thiopental.
For the nerve function assessment, conscious mice were used to perform the tail flick test. The same mice were anaesthetized (65 mg (kg body weight)−1, i.p.) to measure nerve conduction velocities and were then killed by an overdose of thiopental.
Assessment of endothelium-dependent and -independent responses
Skin blood flow was recorded using a laser Doppler multifibre probe (481-1, Perimed, Sweden) connected to a laser Doppler flowmeter (PF5000 Master, Perimed, Sweden). Transcutaneous iontophoresis was applied to a 1.2 cm2 area on the hairless back of the animals. Local iontophoretic drug delivery was chosen to assess the in vivo cutaneous microvascular function while avoiding any systemic effects. Endothelium-dependent responses were assessed using iontophoretic delivery of ACh (2%, Sigma, St Quentin Fallavier, France) with an anodal current application of 100 μA for 10 s, which induces a supra-maximal effect in mice (data not shown). Endothelium-independent responses were assessed using iontophoretic delivery of sodium nitroprusside (SNP) (2%, Nitriate, SERB, Paris, France) with a cathodal current application of 100 μA for 20 s, which induces a supra-maximal effect in mice. In addition, we ascertained the absence of non-specific anodal (100 μA, 10 s) and cathodal (100 μA, 20 s) current effects using deionized water as a vehicle, as previously reported in mice (Sigaudo-Roussel et al. 2004). The laser Doppler signal expressed in arbitrary units (a.u.) was digitized with a 200 Hz sampling frequency using a computerized acquisition system (Biopac, Santa Barbara, CA, USA). Data collection started with a 1 min control period prior to iontophoretic delivery and was continued for 30 min. Vasodilatation in response to ACh and SNP was reported as the maximal percentage increase in skin blood flow from baseline.
PIV assessment
To assess PIV, a weighbridge, adapted at one end to hold the laser Doppler probe (415, Perimed, Sweden), was carefully balanced with the probe placed on the middle of the hairless skull of the mouse. External pressure was increased progressively at 2.2 Pa s−1 (1 mmHg min−1) through the laser Doppler probe, as previously described (Sigaudo-Roussel et al. 2004), until a final pressure of 4 kPa was reached.
Data collection started with a 1 min control period prior to the onset of increasing pressure and was continued for 30 min. PIV was reported as the maximal percentage increase in skin blood flow from baseline in response to local pressure application. In the absence of an increase in skin blood flow in response to local pressure application, the percentage decrease in skin blood flow from baseline was calculated at the local applied pressure corresponding to the maximal amplitude of PIV in untreated mice and expressed as a negative percentage change.
Pharmacological treatments
We used pharmacological inhibitors of constitutive NOS, COX and potassium channels to determine the involvement of NO, PG and EDHF, respectively. Nω-Nitro-l-arginine (LNNA, 20 mg kg−1, i.p., Sigma, St Louis, MO, USA), a specific inhibitor of constitutive NOS, was injected 30 min prior to the iontophoretic delivery of ACh. Indomethacin (INDO, 5 mg kg−1, i.p., Sigma), a non-specific inhibitor of COX, was injected 30 min prior to the iontophoretic delivery of ACh. Dual inhibition using LNNA and INDO (LNNA + INDO) was assessed to evaluate the interaction between NOS and COX involved in the response to ACh, as well as in PIV.
The blockade of potassium channels by APA + ChTX has been well documented in isolated vessels and is recognized as an efficient blocker of responses to EDHF (Edwards et al. 1998; Fisslthaler et al. 1999; Busse et al. 2002). Therefore the combination of APA (0.5 mg kg−1) and ChTX (0.15 mg kg−1), calculated to achieve concentrations equivalent to those used for a period of 20 min in our previous study (Garry et al. 2003), was used to evaluate the involvement of EDHF in the response to ACh and in PIV. Since the blockade of EDHF by the combination of APA + ChTX was shown to be a localized effect (Parkington et al. 2002), the injection was performed very close to the stimulated site. Because a subcutaneous injection of saline prior to iontophoresis prevents the molecular diffusion by iontophoresis and, consequently, leads to the absence of vasodilatation in response to ACh (data not shown), APA + ChTX was injected into the proximal part of the tail vein 20 min prior to ACh delivery to allow proper ACh diffusion by iontophoresis. For PIV assessment, APA + ChTX was injected into the skin overlying the skull 30 min prior to local pressure application. A subcutaneous injection of saline prior to local pressure application did not modify the PIV response (data not shown).
