Abstract
Inhibitory patterns of repetitive transcranial magnetic stimulation (rTMS) were applied to pharyngeal motor cortex in order to establish its role in modulating swallowing activity and provide evidence for functionally relevant hemispheric asymmetry. Healthy volunteers underwent single pulse TMS before and for 60 min after differing intensities of 1 Hz rTMS (n = 9, 6 male, 3 female, mean age 34 ± 3 years) or theta burst stimulation (TBS) (n = 9, 6 male, 3 female, mean age 37 ± 4 years). Electromyographic responses recorded from pharynx and hand were used as a measure of cortico-motor pathway excitability. Swallowing behaviour was then examined with a reaction time protocol, before and for up to 60 min after the most effective inhibitory protocol (1 Hz) applied to each hemisphere. Interventions were conducted on separate days and compared to sham using ANOVA. Only high intensity 1 Hz rTMS consistently suppressed pharyngeal motor cortex immediately and for up to 45 min (−34 ± 7%, P ≤ 0.001). Adjacent hand and contralateral pharyngeal motor cortex showed no change in response (−15 ± 12%, P = 0.14 and 15 ± 12%, P = 0.45, respectively). When used to unilaterally disrupt each hemisphere, rTMS to pharyngeal motor cortex with the stronger responses altered normal (−12 ± 3%, P ≤ 0.001) and fast (−9 ± 4%, P ≤ 0.009) swallow times, not seen following rTMS to the contralateral cortex or after sham. Thus, suppression of pharyngeal motor cortex to rTMS is intensity and frequency dependent, which when applied to each hemisphere reveals functionally relevant asymmetry in the motor control of human swallowing.
Swallowing represents a fundamental yet effortless motor activity which, in contrast to most somatic functions, has bilateral cerebral representation. It is now well established that the sensorimotor cortex plays a role in the regulation of swallowing, acting at both volitional and reflexive levels (Miller, 1982; Martin & Sessle, 1993). Of relevance, many non-invasive human brain imaging techniques including positron emission tomography, functional magnetic resonance imaging and magnetoencephalography have been applied to the study of swallowing (Hamdy et al. 1999a,b; Mosier et al. 1999; Zald & Pardo, 1999; Hartnick et al. 2001; Kern et al. 2001a,b; Loose et al. 2001; Mosier & Bereznaya, 2001; Suzuki et al. 2003; Furlong et al. 2004). Almost universally, these studies have reported varying degrees of lateralized activation in a number of brain regions, most consistently the sensorimotor cortex, insula and cerebellum. These observations are also in keeping with non-invasive transcranial magnetic stimulation (TMS) based mapping studies which demonstrate that the motor cortex representation for swallowing displays territorial asymmetry, inferring a possible functional hemispheric dominance. Despite these observations, there remains controversy about the relative hemispheric contributions controlling swallowing and specifically whether the presence of lateralization for swallowing functions truly exists. Indeed, there are no neuroanatomical data looking directly at fibre tracts for the pharyngeal musculature to determine evidence for asymmetry. The functional data that does exist are somewhat circumstantial, and rely on inference from stroke studies, where dysphagia is a common consequence of unilateral brain damage, and these patients appear to have reduced corticobulbar pathway responsitivity to TMS of the undamaged hemisphere compared to nondysphagic control patients, implying damage has occurred in a swallowing dominant hemisphere (Hamdy et al. 1997).
Recently an exciting new approach to non-invasively probing brain function, known as repetitive TMS (rTMS), has generated much interest since it can cause immediate and lasting changes in the excitability of focal cortical areas at the site of stimulation (Pascual-Leone et al. 1994; Chen et al. 1997; Berardelli et al. 1998; Maeda et al. 2000; Siebner et al. 2000; Gerschlager et al. 2001; Modugno et al. 2001; Touge et al. 2001). A more recent development, rTMS-induced ‘virtual lesioning’, has provided a novel and relatively safe approach to temporarily and reversibly disrupt focal regions of brain both in health and disease with measurable alterations in behaviour, reminiscent of observations seen with the more invasive Wada Test (Benke et al. 2006). Virtual lesions have been applied to the study of speech (Pascual-Leone et al. 1991), vision (Cohen et al. 1997), pain (Andre-Obadia et al. 2006) and motor physiology (Huang et al. 2005), but have never been applied to the study of bilaterally innervated midline musculature.
To date, the motor cortex role in the control of human swallowing remains incompletely understood. Some authors suggest that the primary motor cortex may act as an initiator of volitional swallowing (Martin & Sessle, 1993), whereas others suggest swallowing is primarily initiated in structures such as the insula and cingulate/supplementary motor cortex (Watanabe et al. 2004). This may imply that whilst the primary motor cortex is active during swallowing, it may play a more executive role, possibly through a balance of excitatory and inhibitory mechanisms to the brainstem. Indeed, in animals, stimulation of the cortical chewing cortex will actively inhibit reflexive swallowing responses (Lamkadem et al. 1999). Thus, by disrupting pharyngeal motor cortex, and assessing the behavioural response, it should be possible to further elucidate the relative contribution of human motor cortex in the regulation of swallowing.
The aim of this study was to assess the effects of suppressing swallowing cortico-bulbar excitability using two inhibitory protocols: 1 Hz rTMS (Maeda et al. 2000; Muellbacher et al. 2000; Hilgetag et al. 2001) and the modified theta burst stimulation (TBS) protocol (Huang et al. 2005). We hypothesized that unilaterally inhibiting pharyngeal motor cortex would reciprocally alter excitability in the contralateral (unconditioned) hemisphere. In an additional set of experiments, we also assessed the effects of applying a ‘virtual lesion’ to the motor cortical regions controlling the pharyngeal musculature of both hemispheres, with the hypothesis that a virtual lesion applied to the stronger (or dominant) pharyngeal motor cortex would produce physiologically measurable effects on swallowing function not seen when applied to the contralateral weaker (or non-dominant) pharyngeal cortex.
Methods
Participants
Healthy adult volunteers (n = 13, nine male, four female, age range 24–58 years) participated in a complex series of rTMS studies. Protocol 1: 1 Hz rTMS; n = 9 (six male, three female, mean age 36 years). Protocol 2: TBS; n = 9 (six male, three female, mean age 39 years). Protocol 3: Swallowing behaviour; n = 9 (six male, three female, mean age 36 years).
All volunteers met previously established inclusion criteria (Wassermann, 1998). Experiments were approved by a local research ethics committee and were performed in accordance with the Declaration of Helsinki. All studies were conducted in the clinical laboratory of the Gastrointestinal Physiology department at Hope Hospital.
Electromyographic (EMG) measurements
Motor evoked potentials (MEPs) to TMS were recorded from pharynx and thenar eminence of the hand. Pharyngeal MEPs (PMEPs) were recorded using a pair of bipolar ring electrodes built into a 3 mm diameter intraluminal catheter (Gaeltec, Dunvegan, Isle of Skye, IV55 8 GU, UK) passed into the oropharynx either 11–14 cm transnasally or 13–17 cm transorally according to subject preference. Thenar MEPs (TMEPs) were recorded using gel electrodes (H69P, Tyco Healthcare, Gosport, UK) placed 1 cm apart over the thenar eminence muscle contralateral to stimulated pharyngeal motor cortex.
