Abstract
Cutaneous vasoconstrictor responsiveness may be impaired by substance(s) directly or indirectly responsible for cutaneous active vasodilatation. In this study, we tested the hypothesis that endogenous nitric oxide (NO) attenuates the reduction in cutaneous vascular conductance (CVC) during an orthostatic challenge combined with whole-body heating, as well as during whole-body cooling. In protocol 1, healthy subjects were pretreated with an intradermal injection of botulinum toxin A (BTX) to block the release of neurotransmitters from nerves responsible for cutaneous active vasodilatation. On the experimental day, a microdialysis probe was placed at the BTX-treated site as well as at two adjacent untreated sites. NG-nitro-l-arginine methyl ester (l-NAME, 10 mm) was perfused through the probe placed at the BTX-treated site and at one untreated site. After confirmation of the absence of cutaneous vasodilatation at the BTX site during whole-body heating, adenosine was infused through the microdialysis probe at this site to increase skin blood flow to a level similar to that at the untreated site. Subsequently, 30 and 40 mmHg lower-body negative pressures (LBNPs) were applied. The reduction in CVC to LBNP was greatest at the BTX-treated site (15.0 ± 2.4% of the maximum level (% max)), followed by the l-NAME-treated site (11.3 ± 2.6% max), and then the untreated site (3.8 ± 3.0% max; P < 0.05 for all comparisons). In protocol 2, two microdialysis membranes were inserted in the dermal space of one forearm. Adenosine alone was infused at one site while the other site received adenosine and l-NAME. The reduction in CVC in response to whole-body cooling was significantly greater at the l-NAME-treated site than at the adjacent adenosine alone site. These results suggest that endogenous NO is capable of attenuating cutaneous vasoconstrictor responsiveness.
Elevations in internal temperature during heat stress increase skin blood flow through a combination of withdrawal of adrenergic cutaneous vasoconstrictor activity and the engagement of a separate non-adrenergic cutaneous active vasodilator activity (Johnson & Proppe, 1996). Pronounced heat stress can increase cardiac output to up to 13 l min−1 with 50% or more of that value being distributed to skin (Rowell et al. 1969; Rowell, 1986). Under these conditions, the control of cutaneous vascular conductance (CVC) becomes an important factor in the maintenance of blood pressure. Consistent with this idea, CVC typically decreases during head-up tilt or lower-body negative pressure (LBNP) while subjects are heat stressed (Lind et al. 1968; Johnson et al. 1973; Kellogg et al. 1990; Crandall et al. 1996; Shibasaki et al. 2006).
The reduction in CVC in response to an orthostatic challenge can occur through the engagement of vasoconstrictor activity and/or by withdrawal of the active vasodilator system. Kellogg et al. (1990) proposed that withdrawal of the cutaneous active vasodilator activity was the primary mechanism for reductions in CVC during LBNP of heat-stressed subjects; whereas we (Shibasaki et al. 2006) recently found that the cutaneous vasoconstrictor activity could be engaged during an orthostatic challenge in this thermal condition if the active vasodilator system was blocked. A possible explanation for these apparently contradictory findings (Kellogg et al. 1990; Shibasaki et al. 2006) may be that substances released from the cutaneous active vasodilator system reduce the effectiveness of the vasoconstrictor system by impairing neurotransmitter release and/or effectiveness of the vasoconstricting neurotransmitters. Thus, if neurotransmitters from the active vasodilator system were prevented from being released, reductions in CVC during the orthostatic challenge due to increased cutaneous vasoconstrictor activity may become unmasked.
Although the neurotransmitter(s) responsible for cutaneous active vasodilatation remains unclear, nitric oxide (NO) has been shown to contribute to the active vasodilator response during whole-body heating (Kellogg et al. 1998; Shastry et al. 1998; Shibasaki et al. 2002). It is interesting that NO has been reported to attenuate sympathetically mediated vasoconstriction (Greenberg et al. 1990; Zanzinger et al. 1994; Habler et al. 1997; Costa et al. 2001; Chavoshan et al. 2002; Kolo et al. 2004). Consistent with those observations, we found that exogenous NO, via local administration of the NO donor sodium nitroprusside (SNP), attenuated cutaneous vasoconstrictor responses during whole-body cold stress (Durand et al. 2005). Despite these observations, it remains unknown whether endogenous NO in the skin is capable of attenuating cutaneous vasoconstrictor responsiveness. Thus the purpose of this study was to test the hypothesis that endogenous NO attenuates cutaneous vasoconstrictor responsiveness.