Assessment of nerve function
We measured tail flick latencies and sciatic nerve conduction velocities in untreated young adult (n = 10) and old (n = 10) mice to verify that the mice used in the study were without significant functional alteration of small or large nerve fibres. For the tail flick test, conscious mice were gently maintained in a restrainer, and a light was focused onto the dorsal surface of the tail. Tail flick latency up to a cut-off time of 10 s was measured. Four trials were completed with a minimum of 5 min intervals between trials to prevent sensitization and the average of these four trials was calculated for each animal.
Following the tail flick test, four measurements of nerve conduction velocity were performed in anaesthetized young adult and old mice as previously carried out (Sigaudo-Roussel et al. 2004). The average of the four measurements was calculated. In brief, after general anaesthesia (65 mg kg−1, i.p.), motor nerve conduction velocity was assessed by stimulating at the exposed sciatic notch and knee while recording the M-wave (compound muscle action potential) from the tibial-innervated dorsal interossei foot muscles. Sensory nerve conduction velocity was measured between groin and calf. Nerve temperature was monitored by thermocouple probe and was maintained at 37°C with a radiant heat.
Protocol 1: assessment of ACh-induced vasodilatation in young adult and old mice
The cutaneous microvascular responses to the iontophoretic delivery of ACh were measured in untreated young adult (n = 9) and old (n = 9) mice, in young adult (n = 9) and old (n = 9) mice treated with LNNA, in young adult (n = 9) and old (n = 9) mice treated with INDO, in young adult (n = 5) and old (n = 9) mice treated with LNNA + INDO, and in young adult (n = 9) and old (n = 5) mice treated with APA + ChTX.
Protocol 2: assessment of SNP-induced vasodilatation in young adult and old mice
The cutaneous microvascular responses to the iontophoretic delivery of SNP were measured in untreated young adult (n = 9) and old (n = 9) mice.
Protocol 3: assessment of PIV in young adult and old mice
The cutaneous microvascular responses to local pressure application were measured in untreated young adult (n = 13) and old (n = 13) mice, in young adult (n = 5) and old (n = 9) mice treated with LNNA + INDO, and in young adult (n = 6) and old (n = 5) mice treated with APA + ChTX.
Data analysis
The laser Doppler signals were averaged every 10 s to reduce the variability due to instantaneous vasomotion. Results in the text and the figures are presented as mean ± s.e.m. Student's unpaired t test was used to test for significant differences between two groups. Student's paired t test was used to evaluate the significance of changes within a group. To test for significant differences among groups with different inhibitors, we performed one-way ANOVA with Dunnett's multiple-comparison test (untreated mice as controls). Differences were considered significant when P < 0.05.
Results
Effects of the treatments on ABP and skin blood flow
Basal ABP was not different between untreated young adult (82 ± 2 mmHg, n = 31) and old (80 ± 2 mmHg, n = 31) mice. In comparison to untreated mice, the increase in ABP due to NOS inhibition was not different between young adult and old mice following LNNA (121 ± 7 mmHg, n = 9 versus 119 ± 10 mmHg, n = 9) and LNNA + INDO (126 ± 9 mmHg, n = 10 versus 124 ± 6 mmHg, n = 18) treatments. No effect in ABP compared to untreated mice was seen in either young adult or old mice following INDO (86 ± 5 mmHg, n = 9 versus 81 ± 4 mmHg, n = 9) or following APA + ChTX (84 ± 3 mmHg, n = 15 versus 80 ± 2 mmHg, n = 10) treatment. Basal skin blood flow was not different between untreated young adult and old mice prior to iontophoretic drug delivery (33 ± 3 a.u., n = 18 versus 34 ± 6 a.u., n = 18) or local pressure application (75 ± 9 a.u., n = 13 versus 76 ± 5 a.u., n = 13). The difference in the index of skin blood flow between the two vascular beds was due to the use of two different kinds of LDF probes (481-1 and 415). Compared to untreated mice, there was a large reduction in basal skin blood flow following LNNA in young adult mice (−50 ± 5%, n = 9) versus old mice (−23 ± 9, n = 9, P < 0.05 between groups). Similarly, the difference in skin blood flow between INDO-treated and untreated mice was greater for young adult mice (−35 ± 7%, n = 9) than for old mice (−13 ± 7%, n = 9, P < 0.05 between groups). For the combined LNNA + INDO treatment, again the treated young adult mice showed a greater difference from their untreated counterparts (−55 ± 6%, n = 10) than did the old mice (−26 ± 7%, n = 18, P < 0.05 between groups). In contrast, APA + ChTX treatment induced a large decrease in basal skin blood flow in old mice (−46 ± 6%, n = 10), which was not observed in young adult mice (0 ± 3%, n = 15, P < 0.001 between groups).