Electrodes were connected to a preamplifier (CED 1902; Cambridge Electronic Design, Cambridge, UK) with high and low pass filter settings of 200 Hz and 2 kHz, respectively, via connecting cables. Response signals were processed through a 50/60 Hz noise eliminator (‘HumBug’; Quest Scientific, North Vancouver, Canada) to remove any unwanted electrical interference and collected through a laboratory interface (CED micro 1401) at a sampling rate of 5 kHz and recorded using Signal software (v2.13, CED) running on a Pentium III PC.
Transcranial magnetic stimulation (TMS)
Subjects were seated in a comfortable chair with their eyes open. Magnetic stimulation (single monophasic pulses) was performed using a hand-held figure of eight coil (mean 70 mm outer diameter) connected to a Magstim 200 (The Magstim Company, Whitland, UK) over the regions of interest on the scalp, as described by Hamdy et al. (1996). From previous studies the optimal orientation was known to be an antero-posterior direction with the plane of the coil parallel to the scalp surface and the handle/axis of the coil approximately 45 ° to the midsagittal line (Hamdy et al. 1996). The optimal location of the coil was determined by the location on the scalp where magnetic stimulation produced the largest MEPs from the target muscle when the subject was relaxed (the ‘motor hot-spot’). For pharynx, the motor hot-spot in each hemisphere was identified. The hemisphere evoking the largest consistent responses from pharyngeal motor cortex, at the lowest threshold was described as the stronger pharyngeal cortex. Consequently, the contralateral pharyngeal motor cortex was described as the weaker pharyngeal cortex. Resting motor threshold (rMT) was defined as the minimum stimulation intensity over the motor hot-spot required to evoke PMEPs > 20 μV in 5 out of 10 trials. Our initial analysis of the reproducibility of the MT across studies and across investigators showed that there was ≤ 20% differences for both intra-subject and inter-investigator measurements, 95% of the time (Bland & Altman, 1986).
Electrodes were placed over the thenar muscle contralateral to stronger pharyngeal motor cortex. The motor hot-spot for evoking TMEPs was identified and rMT established. Thenar rMT was defined as the minimum stimulation intensity over the motor hot-spot required to evoke TMEPs > 50 μV in 5 out of 10 trials. The peak-to-peak amplitude of MEPs evoked by magnetic stimulation was used to probe the excitability of the motor cortex. Active motor threshold (aMT) was defined as the minimum stimulation intensity over the motor hot-spot required to evoke TMEPs > 200 μV in more than 5 out of 10 trials while the subject was maintaining a voluntary contraction.
Repetitive transcranial magnetic stimulation (rTMS)
RTMS (biphasic) of the cerebral cortex was performed using a Magstim Super Rapid stimulator (The Magstim Company) connected to a figure of eight coil with a 70 mm outer diameter (maximal output of 1.8 tesla), held in an identical orientation to single pulse TMS. Three protocols were tested:
RTMS at 1 Hz was delivered for 10 min (over the stronger (and weaker) pharyngeal motor cortex – see below for reaction time tasks) at 80% (activeLOW) and 120% (activeHIGH) of pharyngeal rMT (on separate days) in two blocks of 300 pulses (5 min each) with a 30 s intertrain interval (600 pulses in total). A Windows 98 laptop running Session 32 software (The Magstim Company) was used to control the rTMS unit.
TBS was performed using the continuous TBS pattern as described by Huang et al. (2005). Briefly, this consisted of three pulses of stimulation delivered at 50 Hz, repeated every 200 ms for a total of 40 s (600 pulses in total) and was generated using Signal software (v2.13, CED). The stimulus intensity was set (for safety reasons) to 80% of aMT of hand and also delivered over the stronger pharyngeal motor cortex.
Sham stimulations were performed over the pharyngeal motor cortex, with the coil tilted on its side, 90 ° to the scalp.
Swallowing reaction times
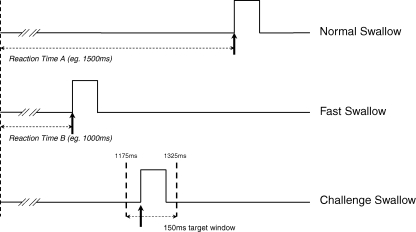
Normal and fast swallowing times were recorded using a second 2 mm diameter catheter with a built in pressure transducer (Gaeltec) to measure swallowing behaviour. Subjects were given 3 ml boluses of mineral water to swallow, delivered directly into the mouth via a plastic catheter connected to a hand held syringe. For normal swallows, subjects were told to perform the swallows at their own pace and as fast as possible for the fast swallows. Following a cue to swallow, the transducer (positioned in the pharynx) would indicate the onset of a pressure wave within the pharynx, set to a predetermined threshold. This catheter was also connected to the Pentium III PC running Signal software (v2.13, CED), via the preamplifier (CED 1902) and laboratory interface (CED micro 1401). Each task was performed 30 times and an electrical trigger was used to cue each swallow. This was achieved through Ag–AgCl cup electrodes, 2 cm apart, applied to the dorsum of the volunteers' hand. Electrodes were connected to an electronic pulse generator (Digitimer, Welwyn Garden City, UK) set to trigger at 10 s intervals for measurements of normal and fast swallows (Fig. 1), and then at random for challenged swallows (see below). The time taken from receiving the electrical cue to the onset of the pharyngeal swallow provided the swallow reaction time. In preliminary studies to establish the feasibility of this method in studying swallowing function, over 20 subjects (10 male and 10 female) were investigated, and the results demonstrated that this approach appeared both reliable and well tolerated.
Figure 1. Normal, fast and challenge swallows.
Illustration demonstrating the recording of normal, fast and challenge swallows.
In addition to measuring normal and fast swallowing times, a challenged swallow task was also created and assessed (Fig. 1). For this, volunteers were asked to swallow post-cue within a 150 ms target time window as indicated by cursors placed on a computer screen, using visual feedback (Signal). This was also performed 30 times. From observations in the above mentioned initial pilot study, a 150 ms window was established and chosen as a period which consistently challenged the subject to achieve an average of 30–50% success rate. The 150 ms target time window was calculated based on the following formula (B + (T/2)) ± 75 ms where A is the mean normal swallow reaction time, B is the mean fast swallow reaction time and T is the time difference between normal and fast swallow reaction time averages (A − B). For example: if A = 1500 ms and B = 1000 ms, then Target window = 1000 + [(1500 − 1000)/2] ± 75 = 1175 to 1325 ms.
Experimental procedures
Protocols 1 and 2: Effects of rTMS intervention on swallowing motor cortex excitability
To elucidate the neurophysiological effects of inhibitory rTMS protocols on pharyngeal motor cortex excitability, volunteers attended on at least two occasions with studies performed a week apart in a double blind, pseudo-randomised order incorporating both active and sham components.