Methods
Eight healthy normotensive subjects participated in the first protocol (protocol 1A) with an average age, height and weight of 32 ± 5 year, 174.6 ± 7.3 cm and 77.7 ± 9.6 kg, respectively. A follow-up study (protocol 1B) was performed on six healthy normotensive subjects with an average age, height and weight of 31 ± 8 year, 175.3 ± 11.1 cm and 71.1 ± 15.4 kg, respectively. A separate group of six healthy normotensive subjects with an average age, height and weight of 34 ± 6 year, 176.1 ± 3.8 cm and 76.4 ± 10.0 kg, respectively, participated in protocol 2. Each subject was informed of the purpose and risks of these studies before providing their written consent. The consent form was approved by the University of Texas South-western Medical Center at Dallas and Presbyterian Hospital of Dallas. All subjects refrained from caffeine, alcohol and exercise 24 h before the study. At least 3 days prior to experimentation, subjects participating in protocol 1 received an intradermal injection of botulinum toxin type A (BTX, 10 units in 0.15 ml normal saline) to locally abolish cutaneous active vasodilatation at that site (Kellogg et al. 1995; Shibasaki et al. 2006).
Measurements
Upon entering the laboratory (ambient temperature, 24–25°C), each subject was instrumented for the measurement of mean skin temperature from the weighted average of six thermocouples placed on the skin (Taylor et al. 1989). The subject was then dressed in a two piece tube-lined water perfusion suit that permitted the control of skin and internal temperatures. The suit covered the entire body surface except for the head, feet and a forearm where skin blood flow probes were placed. A thermistor was placed in the sublingual sulcus to provide an index of internal temperature. Heart rate (HR) was continuously obtained from electrocardiogram (SpaceLabs) with the signal interfaced with a cardiotachometer (CWE). Arterial blood pressure was measured via auscultation of the brachial artery (Suntech). During LBNP, blood pressure was obtained at 1–2 min intervals, while it was continuously recorded if the subject became hypotensive. Mean arterial pressure (MAP) was calculated as one-third of the pulse pressure plus diastolic pressure. Skin blood flow was indexed using multifibre laser Doppler flowmetry (Perimed) from the forearm that was not exposed to the tube-lined suit. CVC was calculated by dividing skin blood flux by MAP. CVC was normalized to the respective maximal vasodilatation values as outlined below. These normalized data are expressed as percentage maximum (% max).
Protocol 1
After putting on the water-perfused suit, subjects were placed in the supine position in an LBNP device that included a bicycle seat for support. The LBNP device was sealed directly to the subject's skin at the iliac crest, thereby minimizing/eliminating cooling associated with LBNP. This device was used to simulate orthostatic stress via application of negative pressure inside the box, which causes a controlled haemorrhage by pooling of blood in the lower extremities. Subjects rested in the supine position while normothermic water (34°C) circulated through the suit.