Assessment of nerve function in young adult and old mice
The tail flick latency was not significantly different between young adult (6.8 ± 0.3 s, n = 10) and old mice (7.8 ± 0.5 s, n = 10). Motor nerve conduction velocity was also not significantly different between young adult (41.2 ± 0.9 m s−1, n = 10) and old mice (40.2 ± 1.2 m s−1, n = 10). Sensory nerve conduction velocity was also not significantly different between young adult (47.3 ± 1.2 m s−1, n = 10) and old mice (45.9 ± 0.8 m s−1, n = 10).
Protocol 1: assessment of ACh-induced vasodilatation in young adult and old mice
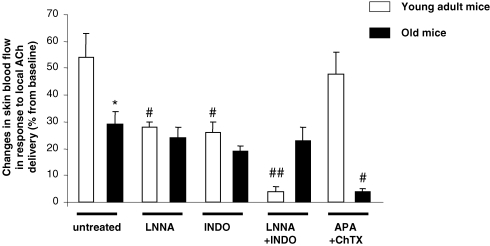
Without pharmacological treatment, the endothelium-dependent vasodilatation in response to iontophoretic delivery of ACh was reduced in old mice (29 ± 5%, n = 9) compared to that in young adult mice (54 ± 9%, n = 9, P < 0.05, Fig. 1).
Figure 1.
Percentage changes in skin blood flow in response to local iontophoretic delivery of acetylcholine (ACh) in young adult and old mice without inhibition (untreated), following the inhibition of NOS using Nω-nitro-l-arginine (LNNA), following the inhibition of COX using indomethacin (INDO), following the combined inhibition of NOS and COX (LNNA + INDO) and following the blockade of endothelium-derived hyperpolarizing factor (EDHF) using apamin and charybdotoxin (APA + ChTX). Error bars represent s.e.m. n = 9 in each group, except for young adult mice treated with LNNA + INDO (n = 5) and old mice treated with APA + ChTX (n = 5). * P < 0.05 untreated old mice compared to untreated young adult mice. # P < 0.05, ## P < 0.01 treated compared to age-matched untreated mice.
In young adult mice, the endothelium-dependent vasodilatation in response to ACh was reduced following NOS inhibition with LNNA (28 ± 2%, n = 9, P < 0.05 representing an inhibition of 48%) and COX inhibition with INDO (26 ± 4%, n = 9, P < 0.05 representing an inhibition of 52%) compared to untreated young adult mice (54 ± 9%, n = 9) and almost abolished following the combined inhibition with LNNA + INDO (4 ± 2%, n = 5, P < 0.01 representing an inhibition of 93%). In contrast, the response to ACh was unchanged by the blockade of EDHF from the combined inhibition by APA + ChTX (48 ± 8%, n = 9) compared to untreated young adult mice (54 ± 9%, n = 9) (Fig. 1).
In old mice, ACh-induced vasodilatation was not significantly affected by the administration of LNNA (24 ± 4%, n = 9), INDO (19 ± 2%, n = 9) or LNNA + INDO (23 ± 5%, n = 9) compared to untreated old mice (29 ± 5%), whereas it was almost abolished (4 ± 1%, n = 5, P < 0.01 representing an inhibition of 86%) following the blockade of EDHF (Fig. 1).
Protocol 2: assessment of SNP-induced vasodilatation in young adult and old mice
Iontophoretic delivery of SNP, an exogenous NO donor, increased skin blood flow in young adult and old mice, showing no difference in endothelium-independent vasodilatation between groups (49 ± 7%, n = 9 in young adult mice versus 44 ± 5%, n = 9 in old mice; P > 0.05 between groups).