Pharyngeal (stronger and weaker hemispheric responses) and thenar motor hot-spots, rMT and aMT were identified using TMS at the start of each study as described earlier. Baseline measurements of cortical excitability were made using single pulse TMS stimuli delivered at rMT and rMT + 10% (stimulator output) at each of the three sites (stronger and weaker pharyngeal motor cortices and hand motor cortex), with a 5 s interval between each stimulus (60 stimuli in total). Following baseline measurements of cortical excitability, volunteers received one of five rTMS interventions delivered over the stronger pharyngeal motor hot-spot: (i) 1 Hz rTMS activeLOW, (ii) 1 Hz rTMS activeHIGH, (iii) sham 1 Hz rTMS, (iv) active cTBS, or (v) sham cTBS.
Once completed, cortical excitability measurements (identical to baseline), were taken immediately after and every 15 min post-intervention for 1 hour. These times were chosen on the basis of previous studies which have demonstrated that rTMS of both the pharyngeal motor cortex and hand motor cortex can alter cortical excitability for at least 1 hour (Gow et al. 2004; Huang et al. 2005).
Protocol 3: Effects of rTMS on swallowing behaviour
In order to assess whether inhibition of motor cortex excitability has modulatory effects on swallowing, a further series of rTMS studies were performed, using the protocol shown in protocols 1 and 2 which induced the greatest degree of cortical inhibition (1 Hz rTMS activeHIGH). Volunteers attended on three occasions with studies performed a week apart in a double blind, pseudo-randomised order incorporating both active and sham components.
Pharyngeal (stronger and weaker hemispheric responses) motor hot-spots and rMT were identified using TMS at the start of each study as described earlier. Baseline swallowing reaction time tasks were then performed. Volunteers were asked to perform three different swallow tasks: normal, fast and challenged swallowing. Each swallowing task was performed 30 times and volunteers were given 3 ml of mineral water to swallow prior to each cue. Normal and fast swallows were performed first and allowed online calculation of the 150 ms challenge swallow target time window as described earlier. Challenge swallows were conducted last and performance was scored based on the number of swallows initiated within the target time window. After recording baseline swallowing data, volunteers received each of three rTMS ‘virtual lesion’ interventions: (i) active rTMS to pharyngeal motor cortex with the stronger responses, (ii) Active rTMS to contralateral pharyngeal motor cortex with the weaker responses, and (iii) sham rTMS.
Following the intervention, measurements of swallowing behaviour (identical to baseline) were repeated immediately after, at 30 and 60 min post-rTMS.
Data analysis
MEP and behavioural data were analysed separately using repeated measures ANOVA.
MEP data
The peak-to-peak amplitude of MEPs evoked by magnetic stimulation was used as a measure of motor cortex excitability. Baseline MEP data for all interventions were compared using a Mann–Whitney test. The mean (raw) amplitude and latency values were separately analysed using a repeated measures ANOVA (SPSS 14.0) with time (six levels: baseline, 0, 15, 30, 45 and 60 min post-rTMS) and intervention (5 levels: 1 Hz activeHIGH, 1 Hz activeLOW, TBS, 1 Hz sham, TBS sham) as within-subject factors.
Swallowing behaviour data
The mean (raw) values of normal and fast swallowing times were analysed for each time interval pre- and post-rTMS for each subject. For challenged swallows, the percentage number of correctly timed swallows was calculated for each subject. Motor threshold and baseline behavioural data were analysed with Wilcoxon's signed rank test. Swallowing times and challenged swallow results were then analysed separately for each rTMS intervention (active stronger responses, active weaker responses and sham) using a repeated measures ANOVA (SPSS 14.0) with time (four levels: baseline, 0, 30 and 60 min post-rTMS) and stimulation site (three levels: strong pharyngeal response, weak pharyngeal response and thenar) as within-subject factors.
Non-sphericity was corrected using Greenhouse–Geisser when necessary. When a significant interaction was present, separate ANOVAs with time as a within-subject factor were performed to characterize time-dependent changes in performance. Post hoc paired-sample t tests were then performed to explore the strength of main effects and the patterns of interaction between experimental factors. A P-value of ≤ 0.05 was used to indicate statistical significance. All data are presented as group means ± standard errors of the mean (s.e.m.) unless stated otherwise.
Results
Both TMS and rTMS were well tolerated by all subjects with no reported adverse effects. Of the 13 subjects recruited, eight were judged to have stronger responses from pharyngeal motor cortex in the right hemisphere and five in the left hemisphere. In one subject, the rTMS threshold was too high to safely and reliably induce inhibition and therefore removed from any further analysis. The mean mediolateral and anteroposterior scalp positions (referenced to the vertex) where rTMS was applied to pharyngeal motor cortex of each hemisphere were approximately 2.5–4 cm.
During Protocols 1 and 2, the mean (± s.e.m.) rMT to evoke responses from the stronger and weaker pharyngeal motor cortices and ipsilateral hand motor cortex were 74 ± 1%, 78 ± 1% and 47 ± 2%, respectively. The mean intensity of cortical stimulation for the 1 Hz rTMS (activeLOW/activeHIGH) and TBS interventions were 67 ± 5%, 82 ± 5% and 45 ± 2%, respectively.
During Protocol 3, the mean (± s.e.m.) rMT to evoke the stronger pharyngeal motor responses in the three studies (rTMS to strong/weak hemispheres and sham) were 62 ± 2, 65 ± 3 and 68 ± 3% of stimulator output, respectively. The mean (± s.e.m.) rMT to evoke the weaker pharyngeal motor responses in the three studies were 70 ± 2, 78 ± 3 and 74 ± 3% of stimulator output, respectively.
As expected, rMT for the pharyngeal motor cortex with the weaker responses was higher than for the stronger responses, P ≤ 0.001 (two sided Wilcoxon's signed ranks test).
Effects of rTMS intervention on pharyngeal motor cortex excitability (hemisphere with the stronger responses alone)
Amplitude
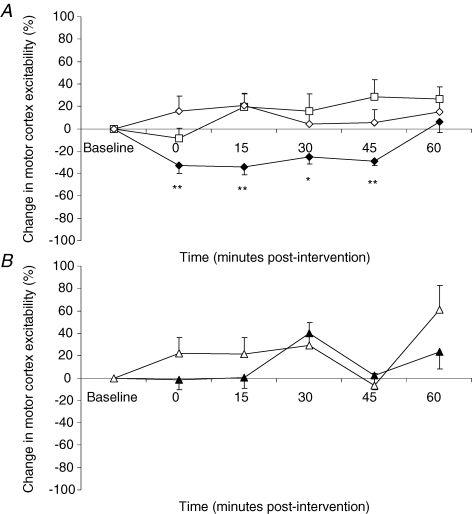
Mean changes in amplitude from baseline following both active and sham arms of the 1 Hz rTMS interventions and TBS interventions are shown in Fig. 2A and B, respectively. Mann–Whitney tests comparing absolute baseline values for each of the conditions did not reveal any significant differences. Repeated measures ANOVA comparing the effects of the different interventions on PMEP amplitudes revealed a significant effect of intervention (F3,44 = 3.5, P = 0.028), time (F3,52 = 4.7; P = 0.006) and time × intervention (F20,340 = 2.5; P ≤ 0.001). Separate follow-up ANOVAs with time as a within-subject factor were performed to characterize time-dependent changes in PMEP amplitude and revealed that responses were decreased from baseline only following activeHIGH 1 Hz rTMS (F5,102 = 6.6; P ≤ 0.001). Post hoc t tests performed at each time point, revealed that PMEPs were inhibited immediately and for up to 45 min post-rTMS (maximum = −34 ± 7%, P ≤ 0.001). There were no significant rTMS associated changes following activeLOW 1 Hz rTMS, 1 Hz sham, or following either active or sham TBS and were therefore not considered for any further analysis.
