Abstract
Long-term depression (LTD) of excitatory transmission at cerebellar parallel fibre–Purkinje cell synapses is a form of synaptic plasticity crucial for cerebellar motor learning. Around the postsynaptic membrane of these synapses, B-type γ-aminobutyric acid receptor (GABABR), a Gi/o protein-coupled receptor for the inhibitory transmitter GABA is concentrated and closely associated with type-1 metabotropic glutamate receptors (mGluR1) whose signalling is a key factor for inducing LTD. We found that in cultured Purkinje cells, GABABR activation enhanced LTD of a glutamate-evoked current (LTDglu), increasing the magnitude of depression. It has been reported that parallel fibre–Purkinje cell synapses receive a micromolar level of GABA spilt over from the synaptic terminals of the neighbouring GABAergic interneurons. This level of GABA was able to enhance LTDglu. Our pharmacological analyses revealed that the βγ subunits but not the α subunit of Gi/o protein mediated GABABR-mediated LTDglu enhancement. Gi/o protein activation was sufficient to enhance LTDglu. In this respect, LTDglu enhancement is clearly distinguished from the previously reported GABABR-mediated augmentation of an mGluR1-coupled slow excitatory postsynaptic potential. Baclofen application for only the induction period of LTDglu was sufficient to enhance LTDglu, suggesting that GABABR signalling may modulate mechanisms underlying LTDglu induction. Baclofen augmented mGluR1-coupled Ca2+ release from the intracellular stores in a Gi/o protein-dependent manner. Therefore, GABABR-mediated LTDglu enhancement is likely to result from augmentation of mGluR1 signalling. Furthermore, pharmacological inhibition of GABABR reduced the magnitude of LTD at parallel fibre–Purkinje cell synapses in cerebellar slices. These findings demonstrate a novel mechanism that would facilitate cerebellar motor learning.
In the cerebellar cortex, individual Purkinje cells integrate excitatory synaptic inputs from numerous parallel fibres, the axons of granule cells which receive mossy fibre inputs that convey sensory information arising from various parts of the body and motor command signals from the upper centres (Thach et al. 1992; Llinas et al. 2003). The efficacy of transmission at certain parallel fibre–Purkinje cell synapses undergoes long-term depression (LTD) following correlated transmission at these synapses and climbing fibre–Purkinje cell synapses (Ito, 2002). Cerebellar LTD modifies information flow along the cerebellum and is therefore crucial for cerebellar motor learning (Ito, 2002).
Purkinje cells express a high density of B-type γ-aminobutyric acid receptor (GABABR), a Gi/o protein-coupled receptor for the inhibitory neurotransmitter GABA (Jones et al. 1998; Kaupmann et al. 1998; Kuner et al. 1999). Interestingly, GABABR is concentrated on the annuli of parallel fibre-innervated dendritic spines (Ige et al. 2000; Kulik et al. 2002) where LTD occurs. When the neighbouring interneurons are stimulated at a relatively high frequency, GABABR in the dendritic spines may receive 5–10 μm GABA spilt over from the synapses of the interneurons (Dittman & Regehr, 1997). GABABR has a high enough affinity (EC50, ∼1 μm) to sense this level of GABA (Sodickson & Bean, 1996). GABABR activated by spilt-over GABA could possibly influence the signalling of type-1 metabotropic glutamate receptor (mGluR1), a Gq/11 protein-coupled receptor, because these receptors colocalize at the annuli of Purkinje cell dendritic spines (Lujan et al. 1997; Kulik et al. 2002) and are likely to form complexes (Tabata et al. 2004). mGluR1 signalling is an essential factor for inducing LTD (Conquet et al. 1994; Shigemoto et al. 1994; Ichise et al. 2000; Ito, 2002); this signalling leads to the facilitated endocytosis of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptor at parallel fibre–Purkinje cell synapses (Matsuda et al. 2000; Wang & Linden, 2000; Chung et al. 2003). Therefore, possible influence of GABABR on mGluR1 could modulate the induction of LTD.
In this study, we tested this possibility and explored the underlying mechanism. In cerebellar slice preparations, pharmacological manipulation of GABABR should affect glutamate release by the parallel fibres and climbing fibres through modulating the presynaptic mechanisms (Dittman & Regehr, 1996). This masks the postsynaptic action of GABABR on LTD. Thus, we employed an in vitro experimental system using cultured Purkinje cells, in which parallel and climbing fibre inputs are replaced with local application of glutamate and somatic depolarization, respectively (Linden et al. 1991). In this system, a glutamate-evoked current corresponds to parallel fibre-mediated excitatory postsynaptic current and undergoes LTD following conjunctive glutamate/depolarization stimuli (LTDglu).
We show here that postsynaptic GABABR activation enhances LTDglu, increasing the magnitude of depression. Our pharmacological analyses indicate that this LTDglu enhancement is likely to result from Gi/o protein-mediated augmentation of mGluR1 signalling. Furthermore, we show that GABABR indeed influences LTD of parallel fibre–Purkinje cell excitatory postsynaptic currents (EPSCs) in cerebellar slices.
These findings together demonstrate a novel mechanism that would facilitate cerebellar motor learning.