Protocol 3: assessment of PIV in young adult and old mice
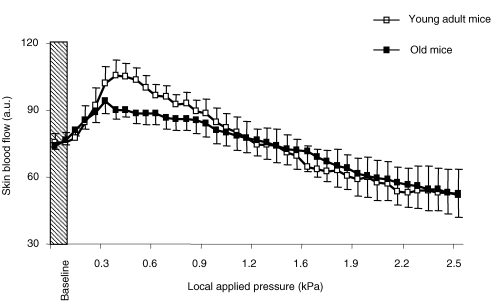
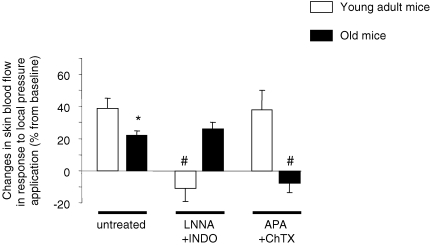
In young adult mice (n = 13), skin blood flow increased progressively in response to a local applied pressure and reached a maximal increase from baseline (PIV of 39 ± 6%) at 0.39 ± 0.05 kPa (Fig. 2). In contrast, skin blood flow decreased in response to local pressure application in young adult mice treated with LNNA + INDO (n = 5). At 0.39 kPa, the decrease in skin blood flow from baseline was −11 ± 8% (Fig. 3). In young adult mice treated with APA + ChTX (n = 6), skin blood flow increased in response to local pressure application and reached its maximal value at 0.35 ± 0.04 kPa, representing a PIV of 38 ± 12% that was not different from PIV in untreated young adult mice (39 ± 6%, Fig. 3).
Figure 2.
Changes in skin blood flow, expressed in arbitrary units (a.u.), in response to local pressure application in untreated young adult and old mice (n = 10 in each group). Error bars represent s.e.m.
Figure 3.
Percentage changes in skin blood flow in response to local pressure application in young adult and old mice without inhibition (untreated, n = 10 in each group), following the inhibition of NOS and COX using Nω-nitro-l-arginine and indomethacin (LNNA + INDO, n = 5 in young adult mice and n = 8 in old mice) and following the blockade of endothelium-derived hyperpolarizing factor (EDHF) using apamin and charybdotoxin (APA + ChTX, n = 6 in young adult mice and n = 5 in old mice). Error bars represent s.e.m. * P < 0.05 old mice compared to young adult mice. # P < 0.05 treated compared to age-matched untreated mice.
In old mice (n = 13), skin blood flow increased in response to local pressure application, reaching its maximal value at 0.27 ± 0.05 kPa, corresponding to a PIV of 22 ± 3%, which was reduced compared to that in untreated young adult mice (P < 0.05) (Figs 2 and 3). In old mice treated with LNNA + INDO (n = 9), skin blood flow increased in response to local pressure application and reached its maximal value at 0.22 ± 0.02 kPa, corresponding to a PIV of 26 ± 4%, which was not different from PIV in untreated old mice (Fig. 3). In contrast, skin blood flow decreased in response to local pressure application in old mice treated with APA + ChTX (n = 5). At 0.27 kPa, the decrease in skin blood flow from baseline was −8 ± 6% (Fig. 3).
Discussion
Our results demonstrated that, in the cutaneous microcirculation, EDHF partially counteracted the reduction in NO and PG function seen in old mice. The enhanced EDHF activity due to ageing may thereby preserve to some extent ACh-induced vasodilatation and PIV in old mice.
The present results showed that vasodilatation resulting from iontophoretic delivery of ACh was reduced in old mice, while the vasodilator response to SNP remained unchanged. This demonstrates that the reduction of the response to ACh in old mice was not due to vascular smooth muscle impairment but rather to an endothelial dysfunction, as already reported in humans (Algotsson et al. 1995; Rossi et al. 2002; Tao et al. 2004) and animals (Muller-Delp et al. 2002; Woodman et al. 2003). This endothelial dysfunction could explain the reduction of PIV observed in old mice, since an intact endothelial function is crucial for maximal PIV development (Fromy et al. 2000; Sigaudo-Roussel et al. 2004; Demiot et al. 2006a).
The age-related endothelial dysfunction assessed in vivo is in accordance with previous in vitro studies. Different hypotheses have been raised to explain the underlying cellular and molecular mechanisms associated with age-related endothelial dysfunction, such as reduced expression and/or activity of endothelial nitric oxide synthase (eNOS; Cernadas et al. 1998), decreased NO access to smooth muscle cells because of thickening of endothelial and smooth muscle layers with age (Marin, 1995), or reduced prostanoid-dependent vasodilatation with healthy ageing due to an increase in thromboxane vasoconstrictor activity and/or decreased prostacyclin-mediated vasodilator activity (Taddei et al. 1997; Buus et al. 2000; Heymes et al. 2000). However, the mechanisms for the detrimental effects of ageing on endothelium-dependent vasodilatation are not totally clear, but the changes in the contribution of each endothelial factor in vasodilatation with ageing (Matz & Andriantsitohaina, 2003) might serve to explain this age-related endothelial dysfunction.