Figure 2. Effects of rTMS intervention on pharyngeal motor cortex excitability.
A, group mean ± s.e.m. (%) change in PMEP amplitudes from baseline following 1 Hz rTMS activeHIGH (♦), activeLOW (□) and sham (⋄) interventions over stimulated pharyngeal motor cortex. PMEPs are inhibited by only activeHIGH 1 Hz rTMS, immediately and for up to 45 min (*P ≤ 0.004, **P ≤ 0.001, post hoc t tests). B, group mean ± s.e.m. (%) change in PMEP amplitudes from baseline following active (▴) and sham (▵) TBS interventions over the stimulated pharyngeal motor cortex. Despite receiving the same number of pulses as activeHIGH 1 Hz rTMS, no inhibitory effects are observed after TBS.
Latency
Mean response latencies at baseline and each time point for pharyngeal motor cortex following 1 Hz rTMS and TBS are shown in Table 1. A repeated measures ANOVA comparing the effects of the different interventions on PMEP latencies revealed a significant time effect (F5,85 = 8.4, P ≤ 0.001) with a small but consistent lengthening of latency. There was no effect of intervention (F3,42 = 0.25, P = 0.8) or time × intervention (F20,340 = 1.7, P = 0.1). Separate follow-up ANOVAs however, did not reveal any significant effects on response latencies (activeHIGH 1 Hz F5,102 = 2.2, P = 0.06; activeLOW 1 Hz F5,102 = 0.6, P = 0.7; TBS F5,102 = 0.5, P = 0.8; 1 Hz Sham F5,102 = 0.2, P = 0.97; TBS Sham F5,102 = 0.76, P = 0.6).
Table 1.
Pharyngeal and thenar motor evoked potential latencies
| Baseline | 0 min | 15 min | 30 min | 45 min | 60 min | |
|---|---|---|---|---|---|---|
| ActiveHIGH 1 Hz | ||||||
| Pharynx – strong | 8.8 ± 0.1 | 9.4 ± 0.1 | 9.4 ± 0.1 | 9.1 ± 0.1 | 9.3 ± 0.2 | 9.2 ± 0.2 |
| Pharynx – weak | 9.0 ± 0.1 | 9.3 ± 0.2 | 9.3 ± 0.2 | 9.0 ± 0.1 | 9.2 ± 0.1 | 9.2 ± 0.2 |
| Hand | 22.3 ± 0.3 | 22.1 ± 0.3 | 22.5 ± 0.3 | 22.7 ± 0.4 | 22.6 ± 0.5 | 22.7 ± 0.4 |
| ActiveLOW 1 Hz | ||||||
| Pharynx – strong | 9.4 ± 0.2 | 9.7 ± 0.2 | 9.8 ± 0.3 | 9.8 ± 0.2 | 10.0 ± 0.2 | 9.7 ± 0.2 |
| Pharynx – weak | 10.0 ± 0.2 | 10.0 ± 0.2 | 9.7 ± 0.2 | 9.9 ± 0.2 | 9.7 ± 0.2 | 9.7 ± 0.2 |
| Hand | 22.4 ± 0.6 | 22.2 ± 0.4 | 22.7 ± 0.6 | 22.9 ± 0.6 | 23.1 ± 0.6 | 22.8 ± 0.7 |
| Sham 1 Hz | ||||||
| Pharynx – strong | 9.0 ± 0.2 | 9.0 ± 0.2 | 9.0 ± 0.2 | 9.1 ± 0.2 | 9.2 ± 0.2 | 9.1 ± 0.2 |
| Pharynx – weak | 9.1 ± 0.1 | 8.9 ± 0.1 | 9.2 ± 0.1 | 9.1 ± 0.1 | 9.3 ± 0.1 | 9.2 ± 0.2 |
| Hand | 22.6 ± 0.4 | 22.9 ± 0.4 | 22.7 ± 0.4 | 23.0 ± 0.5 | 23.1 ± 0.4 | 23.2 ± 0.4 |
| Active TBS | ||||||
| Pharynx – strong | 8.8 ± 0.2 | 9.1 ± 0.2 | 9.0 ± 0.2 | 9.1 ± 0.2 | 9.3 ± 0.2 | 9.2 ± 0.2 |
| Pharynx – weak | 8.9 ± 0.2 | 8.9 ± 0.2 | 9.0 ± 0.2 | 9.1 ± 0.2 | 9.2 ± 0.3 | 9.3 ± 0.2 |
| Hand | 22.3 ± 0.3 | 22.2 ± 0.3 | 22.7 ± 0.4 | 22.6 ± 0.5 | 23.1 ± 0.3 | 22.7 ± 0.4 |
| Sham TBS | ||||||
| Pharynx – strong | 8.9 ± 0.2 | 9.0 ± 0.1 | 9.1 ± 0.2 | 8.8. ± 0.1 | 9.2 ± 0.2 | 9.0 ± 0.2 |
| Pharynx – weak | 9.1 ± 0.2 | 9.0 ± 0.2 | 9.1 ± 0.2 | 9.0 ± 0.2 | 9.0 ± 0.2 | 9.0 ± 0.2 |
| Hand | 22.7 ± 0.4 | 22.9 ± 0.3 | 22.7 ± 0.4 | 22.9 ± 0.4 | 22.9 ± 0.4 | 22.6 ± 0.4 |
Cortico-pharyngeal and thenar latencies for each site, across each time point before and after both 1 Hz rTMS and TBS. Data (ms) are presented as mean ± s.e.m.
Effects of activeHIGH 1 Hz rTMS on pharyngeal motor cortex excitability (comparing hemispheres with stronger and weaker responses)
Having demonstrated that high intensity 1 Hz rTMS had an effect upon PMEPs evoked from the hemisphere with the stronger pharyngeal responses, this was further examined in the (unstimulated) hemisphere with the weaker responses.