Methods
Cell Culture
Cerebellar cell culture was prepared as described elsewhere (Tabata et al. 2000). Briefly, perinatal C57BL/6 embryos were caesarean-sectioned from pregnant mice deeply anaesthetized and killed with diethylether or isoflurane. The embryos were deeply anaesthetized by cooling in chilled phosphate-buffered saline and then killed by decapitation. Cerebellar neurons were obtained from these embryos, dissociated with trypsin, and plated on plastic dishes or low-fluorescence plastic films (Sumilon, MS-92132, Sumitomo, Tokyo, Japan). The cells were cultured in a low-serum medium for 9–22 days. Purkinje cells were identified by their large somata (18 μm or greater) and/or thick primary dendrites. This procedure fully conforms to the guidelines administered by the committee on animal experiments of Osaka University.
Slice preparation
Cerebellar slices were prepared as described elsewhere (Kakizawa et al. 2000; Kakizawa et al. 2005; Kakizawa et al. 2007). Briefly, 3-week-old C57BL/6 mice were deeply anaesthetized with diethylether and then killed by decapitation. Cerebella obtained from these mice were sliced along the parasagittal planes to a thickness of 250 μm. This procedure fully conforms to the guidelines established by the Animal Welfare Committee of the University of Tokyo.
Electrophysiology
Somatic whole-cell voltage-clamp recordings were made from cultured Purkinje cells in dishes, using a perforated-patch technique (holding potential after the correction of a liquid junction potential between the pipette and bath solutions, −70 mV). The recording pipette contained (mm): 95 Cs2SO4, 15 CsCl, 0.4 CsOH, 8 MgCl2, 10 Hepes and 500 μg ml−1 amphotericin B (pH 7.35). The bath was perfused at a rate of 1–2 ml min−1 with a saline consisting of (mm): 140 NaCl, 5 KCl, 2 CaCl2, 0.8 MgCl2, 10 Hepes, 0.5 μm tetrodotoxin, and 10 μm (−)-bicuculline methochloride (pH 7.3, 25°C). Signals were sampled at 5 kHz, using an EPC-8 amplifier (HEKA, Lambreht, Germany) driven by PULSE software (versions 8.77, HEKA).
In the measurements using cerebellar slices, somatic whole-cell voltage-clamp recordings (holding potential after the correction of the liquid junction potential, −80 to −90 mV) were made from Purkinje cells, using pipettes (2.5–3.5 MΩ) containing (mm): 60 CsCl, 40 caesium d-gluconate, 20 TEA-Cl, 1 EGTA, 4 MgCl2, 4 ATP, 0.4 GTP, and 30 Hepes (pH 7.3). The bath solution contained (mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3 and 20 glucose (24–25°C), which was bubbled continuously with a mixture of 95% O2 and 5% CO2. Bicuculline (10 μm) was always present in the saline to block spontaneous inhibitory postsynaptic currents. Parallel fibre–Purkinje cell EPSCs were monitored delivering electrical pulses (duration, 0.1 ms) to the parallel fibres through a glass pipette filled with the bath solution at 0.1 Hz except for the period of LTD induction. The intensity of the pulses was adjusted as to evoke parallel fibre–Purkinje cell EPSCs with a basal amplitude of 100–150 pA. Signals were sampled at 20 kHz, using an EPC-9 amplifier (HEKA). After stable basal recording was obtained for at least 10 min, LTD was induced with a conjunctive stimulation protocol stated in the corresponding figure legend.
Ca2+ imaging
Cerebellar neurons on a film were incubated with fura-2 acetoxymethyl ester (5 μm) at 37°C for 15 min. Then, the film was placed on a glass-based recording chamber and perfused at a rate of ∼0.8 ml min−1 with saline (see above) supplemented with 6-nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione (NBQX) and (3-[(R)-2-carboxypiperazin-4-yl]propyl-1-phosphonic acid (R-CPP). Intracellular free calcium ion concentration ([Ca2+]i)-dependent fluorescence signals were captured at 2–5 Hz, using an imaging system (Polychrome II, TILL, Planegg, Germany) attached to an inverted microscope (IX70 with a ×20 objective lens of NA 0.75, Olympus, Tokyo, Japan). The amplitude of a [Ca2+]i rise was expressed as a change in the ratio of somatic fluorescence signals excited at 340 and 380 nm (exposure duration, 40 and 20 ms, respectively) (F340/F380).
Drug application
Test drugs were dissolved into water to concentrations 1000 times higher than the final levels, kept at 4 or −20°C until use, and diluted into saline on the day of recordings unless otherwise stated. Forskolin and thapsigargin were dissolved into DMSO to concentrations of 5000 and 10 000 times higher than the final levels, respectively, and kept at −20°C until use. Glutamate was applied iontophoretically (30 ms, 0.05 Hz) through a glass pipette filled with 10 mm l-glutamate and 10 mm Hepes (pH adjusted to 7.1 with NaOH, kept at −20°C until use) and located about 20 μm from the branching point of a primary dendrite of the examined cell. The level of ejection current was adjusted (200–800 nA, 30 ms) so that the peak amplitude of basal glutamate-evoked currents were in a range of 100–300 pA. Bath application of baclofen, GABA, mastoparan, and forskolin was done by perfusing the recording chamber at rate of 1–2 ml min−1 with the drug-containing saline. Local application of (R,S) - 3,5-dihydroxyphenylglycine (DHPG), baclofen, and K+-rich saline was done by delivering the drug-containing saline through a wide-tipped pipette located near the examined cell under the control of gravity. In some experiments, pertussis toxin (PTX, reconstituted in the culture medium at a concentration 100 times higher than the final level and kept at 4°C until use) was added to the culture medium 16–24 h before recordings.