In contrast to young adult mice, ACh-mediated vasodilatation and PIV in old mice were not modified by the dual inhibition of NOS and COX. This suggests that there were low tonic levels of NO and PG, and that the process of ageing on endothelial factor levels in the cutaneous microcirculation had already started. Indeed it was found that NO did not directly contribute to ACh-mediated vasodilatation in aged human skin and that older subjects had a diminished PG contribution to ACh-mediated vasodilatation (Holowatz et al. 2005). A generalized abnormality of basal endothelial function in older subjects with similar impairment of NO and PG dilator pathways was shown from measurements of forearm blood flow during intra-arterial infusion of NOS and COX inhibitors (Singh et al. 2002). The age-related decrement in endothelium-dependent dilatation due to impairment of NO and PG release by endothelium was also reported for the skeletal muscle vasculature (Muller-Delp et al. 2002; Woodman et al. 2003).
Although resistant to acute inhibition of NOS and COX, the responses to ACh and PIV were abolished by the combination of APA + ChTX in old mice, which is a characteristic of EDHF-mediated responses (Waldron & Cole, 1999). This finding indicates a significant contribution made by EDHF to ACh-mediated relaxation and PIV in old mice, one which prevents total abolition of these endothelium-dependent relaxations when ageing impairs NO and PG pathways in the cutaneous microcirculation. These results are in accordance with those reported in in vitro studies, in which EDHF may serve as a back-up vasodilator in situations associated with an altered bioavailability of NO (McCulloch et al. 1997; Nishikawa et al. 2000), as well as in renal arteries from aged WKY rats (Bussemaker et al. 2003). In pathophysiological states, this back-up mechanism operates to preserve endothelial-dependent vasodilatation, as when the NO pathway is impaired due to hypertension (Kemp et al. 1995; Katusic, 2002; Sofola et al. 2002), hypercholesterolaemia (Kagota et al. 1999) or diabetes (De Vriese et al. 2000). It was also reported that EDHF might compensate for the lack of NO and preserve endothelium-dependent relaxation in eNOS knockout mice (Waldron et al. 1999; Brandes et al. 2000; Ding et al. 2000). EDHF appears to be at least as important as endothelium-derived NO in mediating agonist-induced vasodilatation in the mouse in vivo or in vitro, since EDHF and endothelium-derived NO can each completely compensate for the lack of the other (Brandes et al. 2000; Fitzgerald et al. 2007). In these situations, NO and EDHF appear to operate simultaneously in a non-additive fashion as parallel pathways in ACh-induced vasodilatation in the mouse hindlimb (Fitzgerald et al. 2007), challenging the view that EDHF is merely a back-up system that comes into play under conditions of reduced NO bioavailability. In the present in vivo study, we demonstrated that EDHF did not totally compensate for the loss of NO and PG function in the cutaneous microcirculation in old mice, since PIV and the responses to ACh were reduced compared to NO- and PG-dependent relaxations in young adult mice. Further studies will be needed to clarify the mechanisms underlying EDHF-mediated responses that operate in the ageing process.
The contribution of EDHF-mediated responses to endothelium-dependent relaxation increases as the vessel size decreases (Garland et al. 1995), as reported in the mesenteric bed (Hwa et al. 1994; Shimokawa et al. 1996). It has been reported that the combination of APA + ChTX not only inhibits EDHF-mediated responses but also prevents the release of NO (Stankevicius et al. 2006). However, the response to ACh and PIV were both unchanged by APA + ChTX in young adult mice, showing that EDHF was not involved in these responses in the young adult cutaneous microcirculation. Therefore it seems that in our in vivo experimental conditions, APA + ChTX did not block NO-mediated vasodilatation in young adult mice. In a previous study, we demonstrated that NO-mediated PIV was unchanged by APA + ChTX in young adult Wistar rats (Garry et al. 2003), strengthening the conclusion that EDHF is not involved in PIV in physiological conditions. This is also in accordance with the idea that NO inhibits EDHF-mediated relaxation (Olmos et al. 1995; Bauersachs et al. 1996; Nishikawa et al. 2000). The major drawback of studies aiming to determine the relevance of EDHF in physiological states is that the contribution of this factor to endothelium-dependent vasodilatation can be estimated only as the non-NO and non-PG portion of relaxation, i.e. only in the presence of blockade of those other systems. Although whether the contribution of EDHF in endothelial responses is physiological or pathophysiological remains controversial (Triggle et al. 2003), based on our results we believe that EDHF does not contribute to vasodilatation in vivo in the cutaneous microcirculation of young adult mice.