Amplitude
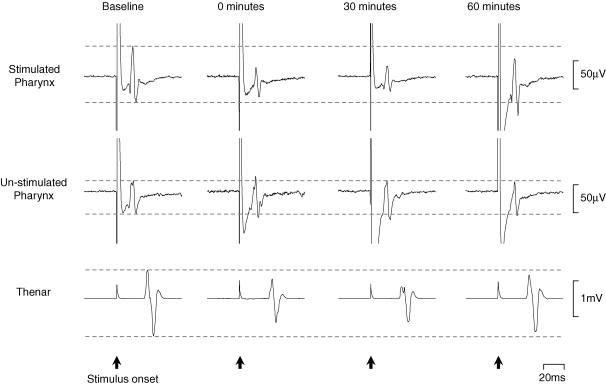
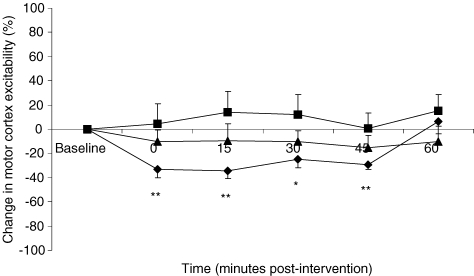
PMEP traces from a representative individual, evoked from both the stronger and weaker hemispheric responses before and after activeHIGH 1 Hz rTMS, are shown in Fig. 3. Mean (%) changes in PMEP amplitudes from baseline following activeHIGH 1 Hz rTMS of the pharyngeal motor cortex from both hemispheres are shown in Fig. 4. A repeated measures ANOVA to assess the effects of activeHIGH 1 Hz rTMS on the two hemispheres revealed a significant effect of time (F3,44 = 4.3; P = 0.013) and a time × hemisphere interaction (F5,85 = 6.4; P ≤ 0.001) thus demonstrating a clear difference in excitability between the two hemispheres after rTMS. There was no effect of hemisphere alone (F1,17 = 2.2, P = 0.155). As before, separate follow-up ANOVAs with time as a within-subject factor were performed to characterize any time-dependent changes in PMEP amplitude, and revealed that responses were decreased from baseline in the stimulated hemisphere with the stronger pharyngeal motor cortical output (F5,102 = 6.6; P ≤ 0.001). Post hoc t tests performed at each time point revealed that PMEPs were inhibited in this hemisphere immediately and for up to 45 min post-rTMS (maximum = −34 ± 7%, P ≤ 0.001). There were no significant rTMS associated changes in the contralateral hemisphere with the weaker pharyngeal motor cortical output.
Figure 3. Changes in MEP amplitudes following activeHIGH 1 Hz rTMS.
Cortically evoked EMG responses in one individual are shown at suprathreshold intensity before and for up to 60 min post-activeHIGH 1 Hz rTMS. Traces are averages of 10 responses. The cortical stimulus was applied at 0 ms. Following rTMS there is an immediate reduction in PMEPs from the stimulated hemisphere and to a lesser extent in TMEPs. No significant increases in PMEPs from the contralateral (un-stimulated) hemisphere are seen.
Figure 4. Effects of activeHIGH 1 Hz rTMS on pharyngeal motor cortex and hand motor cortex excitability.
Group mean ± s.e.m. (%) change in PMEP and TMEP amplitudes from baseline following activeHIGH (♦) 1 Hz rTMS over the stimulated stronger pharyngeal motor cortex. Inhibitory changes in PMEPs evoked from the stronger pharyngeal motor cortex can be seen immediately and for up to 45 min after active rTMS (*P ≤ 0.004, **P ≤ 0.001, post hoc t tests) but are not consistently seen in hand motor cortex (▪). Weaker pharyngeal motor cortex (▴) shows no change in excitability (P = 0.7).
Latency
Mean latencies at baseline and each time point for the pharyngeal motor cortices following 1 Hz rTMS and TBS are shown in Table 1. A repeated measures ANOVA comparing the effects of the different interventions on bihemispheric PMEP latencies revealed a significant time effect (F5,85 = 2.9, P = 0.019) with a small but consistent lengthening of latency. There was no effect of intervention (F1,24 = 0.15, P = 0.8) or time × intervention (F20,340 = 1.4, P = 0.1). Separate follow-up ANOVAs however, did not reveal any significant effects on response latencies (activeHIGH 1 Hz F5,102 = 0.6, P = 0.7; activeLOW 1 Hz F5,102 = 0.4, P = 0.8; TBS F5,102 = 0.4, P = 0.8; 1 Hz Sham F5,102 = 0.9, P = 0.5; TBS Sham F5,102 = 0.2, P = 0.98).
Effects of activeHIGH 1 Hz rTMS on pharyngeal versus hand motor cortex excitability
Amplitude
MEP traces from a representative individual before and after active and sham 1 Hz rTMS are shown in Fig. 3. Mean (%) changes in TMEP amplitude from baseline following activeHIGH 1 Hz rTMS to pharyngeal motor cortex and corresponding hand motor cortex are shown in Fig. 4. A repeated measures ANOVA to assess the effects of 1 Hz rTMS over the pharyngeal area on hand motor cortex excitability revealed a significant effect of site (F1,17 = 23.3, P ≤ 0.001) on cortical excitability but no effect of time (F3,47 = 1.8, P = 0.2) or time × site interactions (F3,48 = 1.0, P = 0.4). As before, separate follow-up ANOVAs with time as a within-subject factor were performed to characterize any time-dependent changes in PMEP amplitude, revealed that responses were decreased following activeHIGH 1 Hz rTMS (F5,102 = 6.6; P ≤ 0.001). There were no significant rTMS associated changes in the hand motor cortex.
Latency
Mean response latencies at baseline and each time point for hand motor cortex following 1 Hz rTMS and TBS are shown in Table 1. A repeated measures ANOVA comparing the effects of the different interventions on TMEP latencies revealed a significant time effect (F3,49 = 5.04, P = 0.004) with a small but consistent lengthening of latency. There was no effect of intervention (F1,23 = 0.62, P = 0.5) or time × intervention (F20,340 = 1.1, P = 0.3). Separate follow-up ANOVAs however, did not reveal any significant effects on response latencies (activeHIGH 1 Hz F5,102 = 0.47, P = 0.8; activeLOW 1 Hz F5,102 = 0.8, P = 0.6; TBS F5,102 = 0.7, P = 0.6; 1 Hz Sham F5,102 = 0.3, P = 0.9; TBS Sham F5,102 = 0.2, P = 0.95).
Effects of a 1 Hz virtual lesion on swallowing behaviour
Having identified that 1 Hz activeHIGH rTMS generated the greatest suppression of swallowing corticomotor excitability, these effects were further investigated. Swallowing reaction times were used to assess swallowing and were recorded through a second 2 mm diameter catheter with a built in pressure transducer. Subjects were given 3 ml boluses of mineral water to swallow, and cued to swallows using a cutaneous electrical pulse, set to trigger at 10 s intervals for measurements of normal and fast swallows, and then at random for challenged swallows.
Normal swallowing reaction time
Group mean changes in normal swallowing reaction times after 1 Hz rTMS over the stronger and weaker pharyngeal motor cortices and after sham are shown in Table 2A, and displayed as percentage change from baseline in Fig. 5. At baseline there was no difference in normal swallow timings across the three rTMS study visits (pharynx – strong versus sham P = 0.95, pharynx – weak versus sham P = 0.2, strong versus weak hemispheres P = 0.2, two sided Wilcoxon's signed ranks test), with an overall mean (± s.e.m.) of 1279 ± 40 ms. However, repeated measures ANOVA revealed a significant intervention (F2,46 = 3.2, P = 0.049), time (F3,69 = 4.2, P = 0.008) and time × intervention (F3,80 = 2.5, P = 0.05) effect indicating a strong effect of intervention on normal swallowing behaviour.
Table 2.