Data analysis
The peak amplitude of a response was measured as a difference from the prestimulus level to the maximal deflection throughout the record (glutamate-evoked current) or during a 10 s (DHPG-evoked [Ca2+]i rises) or 1 s (K+-evoked [Ca2+]i rises) agonist application.
In measurements using cerebellar slices, the amplitudes of parallel fibre–Purkinje cell EPSCs were normalized to mean over a 10 min basal recording. For each Purkinje cell, the magnitude of LTD was evaluated as the mean of parallel fibre–Purkinje cell EPSCs over 26–30 min after the conjunctive stimuli. Data were discarded in the following cases: (i) either the series resistance or membrane resistance changed by more than 10% during a recording session, (ii) the slope of parallel fibre–Purkinje cell EPSC amplitude changed by more than 2% during the 10 min basal recording, and/or (iii) the amplitude continued to change over 30 min after the formation of whole-cell configuration (Namiki et al. 2005; Kakizawa et al. 2007).
Groups of numerical data are presented as means ± s.e.m. Statistical differences were examined by two-sided Mann–Whitney's U test unless otherwise stated.
Immunohistochemistry
Cerebellar cells cultured on films were fixed sequentially with 0.1 m sodium phosphate buffer containing 4% paraformaldehyde at room temperature for 30 min and with 100% methanol at room temperature for 10 min. The fixed cells were rinsed with phosphate-buffered saline (PBS) for three times and then treated with PBS containing 0.1% Triton X-100 and 10% normal donkey serum at room temperature for 30 min (Kamikubo et al. 2006). The treated cells were incubated with a primary antibody against phospholipase C (PLC) β3 and PLCβ4 (raised in guinea pigs and rabbits, respectively; Nakamura et al. 2004; Nomura et al. 2007), GABABR1 subunit (GBR1; raised in rabbits; Kulik et al. 2002), mGluR1 (raised in guinea pigs; Tanaka et al. 2000), or calbindin (raised in mice; C9848, Sigma, St Louis, MO, USA) (1 μg ml−1 in the Triton X-100/serum-containing PBS) at 4°C overnight and then treated with a secondary antibody conjugated with indocorbocyanine (Cy3; Jackson ImmunoResearch, West Grove, PA, USA) or Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA) 1: 200 in the Triton X-100/serum-containing PBS at room temperature for 2.5 h. The film was mounted on a glass slide with Perma Fluor Aquaus mounting medium (434980, Thermo, Pittsburgh, PA, USA), and the cells were examined using a confocal laser microscope (LSM510, Carl Zeiss Göttingen, Germany). The fluorescent signals of Cy3 (red) and Alexa Fluor 488 (green) were excited at 543 and 488 nm, respectively.
Results
GABABR activation enhances LTDglu
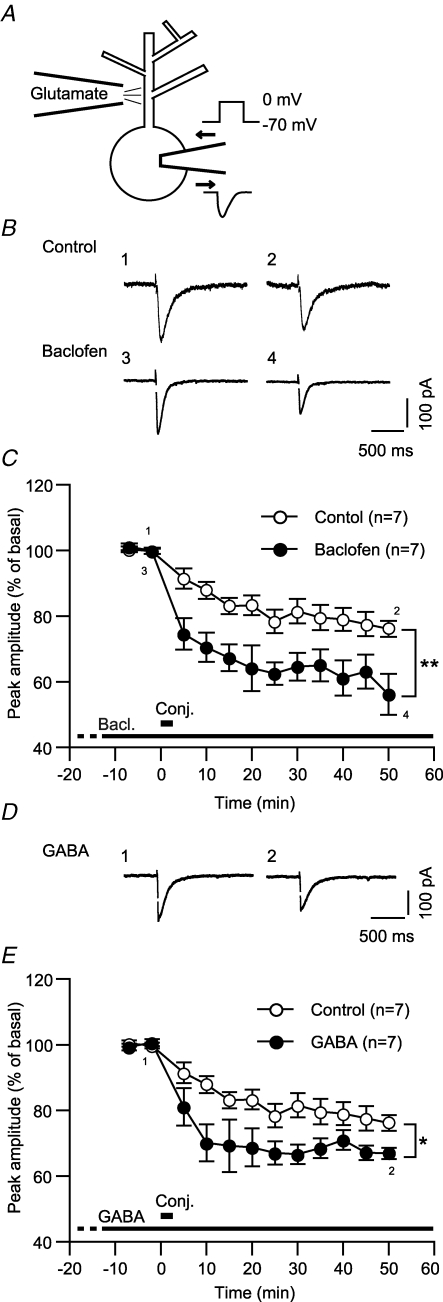
We first assessed whether and how GABABR activation affects LTD of a glutamate-evoked current (LTDglu) in cultured Purkinje cells. We iontophoretically applied glutamate to the dendrite of the examined cell for every 20 s and monitored glutamate-evoked inward currents in a perforated-patch whole-cell mode (holding potential, −70 mV) (Fig. 1A). After the basal recording, we gave six sets of depolarizing voltage steps (0 mV, 3 s) through the recording pipette in conjunction with glutamate iontophoresis (Fig. 1A). In the normal saline (control), the glutamate-evoked current underwent depression lasting over 50 min following the conjunctive stimuli (Fig. 1B and C; ‘Control’). The peak amplitude of the glutamate-evoked current was reduced to 76.2 ± 2.4% of the basal level (n = 7) at the end of the recording sessions (48–52 min after the conjunctive stimuli) (Fig. 1C). We found that GABABR activation enhanced LTDglu, increasing the magnitude of depression (Fig. 1B and C). In the continuous presence of baclofen, a GABABR-selective agonist (3 μm), the peak amplitude was reduced to 56.2 ± 6.2% (n = 7) at the end of the recording sessions (Fig. 1C). This value was significantly smaller than the control (Fig. 1C). LTDglu enhancement emerged immediately after the conjunctive stimuli. As early as 5 min after the conjunctive stimuli, the peak amplitude with baclofen (74.6 ± 4.8%, n = 7) was already significantly smaller than that with the normal saline (91.4 ± 3.0%, n = 7) (Fig. 1C). The early emergence of enhancement indicates that GABABR signalling may modulate the induction process of LTDglu. Moreover, baclofen (3 μm, 15 min) did not reduce the peak amplitude of a glutamate-evoked inward current as its direct effect (102.7 ± 1.6%, n = 7; data not illustrated). Thus, LTDglu enhancement cannot be ascribed to modulation of AMPA-type glutamate receptor channels by GABABR.