Basal skin blood flow was sensitive to the APA + ChTX treatment only in old mice, indicating a large contribution of EDHF in the cutaneous microcirculation in old mice in contrast to young adult mice. It is important to note that this is the first observation of a putative role for EDHF-mediated responses in regulating basal vascular conductance in the cutaneous microcirculation in old mice. In 12-week-old male C57BL/6 mice, Fitzgerald et al. (2007) showed that control of basal vasodilator tone in the hindlimb is dominated by NO. Accordingly, the decrease in basal skin blood flow induced by LNNA, INDO and LNNA + INDO treatments was more pronounced in young adult mice compared to old mice, illustrating the important contributions of NO and PG in the cutaneous microcirculation in young adult mice, but a reduction in old mice. An acute decrease in basal skin blood flow induced by a subcutaneous injection of angiotensin II did not reduce PIV in mice (authors' unpublished observations). Therefore the reduced basal skin blood flow per se induced by different treatments is unlikely to explain the loss of PIV in the present study, strengthening our conclusions regarding the changes in the contribution of each endothelial factor during the ageing process. These pharmacological effects were only observed on basal skin blood flow, suggesting that these changes were limited to the microcirculation. Indeed the different treatments had similar effects on changes in ABP in young adult and old mice, reflecting an absence of NO and PG functional loss in the general systemic circulation with ageing. We reported previously that an acute increase in ABP resulting from noradrenaline infusion did not abolish PIV in rats (Fromy et al. 2007), suggesting that systemic cardiovascular effects of different treatments are unlikely to explain the loss of PIV in the present study.
Regarding nervous function, our results show that ageing did not induce changes in motor or sensory nerve conduction velocities or in tail flick latency, although peripheral neuropathy could become more frequent with ageing (Verdu et al. 2000; Melcangi et al. 2003). However, it was reported that, even in long-lived mice species, neuropathy is less severe than in old rats (Robertson et al. 1993). Moreover there are strain-specific differences contributing to spontaneous age-related peripheral nerve changes. Indeed it was reported that the incidence and severity of nerve lesions in B6C3F1 and C3H mice were significantly greater than in C57BL/6 mice (Tabata et al. 2000). Accordingly, the old C57BL/6 mice used in the present study had little or no nervous system dysfunction. Therefore the reduction of PIV, which relies on intact integrity of both vascular and nervous systems (Demiot et al. 2006b) was highly correlated to reduced ACh-mediated vasodilatation instead of to an alteration of nervous system in old mice.
To our knowledge, this is the first integrative study performed in vivo showing a functional role of EDHF to compensate for vascular complications induced by the endothelial dysfunction associated with ageing. Indeed we demonstrated in vivo that ACh-mediated vasodilatation and PIV are dependent on NO and PG pathways in young adult mice, whereas they are NO- and PG-independent and sensitive to EDHF blockade in old mice. This collection of observations demonstrates that the balance between the different endothelial responses changes in the cutaneous microcirculation with ageing and could account for a limited loss of endothelial responses in old mice. Therefore EDHF appears to serve as a back-up mechanism when ageing causes pathological changes in terms of the ability of NO and PG to relax cutaneous microvessels. The precise mechanism responsible for the compensatory effect of EDHF is unclear and obviously many questions remain to be answered by future studies. A better understanding of EDHF and its partial ability to act as a compensatory mechanism may provide the basis for prevention of vascular complications in elderly people.