Effects of 1 Hz rTMS swallowing behaviour
| Baseline | 0 min | 30 min | 60 min | |
|---|---|---|---|---|
| A | ||||
| Normal swallows | ||||
| Pharynx – strong | 1333 ± 68 | 1201 ± 65 | 1168 ± 71 | 1230 ± 69 |
| Pharynx – weak | 1179 ± 77 | 1209 ± 63 | 1153 ± 73 | 1189 ± 79 |
| Sham | 1326 ± 59 | 1304 ± 61 | 1310 ± 52 | 1303 ± 59 |
| Fast swallows | ||||
| Pharynx – strong | 1122 ± 58 | 1016 ± 54 | 985 ± 47 | 997 ± 54 |
| Pharynx – weak | 926 ± 38 | 917 ± 37 | 946 ± 43 | 935 ± 44 |
| Sham | 1076 ± 52 | 1108 ± 57 | 1118 ± 45 | 1083 ± 46 |
| B | ||||
| Challenge swallows | ||||
| Pharynx – strong | 11 ± 2 | 12 ± 2 | 12 ± 2 | 11 ± 2 |
| Pharynx – weak | 11 ± 2 | 12 ± 2 | 11 ± 2 | 10 ± 2 |
| Sham | 13 ± 2 | 16 ± 2 | 16 ± 2 | 15 ± 1 |
A, group mean normal and fast swallowing reaction times for each time point before and after active and sham 1 Hz rTMS interventions. B, group mean number of correctly timed challenge swallows (out of 30) for each time point before and after active and sham 1 Hz rTMS interventions. All data are presented as mean ± s.e.m. (ms/number of correctly timed swallows).
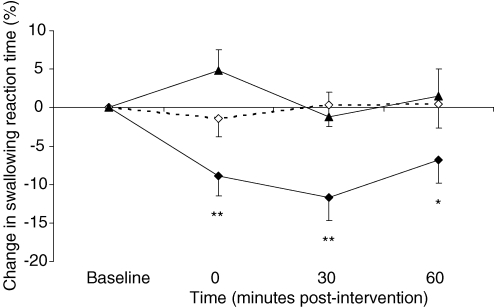
Figure 5. Normal swallows.
Group mean (%) change in normal swallowing reaction times from baseline following active 1 Hz rTMS applied to stronger (♦) and weaker (▴) pharyngeal motor cortices and after sham (⋄). Swallowing reaction times are only decreased following active stimulation over the stronger pharyngeal motor cortex (*P = 0.03, **P ≤ 0.001 post hoc t test).
As before, separate follow-up ANOVAs with time as a within-subject factor were performed to characterize any time-dependent changes in normal swallowing and revealed that times were dramatically reduced following only active rTMS to the stronger pharyngeal motor cortex (F3,69 = 6.4; P ≤ 0.001). Post hoc t tests performed at each time point, revealed that reaction times quickened immediately and for up to 60 min post-rTMS (maximum = −12 ± 3%, P ≤ 0.001). There were no significant effects of rTMS to the weaker pharyngeal motor cortex or after sham.
Fast swallowing reaction time
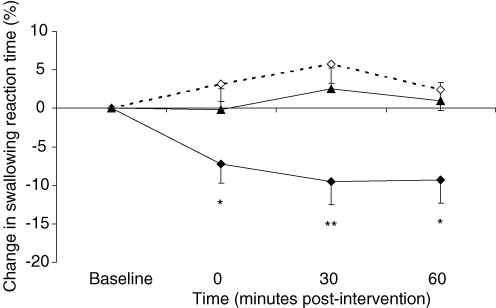
Group mean changes in fast swallowing reaction times after 1 Hz rTMS over stronger and weaker pharyngeal motor cortices and after sham are shown in Table 2A, with percentage change from baseline shown in Fig. 6. At baseline there was no difference in fast swallow timings across the three rTMS study visits (pharynx – strong versus sham P = 0.6, pharynx – weak versus sham P = 0.2, strong versus weak hemispheres P = 0.1, two sided Wilcoxon's signed ranks test), with an overall mean (± s.e.m.) of 1041 ± 30 ms. Repeated measures ANOVA revealed a significant intervention effect (F2,46 = 4.7, P = 0.013) and time × intervention (F3,76 = 4.5, P = 0.005) interaction thereby indicating a clear effect of intervention on fast swallowing behaviour but no effect of time alone (F3,69 = 1.4, P = 0.24). Separate follow-up ANOVAs with time as a within-subject factor were performed to characterize any time-dependent changes in fast swallowing and revealed that fast swallows were also only reduced following active rTMS to the stronger pharyngeal motor cortex (F3,69 = 4.7, P = 0.005). Post hoc t tests performed at each time point revealed that reaction times quickened immediately and for up to 60 min post-rTMS (maximum = −9 ± 4%, P = 0.009). There were no significant changes following rTMS to the weaker pharyngeal motor cortex or after sham.
Figure 6. Fast swallows.
Group mean (%) change in fast swallowing reaction times from baseline following active 1 Hz rTMS applied to stronger (♦) and weaker (▴) pharyngeal motor cortices and after sham (⋄). Swallowing reaction times are decreased only following active stimulation over the stronger pharyngeal motor cortex (*P ≤ 0.04, **P = 0.009 post hoc t test).
Challenged swallow performance
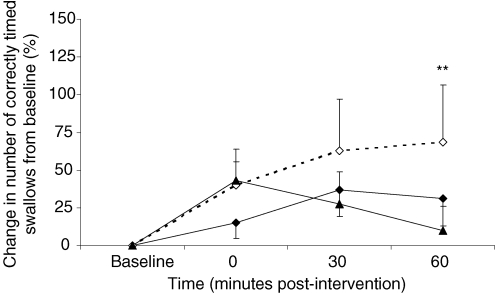
Group mean changes in the number of correctly timed challenge swallows after 1 Hz rTMS to stronger and weaker pharyngeal motor cortices and after sham are shown in Table 2B, with percentage change from baseline shown in Fig. 7. At baseline there was no difference in the number of correctly timed challenge swallows across the three rTMS study visits (pharynx – strong versus sham P = 0.1, pharynx – weak versus sham P = 0.3, strong versus weak hemispheres P = 0.8, two sided Wilcoxon's signed ranks test), with an overall mean (± s.e.m.) of 12 ± 1. Repeated measures ANOVA revealed a significant effect of time (F2,44 = 3.1, P = 0.05) but no effect of intervention (F1,34 = 0.89, P = 0.4) or time × intervention (F3,80 = 1.16, P = 0.3). Separate follow-up ANOVAs with time as a within-subject factor were performed to characterize any time-dependent changes in challenge swallowing and revealed a strong trend to significance with sham only (F3,66 = 2.53, P = 0.065). Post hoc t tests performed on the sham data at each time point revealed a rise in successful trials at 60 min (69 ± 38%, P = 0.04).
Figure 7. Challenge swallows.
Group mean (%) change in successful challenge swallow trials from baseline following active 1 Hz rTMS applied to stronger (♦) and weaker (▴) pharyngeal motor cortices and after sham (⋄). Successful swallow trials show a (anticipated) rise following sham (*P = 0.04 post hoc t test).