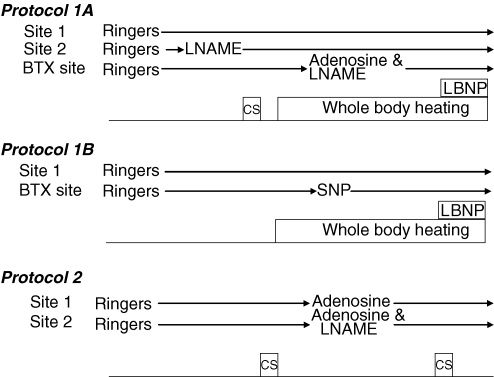
In protocol 1A, three microdialysis probes were placed in the dermal space of the dorsal forearm skin (one at the BTX-treated area and two at adjacent untreated sites) and were perfused with Ringer solution at a rate of 2 μl min−1 (Fig. 1). A multifibre laser-Doppler probe was placed over each microdialysis membrane to monitor cutaneous blood flux. Approximately 90–120 min after microdialysis membrane placement, once the hyperaemic response associated with membrane placement subsided, one of the non-treated sites and the BTX site were perfused with the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 10 mm). At least 15 min after l-NAME infusion, the subjects were exposed to a 3-min cold stress by perfusing water at 5°C through the suit. This was done to confirm that neither l-NAME nor BTX affected cutaneous vasoconstrictor responsiveness, as previously reported (Shibasaki et al. 2006). After this cold stress and subsequent return of skin blood flow to pre-cold stress levels, a heat stress ensued by perfusing water at 46°C through the suit. Although heat stress appropriately increased skin blood flow at the untreated control site, no vasodilatation occurred at the BTX site. Adenosine combined with 10 mm l-NAME was then infused through the BTX site to increase skin blood flow to a similar level relative to the untreated control site. After internal temperature was elevated ∼0.7°C, the temperature of the water perfusing the suit was slightly reduced to attenuate further increases in internal temperature. Typical doses of adenosine ranged from 5.6 × 10−5 to 5.6 × 10−3 m. Upon reaching a steady-state CVC, each subject was exposed to LBNP for 3 min at 30 mmHg below atmospheric pressure, immediately followed by 3 min of 40 mmHg below atmospheric pressure, or until the onset of presyncopal symptoms. LBNP was then stopped. Subjects were then cooled and maximal skin blood flow at each site was identified via administration of 50 mm SNP through the microdialysis membranes.
Figure 1. Schematic illustration of each of the protocols.
l-NAME, NG-nitro-l-arginine methyl ester (10 mm); SNP, sodium nitroprusside (8.4 × 10−5 to 8.4 × 10−3 m); CS, cold stress; LBNP, lower body negative pressure.
As a follow-up study (protocol 1B; see Fig. 1), the procedures outlined in protocol 1A were repeated but using only two microdialysis probes. Instead of administration of adenosine at the BTX site, SNP was infused via the microdialysis probe at the BTX site to increase CVC to a similar level relative to the untreated site via a primarily NO-dependent mechanism. The second site received a continuous infusion of Ringer solution.
Protocol 2
Indirect whole-body cooling reduces CVC solely through engagement of the cutaneous active vasoconstrictor system (Kellogg et al. 1989). This protocol was performed to confirm the effectiveness of endogenous NO in attenuating reductions in CVC during a different vasoconstrictor stimulus relative to protocol 1; that is, by indirect whole-body cooling. Each of the six subjects wore the water-perfused suit and rested in the supine position while normothermic water (34°C) circulated through the suit. Two microdialysis membranes were inserted into dorsal forearm skin. Once the hyperaemic response associated with microdialysis membrane placement subsided, a 3-min cold stress was performed by perfusing water 5 °C through the tube-lined suit. After the cold stress, warm water was perfused through the suit to return skin temperature to pre-cooling levels. Following a 20 min recovery period, a high concentration of adenosine (5.6 × 10−3 m) + l-NAME (10 mm) was administered through one microdialysis membrane, while the second microdialysis membrane received a lower concentration of adenosine (5.6 × 10−4 or 2.8 × 10−3 m) without l-NAME, which was sufficient to increase CVC to a similar level relative to the adenosine + l-NAME site. The objective of this procedure was to cause similar skin blood flows between sites, with one site having an intact endogenous NO system while at the other site, the endogenous NO system was inhibited by l-NAME. After skin blood flow reached a plateau, a second whole-body cold stress was performed. This was followed by identification of maximal skin blood flow via administration of 50 mm SNP through the microdialysis membranes.
Statistical analysis
Data were recorded at 50 Hz (Biopac) and reduced to 20 s averages for each stage (i.e. before cold stress baseline, during cold stress, pre-LBNP and during LBNP for protocol 1A and 1B, and precold baseline and during cold stress for protocol 2). For protocol 1A, one of seven subjects showed presyncopal symptoms early during 40 mmHg LBNP (i.e. within the first ∼30 s). For this subject, data were averaged from the final 20 s at 30 mmHg LBNP. For the two subjects who experienced presyncopal symptoms prior to the end of 40 mmHg LBNP, data were averaged during the 20 s period before LBNP was stopped. Thus for both protocol 1A and 1B, the end point was either the occurrence of presyncopal symptoms or the completion of the prescribed time at 40 mmHg LBNP. The effects of heat stress, relative to preheating baseline, on thermal and haemodynamic variables were compared by Student's paired t tests. In protocol 1A, the reduction (Δ) in CVC in response to LBNP was compared between the untreated, the l-NAME, and the BTX plus adenosine and l-NAME sites via a one-way repeated measures ANOVA. In protocol 1B, the reduction (Δ) in CVC in response to LBNP was compared between the untreated and the BTX plus nitroprusside (BTX + SNP) sites via Student's paired t tests. For protocol 2, the data were averaged from the final 20 s of cold stress. The reduction (Δ) in CVC in response to the cold stress was compared between sites via Student's paired t test. All data are expressed as means ± s.e.m. Statistical significance was set at P < 0.05.