Figure 1. GABABR activation enhances LTDglu in cerebellar Purkinje cells.
A, setting of the measurement of long-term depression of glutamate-evoked current (LTDglu). Glutamate was iontophoretically applied to the first branching point of a dendrite of the examined cell (100–300 pA, 30 ms) for every 20 s. Glutamate-evoked inward currents were monitored under voltage clamp at −70 mV. To induce LTDglu, six sets of depolarizing voltage steps (0 mV, 3 s) were given through the recording pipette in conjunction with glutamate iontophoresis. B and C, baclofen, a GABABR-selective agonist enhanced LTDglu. B, each pair of traces indicates sample glutamate-evoked currents of a cell before and after the conjunctive depolarization/glutamate stimuli in the absence (‘Control’) or continuous presence of baclofen (3 μm). The time when the traces were recorded are indicated by the corresponding integers in the plots (C). C, each plot indicates the time course of the mean peak amplitude of the glutamate-evoked current in the absence or continuous presence of baclofen. Thick bar, timing of the conjunctive stimuli. Dot, data binned for each 5 min period. Error bar, ± s.e.m. *P < 0.05; **P < 0.01, Mann–Whitney's U test. These conventions of data acquisition and representation also apply to the following figures. D and E, GABA, a native GABABR agonist enhanced LTDglu. D, sample responses of a cell before and after the conjunctive stimuli in the continuous presence of GABA (3 μm). E, time courses of the mean peak amplitudes of glutamate-evoked currents in the absence or continuous presence of GABA.
A previous study (Dittman & Regehr, 1997) suggests that the parallel fibre-innervated dendritic spines of Purkinje cells may receive 5–10 μm GABA spilt over from neighbouring inhibitory neurons' synapses in situ. In the continuous presence of a comparable dose (3 μm) of GABA, the peak amplitude of the glutamate-evoked current (66.9 ± 1.7%, n = 7 at 48–52 min after the conjunctive stimuli) was significantly smaller than the control (Fig. 1D and E). Thus, GABABR-mediated LTDglu enhancement could occur under physiological conditions.
The βγ subunits of Gi/o protein may mediate GABABR-mediated LTDglu enhancement
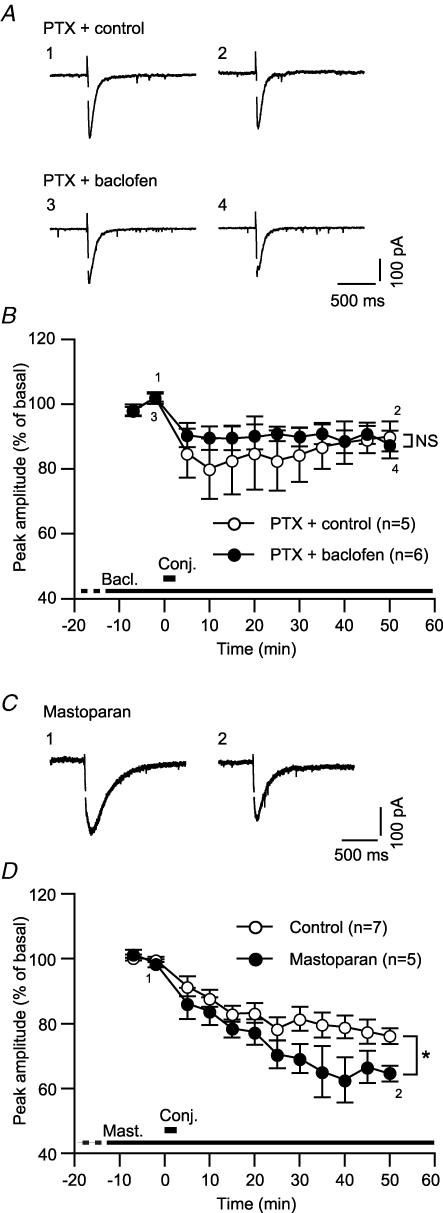
In the following sections, we explored mechanisms underlying GABABR-mediated LTDglu enhancement. To test the involvement of Gi/o protein, the primary messenger coupled to GABABR, we assessed LTDglu in Purkinje cells pretreated with pertussis toxin (PTX), a Gi/o protein inhibitor (500 ng ml−1, > 16 h). This pretreatment is reported to eliminate a Gi/o protein-coupled inwardly rectifying K+ current in Purkinje cells (Tabata et al. 2005). In the normal saline, the conjunctive stimuli induced LTDglu in the PTX-pretreated cells while the magnitude of LTDglu (peak amplitude at 48–52 min after the conjunctive stimuli, 90.1 ± 4.6%, n = 5) was small compared with that in the untreated cells (Fig. 2A and B; cf. Fig. 1C). PTX pretreatment by itself might impede LTDglu (see Discussion). Addition of baclofen (3 μm) to the saline did not increase the magnitude of LTDglu in the PTX-treated cells (87.5 ± 4.3%, n = 6) (Fig. 2A and B). This result suggests the involvement of Gi/o protein in GABABR-mediated LTD enhancement.