Discussion
The results of these experiments demonstrate it is possible to suppress healthy human pharyngeal motor cortex using appropriate patterns and intensities of rTMS. Of the protocols tested, only activeHIGH 1 Hz rTMS successfully reduced cortico-bulbar pathway excitability, an effect not consistently seen with activeLOW 1 Hz or with TBS rTMS. In contrast, the reduction in excitability was not associated with a significant alteration in excitability of the contralateral pharyngeal motor cortex or ipsilateral hand motor cortex. Moreover, our data also show that the areas of motor cortex controlling pharyngeal musculature modulate human swallowing behaviour in a functionally asymmetric manner. Specifically, cortical suppression of the stronger pharyngeal motor cortex (as defined by MEP responses to single pulse TMS) could induce clear changes in swallowing physiology, as measured by a swallowing reaction time protocol, which was not evident when rTMS was applied to the contralateral weaker pharyngeal cortex. These observations are likely to have a neurophysiological basis and therefore merit further discussion.
Intensity and pattern of rTMS
It is interesting to observe that despite delivering the same number of pulses, activeLOW 1 Hz rTMS and TBS were ineffective at inducing inhibition in the pharyngeal motor cortex. The most obvious explanation for this relates to the stimulation intensities applied during these interventions. For safety reasons, rTMS applied using the TBS protocol was limited to 80% (aMT) of hand motor cortex (Huang & Rothwell, 2004; Huang et al. 2005). Importantly, the rMT of pharyngeal motor cortex is inherently higher than that found within hand muscles. Thus, the average intensity at which TBS was applied (45 ± 2%) was considerably lower and likely to be too weak to generate the inhibitory effects, as demonstrated following activeHIGH 1 Hz rTMS, which was applied at intensities more closely related to pharyngeal rMT (82 ± 5%).
Of relevance, a recent report by Martin et al. (2006) on the effects of TBS on a distal and proximal arm muscle, demonstrated that the two motor cortices targeted during TBS responded differently despite similar parameters of stimulation. Since TBS preferentially reduces the amplitude of corticospinal I1 waves (Di Lazzaro et al. 2005), the authors suggest that for proximal muscles, the circuitry between the I1 input and the corticospinal neurone may be less susceptible to the type of inhibition produced by TBS and may also be true for the pharyngeal motor cortex. Moreover, despite the higher intensity of the activeLOW 1 Hz rTMS compared to TBS, this also failed to produce any consistent inhibition, an observation consistent with other reports on the effects low intensity, low frequency rTMS in somatic muscles (Fitzgerald et al. 2002).
Also of interest, we noted a small albeit consistent lengthening of MEP latencies (0.6 ms) over time following rTMS. However, separate follow-up analysis of the data did not reveal any significant interventional effects implying that the MEPs elicited by TMS may have resulted through recruitment of neurones with slower conduction velocities. This finding, however, is entirely consistent with the effects on excitatory post-synaptic potentials from an inhibited cortex, with dampened temporal summation for cortical neurons and interneuronal firing. Indeed, the reverse effect, a shortening of MEP latency to increased temporal summation is commonly observed after excitatory cortical stimulation (Pascual-Leone et al. 1994). Of relevance, response latencies are also observed to be dramatically prolonged after stroke lesions, where both cortical (Brouwer & Schryburt-Brown, 2006) and spinal cord (Valero-Cabre et al. 2001) excitability is greatly inhibited. It would also seem highly unlikely that any inhibition was occurring in downstream structures such as the brainstem, peripheral motoneurones, or the muscle itself, since such an effect would have likely caused inhibition of pathways from each hemisphere, rather than being specific to the hemisphere evoking stronger pharyngeal responses as seen in our study. Nonetheless, in the absence of peripheral nerve recordings in our study, lower level circuitry inhibition remains a possible albeit unlikely scenario.
Evidence for transcallosal interactions in a bilaterally represented motor system
While activeHIGH 1 Hz rTMS clearly decreased excitability of the stimulated pharyngeal motor cortex, the excitability of the homologous contralateral region was not strongly influenced, showing only a small, non-significant increase in excitability. This finding raises an interesting question regarding the hemispheric control of the pharynx: is transcallosal inhibition of less importance in a bilaterally represented hemispheric control system than in a unilaterally controlled system?
Although not tested in this study, previous work using bilateral single pulse TMS to pharyngeal motor cortex (of both hemispheres) has demonstrated that when the two hemispheres are stimulated sequentially at inter-stimulus intervals of 5–10 ms, no convincing inhibition can be demonstrated (Hamdy et al. 1998). This is in contrast to the effects seen in hand muscles, where sequential magnetic stimuli to hand motor cortex of each hemisphere, at inter-stimulus intervals of 5–30 ms, inhibit motor responses, possibly via transcallosal inhibitory interactions that may exist to ensure unilateral movement (Ferbert et al. 1992). These data imply that the inter-hemispheric interactions for midline structures, such as the pharynx and oesophagus, which have bilateral cortical representation, might differ from those which have predominantly unilateral representation. Thus, following inhibition of one hemisphere, changes in the contralateral hemisphere may not have been induced because transcallosal interactions between the two pharyngeal areas are not strongly competitive, and indeed are most likely synergistic. Of relevance, 15 min of suprathreshold 1 Hz rTMS over hand motor cortex has been demonstrated to decrease excitability (Wassermann et al. 1998) and cerebral blood flow (Fox et al. 1997) in the contralateral (unstimulated) homologous region controlling the first dorsal interosseous muscle. The reverse effect, an increase in the excitability of the contralateral homologous hand motor cortex, has also been reported after 30 min of suprathreshold stimulation (Schambra et al. 2003), implying that the modulation of inter-hemispheric fibre activity can be bi-directional. Indeed, as with the ipsilateral inhibitory effects of rTMS, any effects on the contralateral non-stimulated side may also be stimulus intensity dependent with higher intensities more likely to influence excitability transcallosally.
Cortical suppression and its effects on swallowing behaviour
With regards to the behavioural components of our study, 10 min of rTMS to the hemisphere evoking stronger pharyngeal responses resulted in a significant change in swallowing behaviour, observed as a reduction in the response time to evoke a cued swallow, not seen when applied to the hemisphere evoking weaker responses. This differential effect between the two hemispheres provides supportive evidence for functionally relevant asymmetry of motor cortical control for swallowing, which until now had been based on ambiguous interpretation of map hemispheric differences with functional brain imaging. Our data thus give further credence to the notion that hemispheric dominance and non-dominance exists in the control of human swallowing, and sheds light on the role of the motor cortex in regulating swallowing.
Indeed the ‘speeding up’ of the swallow responses seen in our study to motor cortex suppression is reminiscent of the results of a study by Parker et al. (2004) who assessed swallowing using the timed water swallow test in stroke patients. They observed that dysphagic stroke patients with both motor disability and poor awareness of their dysphagia performed swallowing in a less controlled manner, drinking water faster and in larger volumes than those with better awareness. It is therefore conceivable that the shortened swallow timings in our study represent a maladaptive behavioural response, resulting in poorer overall control of the bolus, with higher potential risk of aspiration. If disruption of the sensorimotor cortex is directly linked to quicker, albeit less controlled, swallowing, then this could be interpreted as indicating that pharyngeal motor cortex has significant inhibitory input to the brainstem, and after damage to this region, there is a lack of inhibitory control, resulting in disordered swallowing. Moreover, given that other cortical regions which are thought to initiate swallowing (e.g. insula, cingulated cortex and supplementary motor area) were not directly suppressed by our protocol, it could be speculated that when motor cortex is disengaged from the cortical swallowing network, the behavioural manifestation in healthy subjects is not a delayed swallow but a faster one. However, in the absence of a more detailed evaluation of the differing phases of swallowing, obtainable for example using videofluoroscopy, albeit with the associated problems of radiation exposure, this suggestion must remain speculative.