Results
Protocol 1A
Approximately 90–120 min after placement of the microdialysis probe, baseline CVC (i.e. before cold stress) was similar between sites (Table 1). During cold stress, CVC at all sites significantly decreased, and the magnitude of this reduction was not different between sites (Table 1; P > 0.05).
Table 1.
Cutaneous vascular conductance (CVC) at the untreated, l-NAME, and BTX pretreated sites during cold stress outlined in protocol 1A
| Unit (% max) | Untreated | l-NAME | BTX |
|---|---|---|---|
| Baseline CVC | 14.8 ± 1.9 | 15.7 ± 3.3 | 16.8 ± 2.8 |
| CVC during cold stress | 7.4 ± 1.5* | 8.6 ± 1.8* | 9.7 ± 1.6* |
| ΔCVC | 7.4 ± 1.2 | 7.0 ± 1.8 | 7.1 ± 1.6 |
CVC, cutaneous vascular conductance; ΔCVC, reduction in CVC.
Significantly different from baseline CVC at the different sites; P < 0.05.
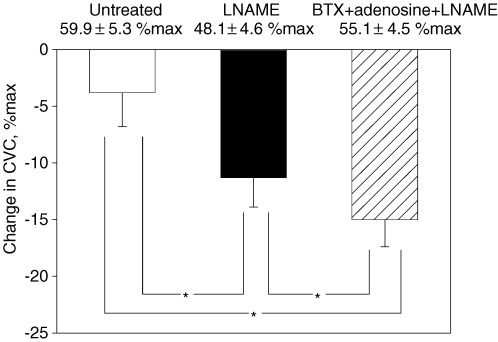
During whole-body heating, mean skin temperature increased from 35.1 ± 0.1°C to 38.4 ± 0.3°C (P < 0.05) and, before application of LBNP, internal temperature increased from 37.0 ± 0.2°C to 37.9 ± 0.2°C (P < 0.05). CVC was significantly elevated at the untreated control and l-NAME sites during whole-body heating, although CVC at the l-NAME site was lower than at the untreated site (59.9 ± 5.3% max versus 48.1 ± 4.6% max, P = 0.05). Prior to adenosine administration at the BTX site, CVC had slightly increased (16.8 ± 2.8% max to 22.4 ± 3.7% max), but remained below CVC at the untreated and l-NAME sites. Infusion of adenosine + l-NAME at the BTX site increased CVC to a similar level relative to the untreated site (55.1 ± 4.5% max versus 59.9 ± 5.3% max, P = 0.43).
LBNP did not significantly change mean skin temperature (38.6 ± 0.3°C to 38.5 ± 0.3°C). LBNP decreased CVC at all sites (Fig. 2) but the reduction in CVC was greatest at the BTX site (15.0 ± 2.4% max), followed by the l-NAME alone site (11.3 ± 2.6% max), and then the untreated site (3.8 ± 3.0% max; P < 0.05 between all site combinations).
Figure 2. Change in cutaneous vascular conductance (CVC) during lower body negative pressure (LBNP) for protocol 1A.
Baseline CVC values prior to LBNP during heat stress are shown at the top of each bar. Baseline CVC values at the BTX site were matched with those at the untreated control site (open bar) by adenosine + l-NAME infusion, although at the l-NAME site (filled bar) CVC was lower than at the other sites. LBNP decreased CVC at all sites. The magnitude of reduction in CVC was significantly greater at the l-NAME site than at the untreated site despite a lower CVC baseline prior to LBNP, while the reduction in CVC at the BTX + adenosine + l-NAME site (hatched bar) was greater than at each of the other sites. *Significantly different between sites; P < 0.05.