References
- Algotsson A, Nordberg A, Winblad B. Influence of age and gender on skin vessel reactivity to endothelium-dependent and endothelium-independent vasodilators tested with iontophoresis and a laser Doppler perfusion imager. J Gerontol A Biol Sci Med Sci. 1995;50:M121–M127. doi: 10.1093/gerona/50a.2.m121. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci U S A. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Bussemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, Brandes RP. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000;129:184–192. doi: 10.1038/sj.bjp.0703041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Gauthier KM. What is new in endothelium-derived hyperpolarizing factors? Curr Opin Nephrol Hypertens. 2002;11:177–183. doi: 10.1097/00041552-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- Demiot C, Fromy B, Saumet JL, Sigaudo-Roussel D. Preservation of pressure-induced cutaneous vasodilation by limiting oxidative stress in short-term diabetic mice. Cardiovasc Res. 2006a;69:245–252. doi: 10.1016/j.cardiores.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Demiot C, Tartas M, Fromy B, Abraham P, Saumet JL, Sigaudo-Roussel D. Aldose reductase pathway inhibition improved vascular and C-fiber functions, allowing for pressure-induced vasodilation restoration during severe diabetic neuropathy. Diabetes. 2006b;55:1478–1483. doi: 10.2337/db05-1433. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio A, Cherubini A, Volpato S, Sparvieri E, Lauretani F, Franceschi C, Senin U, Abate G, Paganelli R, Martin A, Andres-Lacueva C, Ferrucci L. Markers of inflammation, vitamin E and peripheral nervous system function: the InCHIANTI study. Neurobiol Aging. 2006;27:1280–1288. doi: 10.1016/j.neurobiolaging.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kubes P, Triggle C. Potassium- and acetylcholine-induced vasorelaxation in mice lacking endothelial nitric oxide synthase. Br J Pharmacol. 2000;129:1194–1200. doi: 10.1038/sj.bjp.0703144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Edwards G, Weston AH. EDHF – are there gaps in the pathway? J Physiol. 2001;531:299. doi: 10.1111/j.1469-7793.2001.0299i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- Fitzgerald SM, Bashari H, Cox JA, Parkington HC, Evans RG. Contributions of endothelium-derived relaxing factors to control of hindlimb blood flow in the mouse in vivo. Am J Physiol Heart Circ Physiol. 2007;293:H1072–H1082. doi: 10.1152/ajpheart.00072.2007. [DOI] [PubMed] [Google Scholar]
- Fromy B, Abraham P, Saumet JL. Non-nociceptive capsaicin-sensitive nerve terminal stimulation allows for an original vasodilatory reflex in the human skin. Brain Res. 1998;811:166–168. doi: 10.1016/s0006-8993(98)00973-1. [DOI] [PubMed] [Google Scholar]
- Fromy B, Merzeau S, Abraham P, Saumet JL. Mechanisms of the cutaneous vasodilator response to local external pressure application in rats: involvement of CGRP, neurokinins, prostaglandins and NO. Br J Pharmacol. 2000;131:1161–1171. doi: 10.1038/sj.bjp.0703685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromy B, Sigaudo-Roussel D, Baron C, Roquelaure Y, Leftheriotis G, Saumet JL. Neuroendocrine pathway involvement in the loss of the cutaneous pressure-induced vasodilatation during acute pain in rats. J Physiol. 2007;579:247–254. doi: 10.1113/jphysiol.2006.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Garry A, Merzeau S, Fromy B, Saumet J. EDHF involvement in skin pressure-induced vasodilatation. In: Vanhoutte PM, editor. EDHF 2002. London: Taylor & Francis; 2003. pp. 151–155. chap. 19. [Google Scholar]
- Heymes C, Habib A, Yang D, Mathieu E, Marotte F, Samuel J, Boulanger CM. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br J Pharmacol. 2000;131:804–810. doi: 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Williams P, Chatterjee M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol Heart Circ Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- Kagota S, Tamashiro A, Yamaguchi Y, Nakamura K, Kunitomo M. Excessive salt or cholesterol intake alters the balance among endothelium-derived factors released from renal arteries in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1999;34:533–539. doi: 10.1097/00005344-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Katusic ZS. Back to the salt mines – endothelial dysfunction in hypertension and compensatory role of endothelium-derived hyperpolarizing factor (EDHF) J Physiol. 2002;543:1. doi: 10.1113/jphysiol.2002.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BK, Smolich JJ, Ritchie BC, Cocks TM. Endothelium-dependent relaxations in sheep pulmonary arteries and veins: resistance to block by NG-nitro-l-arginine in pulmonary hypertension. Br J Pharmacol. 1995;116:2457–2467. doi: 10.1111/j.1476-5381.1995.tb15096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch AI, Bottrill FE, Randall MD, Hiley CR. Characterization and modulation of EDHF-mediated relaxations in the rat isolated superior mesenteric arterial bed. Br J Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin J. Age-related changes in vascular responses: a review. Mech Ageing Dev. 1995;79:71–114. doi: 10.1016/0047-6374(94)01551-v. [DOI] [PubMed] [Google Scholar]
- Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging. 2003;20:527–550. doi: 10.2165/00002512-200320070-00005. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Azcoitia I, Ballabio M, Cavarretta I, Gonzalez LC, Leonelli E, Magnaghi V, Veiga S, Garcia-Segura LM. Neuroactive steroids influence peripheral myelination: a promising opportunity for preventing or treating age-dependent dysfunctions of peripheral nerves. Prog Neurobiol. 2003;71:57–66. doi: 10.1016/j.pneurobio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Munce TA, Kenney WL. Age-specific modification of local cutaneous vasodilation by capsaicin-sensitive primary afferents. J Appl Physiol. 2003;95:1016–1024. doi: 10.1152/japplphysiol.00934.2002. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol. 2000;279:H459–H465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- Olmos L, Mombouli JV, Illiano S, Vanhoutte PM. cGMP mediates the desensitization to bradykinin in isolated canine coronary arteries. Am J Physiol Heart Circ Physiol. 1995;268:H865–H870. doi: 10.1152/ajpheart.1995.268.2.H865. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Chow JA, Evans RG, Coleman HA, Tare M. Role for endothelium-derived hyperpolarizing factor in vascular tone in rat mesenteric and hindlimb circulations in vivo. J Physiol. 2002;542:929–937. doi: 10.1113/jphysiol.2002.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson J, Zygmunt PM, Hogestatt ED. Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. Br J Pharmacol. 1997;120:1344–1350. doi: 10.1038/sj.bjp.0701032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A, Day B, Pollock M, Collier P. The neuropathy of elderly mice. Acta Neuropathol (Berl) 1993;86:163–171. doi: 10.1007/BF00334883. [DOI] [PubMed] [Google Scholar]
- Rossi M, Cupisti A, Mariani S, Santoro G, Pentimone F. Endothelium-dependent and endothelium-independent skin vasoreactivity in the elderly. Aging Clin Exp Res. 2002;14:343–346. doi: 10.1007/BF03324460. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Sigaudo-Roussel D, Demiot C, Fromy B, Koitka A, Leftheriotis G, Abraham P, Saumet JL. Early endothelial dysfunction severely impairs skin blood flow response to local pressure application in streptozotocin-induced diabetic mice. Diabetes. 2004;53:1564–1569. doi: 10.2337/diabetes.53.6.1564. [DOI] [PubMed] [Google Scholar]
- Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci (Lond) 2002;102:595–600. [PubMed] [Google Scholar]
- Sofola OA, Knill A, Hainsworth R, Drinkhill M. Change in endothelial function in mesenteric arteries of Sprague-Dawley rats fed a high salt diet. J Physiol. 2002;543:255–260. doi: 10.1113/jphysiol.2002.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankevicius E, Lopez-Valverde V, Rivera L, Hughes AD, Mulvany MJ, Simonsen U. Combination of Ca2+-activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery. Br J Pharmacol. 2006;149:560–572. doi: 10.1038/sj.bjp.0706886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Ikegami H, Kariya K. A parallel comparison of age-related peripheral nerve changes in three different strains of mice. Exp Anim. 2000;49:295–299. doi: 10.1538/expanim.49.295. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Cyclooxygenase inhibition restores nitric oxide activity in essential hypertension. Hypertension. 1997;29:274–279. doi: 10.1161/01.hyp.29.1.274. [DOI] [PubMed] [Google Scholar]
- Tao J, Jin YF, Yang Z, Wang LC, Gao XR, Lui L, Ma H. Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age: comparative study of noninvasive pulse wave analysis and laser Doppler blood flow measurement. Am J Hypertens. 2004;17:654–659. doi: 10.1016/j.amjhyper.2004.03.678. [DOI] [PubMed] [Google Scholar]
- Triggle CR, Hollenberg M, Anderson TJ, Ding H, Jiang Y, Ceroni L, Wiehler WB, Ng ES, Ellis A, Andrews K, McGuire JJ, Pannirselvam M. The endothelium in health and disease – a target for therapeutic intervention. J Smooth Muscle Res. 2003;39:249–267. doi: 10.1540/jsmr.39.249. [DOI] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Waldron GJ, Cole WC. Activation of vascular smooth muscle K+ channels by endothelium-derived relaxing factors. Clin Exp Pharmacol Physiol. 1999;26:180–184. doi: 10.1046/j.1440-1681.1999.03006.x. [DOI] [PubMed] [Google Scholar]
- Waldron GJ, Ding H, Lovren F, Kubes P, Triggle CR. Acetylcholine-induced relaxation of peripheral arteries isolated from mice lacking endothelial nitric oxide synthase. Br J Pharmacol. 1999;128:653–658. doi: 10.1038/sj.bjp.0702858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Selected contribution: Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol. 2003;95:2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]