Figure 2. Gi/o protein mediates GABABR-mediated LTDglu enhancement.
A and B, PTX, a Gi/o protein inhibitor abolished baclofen-induced LTDglu enhancement. A, each pair of traces indicates sample glutamate-evoked currents of a PTX (500 ng ml−1, > 16 h)-pretreated cell before and after the conjunctive depolarization/glutamate stimuli in the absence (‘Control’) or continuous presence (‘Baclofen’) of baclofen (3 μm). B, time courses of the mean peak amplitudes of glutamate-evoked currents in the absence and continuous presence of baclofen. C and D, mastoparan, a Gi/o protein agonist, mimicked GABABR-mediated LTDglu enhancement. C, each pair of traces indicates sample glutamate-evoked currents of a cell before and after the conjunctive depolarization/glutamate stimuli in the absence or continuous presence of mastoparan (1 μm). D, time courses of the mean peak amplitudes of glutamate-evoked currents in the absence (‘Control’) and continuous presence of mastoparan.
In supporting the above result, addition of mastoparan, a Gi/o protein agonist (1 μm) to the saline significantly increased the magnitude of LTDglu (peak amplitude at 48–52 min after the conjunctive stimuli, 64.6 ± 2.4%, n = 5). This drug directly activates Gi/o protein by facilitating GTP binding to Gi/o protein without the aid of G protein-coupled receptors (Higashijima et al. 1988; Shpakov & Pertseva, 2006). Thus, activation of Gi/o protein is sufficient to enhance LTDglu.
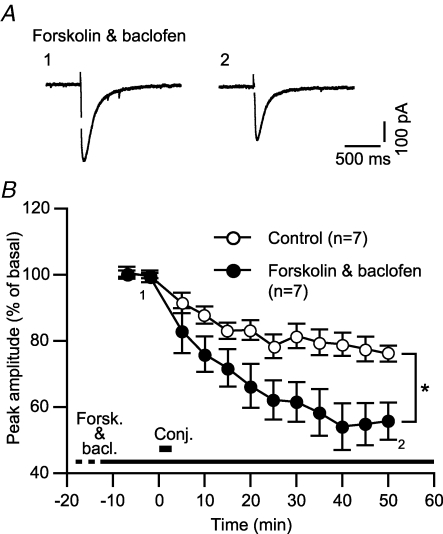
Upon activation, Gi/o protein is cleaved into the α and βγ subunits (Gαi/o and Gβγi/o, respectively). We determined the relative contribution of these subunits to LTDglu enhancement. The main action of active Gαi/o is inhibition of adenylyl cyclase, which results in a reduction in cAMP level. We interfered with this action by adding forskolin (20 μm), a potent activator of adenylyl cyclase to the saline. This dose of forskolin is shown to indeed raise cAMP level in cultured Purkinje cells (Tabata et al. 2007). It is reported that in a neuron, a similar dose (10 μm) of forskolin activates adenylyl cyclase regardless of the presence or absence of micromolar levels of baclofen (Onali et al. 2003). Despite such an effect of forskolin, baclofen enhanced LTDglu (peak amplitude at 48–52 min after the conjunctive stimuli, 55.7 ± 4.3%, n = 7) (Fig. 3A and B). This result suggests that Gβγi/o may primarily mediate LTDglu enhancement.
Figure 3. Gαi/o does not mediate GABABR-mediated LTDglu enhancement.
A and B, forskolin, an adenylyl cyclase activator did not abolish baclofen-induced LTDglu enhancement. A, sample glutamate-evoked currents of a cell before and after the conjunctive depolarization/glutamate stimuli in the continuous presence of forskolin (20 μm) and baclofen (3 μm). B, time courses of the mean peak amplitudes of glutamate-evoked currents in the absence (‘Control’) and the continuous presence (‘Forskolin & Baclofen’) of forskolin (20 μm) and baclofen (3 μm).