Protocol 1B
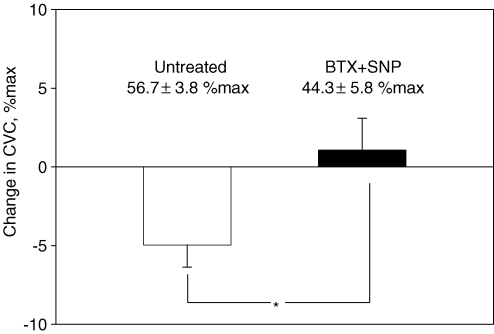
During whole-body heating, mean skin temperature was elevated from 35.0 ± 0.4°C to 37.5 ± 0.3°C (P < 0.05). Before application of LBNP, internal temperature was also elevated from 36.7 ± 0.2°C to 37.3 ± 0.2°C (P < 0.05), while administration of SNP at the BTX site increased CVC to a comparable level relative to the untreated site (44.3 ± 5.8% max versus 56.7 ± 3.8% max, P = 0.085). Skin temperature was slightly decreased during LBNP (37.5 ± 0.1 to 37.4 ± 0.1°C, P = 0.01), but this small change is unlikely to be physiologically significant. The subsequent reduction in CVC caused by LBNP was significantly larger at the untreated site than at the BTX + SNP site (Fig. 3).
Figure 3. Change in cutaneous vascular conductance (CVC) during lower body negative pressure (LBNP) for protocol 1B.
Baseline CVC values prior to LBNP are shown at the top of each bar. Baseline CVC values prior to LBNP during heat stress were similar between sites. LBNP caused CVC to decrease at the untreated site, whereas CVC was unchanged at the BTX + sodium nitroprusside (SNP) site. This resulted in significant differences between CVC responses to LBNP at these sites. *Significantly different compared to the untreated site; P < 0.05.
Protocol 2
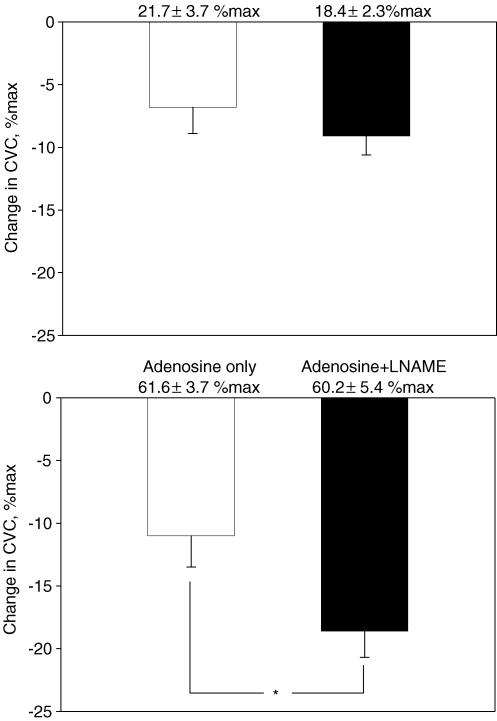
The reduction in skin temperature in response to the cold stress was not significantly different between trials (first cold stress, 34.8 ± 0.1°C to 31.6 ± 0.5°C; second cold stress, 34.9 ± 0.1°C to 31.6 ± 0.3°C; P > 0.05 for both trials). Baseline CVC prior to the first cold stress was similar between sites (21.7 ± 3.7% max versus 18.4 ± 2.3% max). The subsequent reduction in CVC in response to this first cold stress was not significantly different between sites (Fig. 4 upper panel). Administration of adenosine or adenosine + l-NAME similarly increased CVC ∼3-fold (61.6 ± 3.7% max, versus 60.2 ± 5.4% max, respectively; P = 0.72). The reduction in CVC during the ensuing cold stress was significantly greater at the adenosine + l-NAME site relative to the adenosine alone site (Fig. 4 lower panel).
Figure 4. Reduction in cutaneous vascular conductance (CVC) during cold stress before drug infusion (upper panel) and after CVC was increased via adenosine with or without NG-nitro-l-arginine methyl ester (l-NAME; lower panel).