As anticipated, challenged swallowing performance showed a trend to a greater general improvement over the course of the study following sham rTMS compared to real rTMS of either hemisphere. Intriguingly, however, active stimulation of both pharyngeal motor cortices appeared to have a lesser effect on improving performance. Given the more complex nature of the challenged swallow task, it is possible that, in contrast to the initiation of swallowing, both hemispheres may be recruited to execute the task, such that disruption of either motor cortex is enough to affect performance. Indeed, Daniels et al. (2006) using a dual task protocol suggested differing roles between the two hemispheres in the mediation of swallowing and that specific components of swallowing may be preferentially mediated by the left versus the right hemisphere. Specifically both right and left hand finger tapping tasks altered both volume per swallow and number of swallows to straw drinking over a 10 s period. This suggests that certain components of swallowing may have more equally distributed hemispheric control mechanisms or rely on bilateral inputs such that interference to either hemisphere can produce behavioural changes. The challenged swallow task may therefore be such a function that requires more bi-hemispheric swallowing inputs.
In conclusion, the inhibition of human pharyngeal motor cortex using rTMS is intensity dependent and optimally achieved using a high intensity 1 Hz protocol. These effects on excitability lasted for at least 45 min and were localized to the stimulated pharyngeal motor cortex, with little evidence for transcallosal interactions. Our data also provide strong evidence that the motor cortex projections to human pharyngeal musculature show functionally relevant asymmetry that correlates with the already recognized anatomical asymmetry seen with brain imaging. Moreover, our data imply that the motor cortex may have a significant inhibitory role in regulating swallowing behaviour at the level of the brainstem and supports the notion that unilateral brain injury disrupts swallowing because of its lateralized properties. However, this lateralization is likely to have degrees of swallow task specificity, with certain components being more bilaterally organized.
Acknowledgments
S.M. and S.S. were supported by a project grant from the Medical Research Council. E.V. was supported by ADIR Assistance (Isneauville, France). S.J. was supported by a project grant from the Stroke Association. This work was also partially funded by the Royal Society.
References
- Andre-Obadia N, Peyron R, Mertens P, Mauguiere F, Laurent B, Garcia-Larrea L. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol. 2006;117:1536–1544. doi: 10.1016/j.clinph.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Benke T, Koylu B, Visani P, Karner E, Brenneis C, Bartha L, Trinka E, Trieb T, Felber S, Bauer G, Chemelli A, Willmes K. Language lateralization in temporal lobe epilepsy: a comparison between fMRI and the Wada Test. Epilepsia. 2006;47:1308–1319. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Brouwer BJ, Schryburt-Brown K. Hand function and motor cortical output poststroke: Are they related? Arch Phys Med Rehabil. 2006;87:627–634. doi: 10.1016/j.apmr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, Hallett M. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Corey DM, Fraychinaud A, DePolo A, Foundas AL. Swallowing lateralization: the effects of modified dual-task interference. Dysphagia. 2006;21:21–27. doi: 10.1007/s00455-005-9007-2. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, Insola A, Tonali PA, Ranieri F, Huang YZ, Rothwell JC. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565:945–950. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol. 2002;113:1136–1141. doi: 10.1016/s1388-2457(02)00145-1. [DOI] [PubMed] [Google Scholar]
- Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, Martin C, Jerabek P. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport. 1997;8:2787–2791. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- Furlong PL, Hobson AR, Aziz Q, Barnes GR, Singh KD, Hillebrand A, Thompson DG, Hamdy S. Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage. 2004;22:1447–1455. doi: 10.1016/j.neuroimage.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Gow D, Rothwell J, Hobson A, Thompson D, Hamdy S. Induction of long-term plasticity in human swallowing motor cortex following repetitive cortical stimulation. Clin Neurophysiol. 2004;115:1044–1051. doi: 10.1016/j.clinph.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes D, Tallis RC, Thompson DG. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet. 1997;350:686–692. doi: 10.1016/S0140-6736(97)02068-0. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Hobson A, Thompson DG. Sensorimotor modulation of human cortical swallowing pathways. J Physiol. 1998;506:857–866. doi: 10.1111/j.1469-7793.1998.857bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, Tallis RC, Thompson DG. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2:1217–1224. doi: 10.1038/nm1196-1217. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol Gastrointest Liver Physiol. 1999a;277:G219–G225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H215O PET activation. J Neurophysiol. 1999b;81:1917–1926. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- Hartnick CJ, Rudolph C, Willging JP, Holland SK. Functional magnetic resonance imaging of the pediatric swallow: imaging the cortex and the brainstem. Laryngoscope. 2001;111:1183–1191. doi: 10.1097/00005537-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin Neurophysiol. 2004;115:1069–1075. doi: 10.1016/j.clinph.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001a;280:G531–G538. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001b;280:G354–G360. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- Lamkadem M, Zoungrana OR, Amri M, Car A, Roman C. Stimulation of the chewing area of the cerebral cortex induces inhibitory effects upon swallowing in sheep. Brain Res. 1999;832:97–111. doi: 10.1016/s0006-8993(99)01483-3. [DOI] [PubMed] [Google Scholar]
- Loose R, Hamdy S, Enck P. Magnetoencephalographic response characteristics associated with tongue movement. Dysphagia. 2001;16:183–185. doi: 10.1007/s00455-001-0062-z. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Martin PG, Gandevia SC, Taylor JL. Theta burst stimulation does not reliably depress all regions of the human motor cortex. Clin Neurophysiol. 2006;117:2684–2690. doi: 10.1016/j.clinph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8:195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Deglutition. Physiol Rev. 1982;62:129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- Modugno N, Nakamura Y, MacKinnon CD, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC. Motor cortex excitability following short trains of repetitive magnetic stimuli. Exp Brain Res. 2001;140:453–459. doi: 10.1007/s002210100843. [DOI] [PubMed] [Google Scholar]
- Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140:280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope. 1999;109:1417–1423. doi: 10.1097/00005537-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Parker C, Power M, Hamdy S, Bowen A, Tyrrell P, Thompson DG. Awareness of dysphagia by patients following stroke predicts swallowing performance. Dysphagia. 2004;19:28–35. doi: 10.1007/s00455-003-0032-8. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54:956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18:71–77. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Oliveri M, Gangitano M, Pascual-Leone A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport. 2001;12:3845–3848. doi: 10.1097/00001756-200112040-00048. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998;250:141–144. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Abe S, Ishikawa T, Yamada Y, Yamane GY. Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia. 2004;19:100–108. doi: 10.1007/s00455-003-0509-5. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol. 1999;46:281–286. [PubMed] [Google Scholar]