GABABR-mediated LTDglu enhancement is attributable to augmentation of mGluR1 signalling
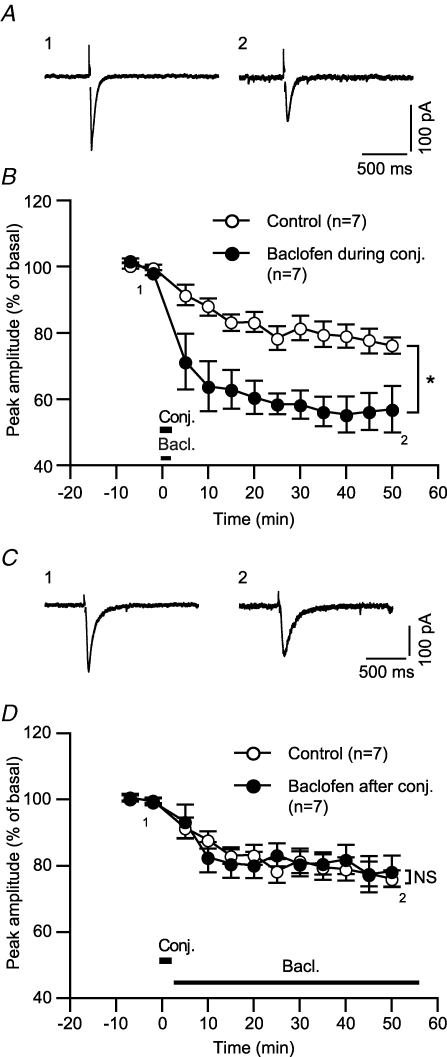
The immediate emergence of GABABR-mediated LTDglu enhancement (see above, Fig. 1A and B) indicates that GABABR signalling may modulate mechanisms underlying LTD induction. This possibility is further supported by experiments with pin-point application of baclofen at either the induction phase or expression/maintenance phase of LTDglu. When applied only during the conjunctive stimuli, baclofen enhanced LTDglu (Fig. 4A and B). By contrast, when applied after the conjunctive stimuli, baclofen failed to enhance LTDglu (Fig. 4C and D).
Figure 4. GABABR activation during the induction phase of LTDglu is sufficient for LTDglu enhancement.
A and B, baclofen applied during the conjunctive depolarization/glutamate stimuli enhanced LTDglu. A, sample glutamate-evoked currents of a cell before and after the conjunctive depolarization/glutamate stimuli with baclofen (3 μm) application during the stimuli. B, time courses of the mean peak amplitudes of glutamate-evoked currents with or without (‘Control’) baclofen application during the conjunction stimuli. C and D, baclofen applied after the conjunctive depolarization/glutamate stimuli did not enhance LTDglu. C, sample glutamate-evoked currents of a cell before and after the conjunctive depolarization/glutamate stimuli with baclofen (3 μm) application after the stimuli. D, time courses of the mean peak amplitudes of glutamate-evoked currents with or without (‘Control’) baclofen application after the conjunction stimuli.
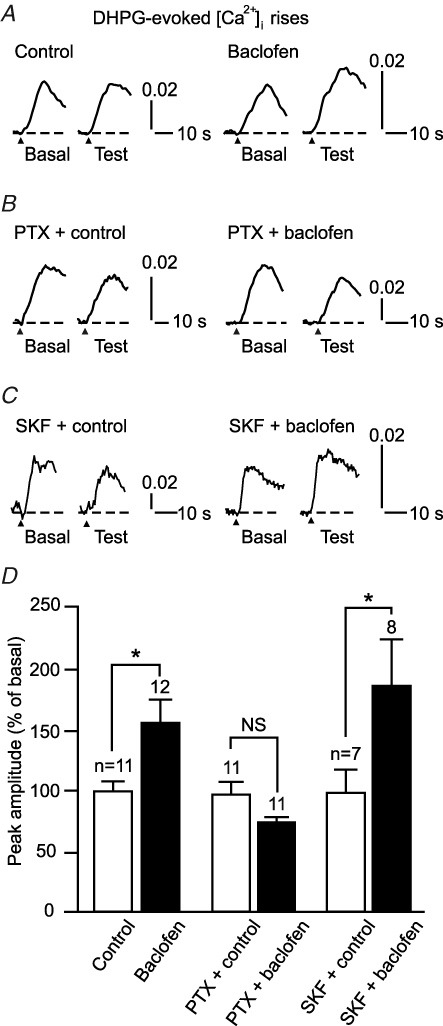
In cultured Purkinje cell preparations, the key factors for triggering LTD induction are glutamate-evoked mGluR1 signalling and depolarization-evoked Ca2+ influx (Linden et al. 1991; Ito, 2002). GABABR could enhance LTDglu by augmenting either or both of these factors. We tested this possibility, using Ca2+ imaging. First, we examined the effect of baclofen on an mGluR1-mediated [Ca2+]i rise evoked by DHPG, a group I mGluR agonist (5 μm, 10 s) (Fig. 5). In the recording conditions used here (the excitability of the cells was pharmacologically attenuated, see Methods), this response mainly reflects Ca2+ release from the intracellular stores (Sato et al. 2004) (see Fig. 7C). mGluR1 promotes the opening of Ca2+-permeable inositol trisphosphate receptor channel (IP3R) on these stores via the Gq/11 protein-phospholipase C (PLC)-IP3 cascade (Ito, 2002). Baclofen (3 μm) but not the normal saline augmented the peak amplitude (expressed in a change in F340/F380) of the [Ca2+]i rise (154.9 ± 19.4%, n = 12 and 98.8 ± 8.0%, n = 11, respectively; Fig. 5A and D). Pretreatment with PTX (500 ng ml−1, > 16 h) abolished this augmentation (Fig. 5B and D). These results suggest that GABABR potentiates mGluR1 signalling via Gi/o protein. The resting [Ca2+]i level (mean F340/F380 over 5 s prior to DHPG application) changed little during application of the normal saline or baclofen (99.2 ± 1.1%, n = 11 and 92.0 ± 3.2%, n = 12, respectively; data not illustrated). Thus, the augmentation cannot be ascribed to [Ca2+]i-dependent facilitation of Ca2+ release (Llano et al. 1994). Moreover, baclofen augmented the [Ca2+]i rise in the presence of SKF96365, an antagonist against receptor-operated Ca2+ channels including TRPC1 (30 μm, Fig. 5C). The extent of this augmentation with SKF96365 (184.2 ± 36.0%, n = 8; Fig. 5D) was comparable with that in the normal saline (Fig. 5A and D). This result suggests that this augmentation is due largely to an increase of Ca2+ release from the intracellular stores; the contribution of an increase of Ca2+ influx through receptor-operated channels might be small although we do not exclude that the [Ca2+]i rise includes such Ca2+ influx. These results suggest that GABABR signalling potentiates mGluR1 signalling via Gi/o protein.