The reduction in CVC during the first cold stress was similar between sites. Adenosine with or without l-NAME increased CVC ∼3-fold from the level before drug infusion. Prior to the second cold stress, although baseline CVC was not different between sites, the reduction in CVC to this cold stress was significantly greater at the adenosine + l-NAME site compared to the adenosine alone site. *P < 0.05.
Discussion
During indirect whole-body heating, NO-related mechanisms contribute to increases in cutaneous blood flow (Kellogg et al. 1998; Shastry et al. 1998, 2000; Shibasaki et al. 2002). A number of studies have shown that NO is capable of attenuating the release of noradrenaline (norepinephrine), reducing the bioactivity of noradrenaline, and/or attenuating vasoconstrictor responses to catecholamines (Greenberg et al. 1990; Zanzinger et al. 1994; Habler et al. 1997; Costa et al. 2001; Chavoshan et al. 2002; Kolo et al. 2004). However, at the time those studies were conducted it was unknown whether NO had similar effects in the skin. To address this, we previously performed a study showing that exogenous NO, via SNP administration, attenuates cutaneous vasoconstrictor responsiveness during cold stress in otherwise normothermic subjects (Durand et al. 2005). Although this finding supports the hypothesis that NO-related mechanisms are capable of attenuating vasoconstrictor responsiveness in the skin circulation, it was unclear whether endogenous NO concentrations are sufficient to attenuate cutaneous vasoconstriction. Thus, the main emphasis of the present study was to identify whether inhibition of endogenous NO production would augment cutaneous vasoconstrictor responses. To that end, the primary findings of this study are that endogenous NO is capable of attenuating cutaneous vasoconstrictor responsiveness to LBNP while subjects are heat stressed as well as in response to whole-body cooling. This conclusion is evidenced by (1) a greater reduction in CVC at the l-NAME-treated site relative to the untreated site during LBNP in protocol 1A, (2) an absence of a reduction in CVC during LBNP at the BTX + SNP site in protocol 1B, and (3) a greater reduction in CVC at the adenosine + l-NAME site relative to the adenosine alone site during cold stress in protocol 2. Together, these results strongly suggest that endogenous NO is capable of attenuates cutaneous vasoconstriction during LBNP in the heat stressed individual as well as attenuates cutaneous vasoconstriction during brief whole-body cooling of the otherwise normothermic individual.
The exact mechanism(s) by which cutaneous active vasodilatation occurs during a heat stress remains unresolved, although it has been suggested that NO contributes to some of the response (Kellogg et al. 1998; Shastry et al. 1998; Bennett et al. 2003; Kellogg, 2006; Wong et al. 2006). Sympathetic cholinergic nerves co-release neuropeptides, such as vasoactive intestinal peptide (VIP) and calcitonin gene-related peptide (CGRP), which are thought to cause cutaneous vasodilatation (Kellogg, 2006). These substances may also attenuate cutaneous vasoconstrictor responsiveness either directly or perhaps through NO-related mechanisms. In order to investigate the proposed hypothesis, in protocol 1 BTX was administered to block the release of neurotransmitters from cholinergic nerves known to cause active vasodilatation (Kellogg et al. 1995; Shibasaki et al. 2006). However, during a subsequent heat stress CVC would not increase at this blocked site thereby making it challenging to compare vasoconstrictor responses to LBNP between BTX and untreated sites. To address this flow-related concern, adenosine + l-NAME was administered at the BTX site such that CVC at that site was similar to CVC at the untreated site during the heat stress. At a third site, NO synthase alone was blocked, while neurotransmitters responsible for active vasodilatation were still released. A significant difference in the magnitude of cutaneous vasoconstriction in response to LBNP between each of these sites reveals insightful information. The largest reduction in CVC occurred at the BTX + adenosine + l-NAME site and this response was greater than the reduction in CVC at the l-NAME alone site. Given that NO synthase was similarly inhibited at both sites, what then could be the mechanism of the differences in vasoconstrictor responsiveness between these sites? A strong possibility is that, in addition to NO, neurotransmitters released from cholinergic vasodilator nerves responsible for active cutaneous vasodilatation attenuate cutaneous vasoconstrictor responsiveness via NO-independent mechanisms. Thus, when the release of these neurotransmitters was blocked with BTX, the cutaneous vasoconstrictor system had the greatest capacity to reduce CVC. Differences in vasoconstrictor responsiveness between the untreated and the l-NAME-treated sites provides evidence that endogenous NO also has the capacity to attenuate reductions in CVC in response to a vasoconstrictor stimulus. Together, the results from protocol 1A suggest that both endogenous NO and neurotransmitters responsible for cutaneous vasodilatation are capable of attenuating cutaneous vasoconstrictor responsiveness.
As outlined above, adenosine was administered to increase CVC at the BTX site to a level similar relative to the control site. Many vasodilators, including adenosine, alter neurotransmission and subsequent noradrenaline release from adrenergic nerves (Vanhoutte et al. 1981); although these findings have not been confirmed in human skin. Nevertheless, adenosine has been shown to attenuate noradrenaline release from stimulated adrenergic nerves (Vanhoutte et al. 1981). It is important to emphasize that if adenosine attenuated noradrenaline release during LBNP, the magnitude of cutaneous vasoconstriction at the BTX site would underestimate the actual vasoconstrictor response had adenosine not impaired noradrenaline release.
To confirm the effectiveness of endogenous NO in attenuating reductions in CVC during a vasoconstrictor stimulus, a follow-up protocol (protocol 1B) was performed in which the NO donor SNP was administered rather than adenosine at the BTX site. In contrast to a pronounced reduction in CVC in response to LBNP at the BTX + adenosine + l-NAME site (see Fig. 2), the reduction in CVC in response to LBNP at the BTX + SNP site was completely abolished (see Fig. 3). These combined findings further support the argument that the cutaneous vasoconstrictor system can be engaged while subjects are heat stressed but the effectiveness of that system is greatly reduced or nullified by neurotransmitters and associated ‘downstream’ substances, perhaps NO, that are responsible for cutaneous active vasodilatation during a heat stress.
Previously, Kellogg et al. (1990) reported that the reduction in CVC during LBNP of heat stressed subjects occurred solely through withdrawal of active cutaneous vasodilator activity. This conclusion was based upon similar reductions in CVC during LBNP at a site where the cutaneous vasoconstrictor system was blocked relative to an unblocked control site. The present findings are not in contrast to those of Kellogg et al. (1990). Rather, results from the present study suggest that NO, and perhaps other substances released from BTX-sensitive nerves, may inhibit the responsiveness of the cutaneous vasoconstrictor system. Therefore, during LBNP of heat stressed subjects, at an intact site, the majority or all of the reduction in CVC is caused by withdrawal of the active vasodilatation system, with little or no influence of the cutaneous vasoconstrictor system. However, when the active vasodilator system is blocked and the accompanying inhibition of the cutaneous vasoconstrictor system is removed, the capacity of the vasoconstrictor system to reduce CVC is manifested.
In protocol 1A we presume that the reduction in CVC during LBNP at the BTX site, where the cutaneous active vasodilator system was abolished, was solely due to augmented cutaneous vasoconstrictor activity. However, we have no direct evidence to confirm this expectation. In contrast, reductions in CVC during whole-body cooling are recognized to occur solely through engagement of the cutaneous active vasoconstrictor system (Kellogg et al. 1989). Thus, protocol 2 was performed to confirm the findings of protocol 1A that NO is capable of attenuating the responsiveness of the cutaneous vasoconstrictor system. To accomplish this objective, CVC was elevated to a similar extent at adjacent sites via administration of adenosine. In pilot studies, we observed that cutaneous vasodilatation as a result of adenosine infusion through the microdialysis probe partially includes NO-mediated vasodilatation, probably via shear stress-induced NO release. To account for this, at one site NO synthase was inhibited via co-administration of l-NAME. Given this effect of NO in contributing to adenosine-mediated cutaneous vasodilatation, higher doses of adenosine were necessary at this site to cause similar dilatation relative to the adjacent adenosine alone site. The subjects were then exposed to a whole-body cold stress with the expectation that the reduction in CVC would be greatest at the site where NO synthase was inhibited, which was consistent with the observed findings (see Fig. 4). These findings are also consistent with previous findings where we reported that the magnitude of cutaneous vasoconstriction to whole-body cooling was attenuated at sites receiving exogenous NO via SNP administration (Durand et al. 2005). Together, these findings strongly support the hypothesis that endogenous NO is capable of attenuating cutaneous vasoconstrictor responsiveness.