Figure 5. GABABR activation augments mGluR1 signalling in a Gi/o protein-dependent manner.
A–D, baclofen induced a Gi/o protein-dependent augmentation of a DHPG-evoked [Ca2+]i rise that reflects mGluR1-coupled intracellular Ca2+ store release. A–C, sample responses of cells untreated (A), pretreated with PTX (500 ng ml−1, > 16 h; B), and perfused with SKF96365 (30 μm, C). Each pair of traces indicates sample DHPG (5 μm, 10 s)-evoked responses of a cell before (‘Basal’) and after (‘Test’) a 12 min application of the normal saline (‘Control’) or baclofen (3 μm). Arrowhead, DHPG onset. Vertical scale bar, change in F340/F380. D, mean peak amplitudes of the DHPG-evoked [Ca2+]i rise after application of the normal saline or baclofen (expressed as percentage of the basal levels).
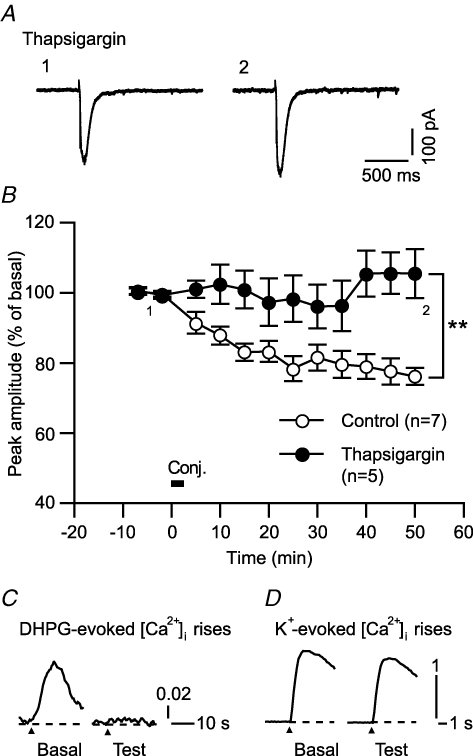
Figure 7. mGluR1-mediated Ca2+ release from the intracellular stores is important for inducing LTDglu.
A and B, thapsigargin (endoplasmic reticular Ca2+-ATPase inhibitor), which was expected to deplete Ca2+ in the intracellular stores, abolished LTDglu. A, sample glutamate-evoked currents of a cell before and after the conjunctive depolarization/glutamate stimuli in the continuous presence of thapsigargin (1 μm). B, time courses of the mean peak amplitudes of glutamate-evoked currents in the absence and continuous presence of thapsigargin. C, thapsigargin indeed abolished mGluR1-mediated Ca2+ release from the intracellular stores. Traces indicate sample DHPG (5 μm, 10 s)-evoked [Ca2+]i rises of a cell before and after application of thapsigargin (1 μm, 12 min). Similar results were obtained from 4 cells. D, tharpsigargin did not abolish depolarization-evoked Ca2+ influx. Traces indicate sample K+ (75 mm, 1 s)-evoked [Ca2+]i rises of a cell before and after application of thapsigargin (1 μm, 12 min). Similar results were obtained from 9 cells.