Conclusion
The results of the present study suggest that the cutaneous vasoconstrictor system is engaged during a hypotensive challenge such as LBNP in heat-stressed humans, but responses of that system can be attenuated through endogenous NO production and perhaps through other substances released from the active vasodilator nerve. Given that during a heat stress, up to 50% of cardiac output is directed towards the skin (Rowell et al. 1969; Rowell, 1986), control of CVC becomes vital in the regulation of blood pressure during a hypotensive challenge. A contributing factor resulting in heat stress-induced orthostatic intolerance may be an effect of the cutaneous vasodilator system impairing cutaneous vasoconstrictor responses. This would result in insufficient cutaneous vasoconstriction during these combined stresses, leading to compromised blood pressure regulation and ultimately reduced orthostatic tolerance.
Acknowledgments
We thank the subjects for their participation in the study and Kimberly Williams and Marilee Brown for skilful nursing assistance. The research project was funded in part by a grant from National Institutes of Health-National Heart, Lung, and Blood Institute (HL61388, HL67422 and HL84072 to C.G.C.) and from the Grant-in-Aid for the Encouragement of Young Scientists from the Japanese Society for the Promotion of Science (17790162 to M.S.).
References
- Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol. 2003;552:223–232. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Christensen NJ, Farley G, Biaggioni I. NO modulates noradrenaline release in human skeletal muscle: implications for neural preconditioning. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1494–R1498. doi: 10.1152/ajpregu.2001.280.5.R1494. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Johnson JM, Kosiba WA, Kellogg DL., Jr Baroreceptor control of the cutaneous active vasodilator system. J Appl Physiol. 1996;81:2192–2198. doi: 10.1152/jappl.1996.81.5.2192. [DOI] [PubMed] [Google Scholar]
- Durand S, Davis SL, Cui J, Crandall CG. Exogenous nitric oxide inhibits sympathetically mediated vasoconstriction in human skin. J Physiol. 2005;562:629–634. doi: 10.1113/jphysiol.2004.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SS, Diecke FP, Peevy K, Tanaka TP. Release of noradrenaline from adrenergic nerve endings of blood vessels is modulated by endothelium-derived relaxing factor. Am J Hypertens. 1990;3:211–218. doi: 10.1093/ajh/3.3.211. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Wasner G, Janig W. Attenuation of neurogenic vasoconstriction by nitric oxide in hindlimb microvascular beds of the rat in vivo. Hypertension. 1997;30:957–961. doi: 10.1161/01.hyp.30.4.957. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol. 1973;35:798–803. doi: 10.1152/jappl.1973.35.6.798. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology section 4 Environmental Physiology. New York, NY: Oxford University Press; 1996. pp. 215–243. [Google Scholar]
- Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilatation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilatation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol. 1989;257:H1599–H1606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Baroreflex control of the cutaneous active vasodilator system in humans. Circ Res. 1990;66:1420–1426. doi: 10.1161/01.res.66.5.1420. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilatation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of noradrenaline resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol. 2004;286:H296–H303. doi: 10.1152/ajpheart.00668.2003. [DOI] [PubMed] [Google Scholar]
- Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation Regulation During Physical Stress. New York: Oxford University Press; 1986. [Google Scholar]
- Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilatation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–472. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Davis SL, Cui J, Low DA, Keller DM, Durand S, Crandall CG. Neurally mediated vasoconstriction is capable of decreasing skin blood flow during orthostasis in the heat-stressed human. J Physiol. 2006;575:953–959. doi: 10.1113/jphysiol.2006.112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilatation during heat stress. J Appl Physiol. 2002;93:1947–1951. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Verbeuren TJ, Webb RC. Local modulation of adrenergic neuroeffector interation in the blood vessel wall. Physiol Rev. 1981;61:151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Williams SJ, Minson CT. Minimal role for H1 and H2 histamine receptors in cutaneous thermal hyperemia to local heating in humans. J Appl Physiol. 2006;100:535–540. doi: 10.1152/japplphysiol.00902.2005. [DOI] [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J, Seller H. Inhibition of sympathetic vasoconstriction is a major principle of vasodilatation by nitric oxide in vivo. Circ Res. 1994;75:1073–1077. doi: 10.1161/01.res.75.6.1073. [DOI] [PubMed] [Google Scholar]