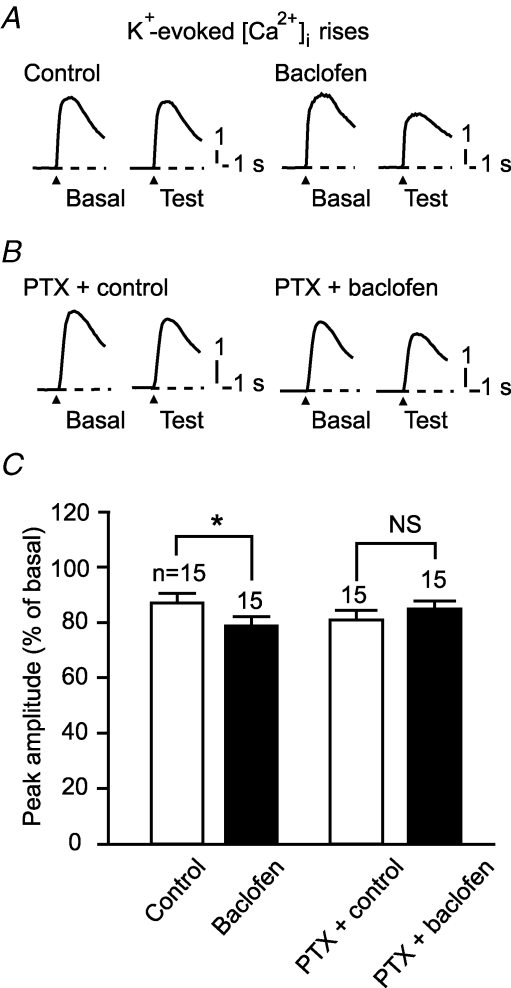
Second, we examined the effect of baclofen on a [Ca2+]i rise evoked by application of K+-rich saline ([K+] = 75 mm, 1 s) (Fig. 6). This response was susceptible to ω-agatoxin IVA (100 nm), an antagonist against the dominant type (P/Q-type) of voltage-gated Ca2+ channels in Purkinje cells (n = 6, data not illustrated), suggesting that this response reflects Ca2+ influx through voltage-gated channels. Baclofen did not augment the K+-evoked [Ca2+]i rise but rather reduced it (peak amplitude, 79.0 ± 2.8%, n = 15) (Fig. 6A and C). Pretreatment with PTX (500 ng ml−1, > 16 h) abolished the baclofen-induced reduction of the K+-evoked [Ca2+]i rise (Fig. 6B and C).
Figure 6. GABABR activation does not augment depolarization-evoked Ca2+ influx.
A–C, baclofen did not augment but rather reduced a [Ca2+]i rise evoked by the K+-rich saline ([K+] = 75 mm, 1 s). A and B, sample responses of untreated or PTX (500 ng ml−1, > 16 h)-pretreated cells. Each pair of traces indicates sample K+-evoked [Ca2+]i rises of a cell before and after a 12 min application of the normal saline (‘Control’) or baclofen (3 μm). Arrowhead, K+-rich saline onset. C, mean peak amplitudes of the DHPG-evoked [Ca2+]i rise after application of the normal saline or baclofen (expressed as percentage of the basal levels).
The results in Fig. 5 imply that GABABR-mediated LTDglu enhancement could be due partly to augmentation of mGluR1-coupled Ca2+ release from the intracellular stores. This notion would be in a good agreement with previous reports that mGluR1-coupled Ca2+ release is essential for inducing LTD in cerebellar slices (e.g. Inoue et al. 1998). However, there is a report (Narasimhan et al. 1998) that this is not the case in certain cultured Purkinje cell preparations. We checked the dependence of LTDglu upon mGluR1-coupled Ca2+ release in our cultured Purkinje cell preparations (Fig. 7). We depleted Ca2+ from the intracellular stores by adding thapsigargin, an endoplasmic reticular Ca2+-ATPase inhibitor, to the saline. Under this condition, the conjunctive stimuli failed to induce LTDglu (105.5 ± 7.0% at 48–52 min after the conjunctive stimuli, n = 5; Fig. 7A and B). Thapsigargin (1 μm, 12 min) suppressed the DHPG (5 μm, 10 s)-evoked [Ca2+]i rise as its direct effect (peak amplitude, 13.6 ± 3.6%, n = 4; Fig. 7C), confirming that thapsigargin indeed abolished mGluR1-mediated Ca2+ release from the intracellular stores. On the other hand, thapsigargin (1 μm, 12 min) little affected the K+ (75 mm, 1 s)-evoked Ca2+ influx (98.8 ± 8.0%, n = 9, Fig. 7D). These results suggest that mGluR1-coupled Ca2+ release from the intracellular stores is important for inducing LTDglu at least in our preparations.
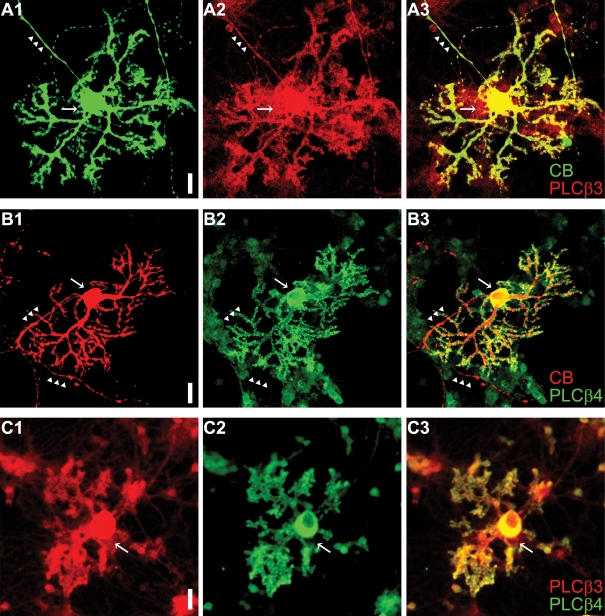
The result in Fig. 3 suggests that Gβγi/o mediates GABABR-mediated LTDglu enhancement. In various cell types, Gβγi/o augments Gq/11 protein-coupled Ca2+ release from the intracellular stores by priming some PLC isoforms including PLCβ3 (for review, Park et al. 1993; Selbie & Hill, 1998). Such priming could underlie GABABR-mediated augmentation of mGluR1-coupled Ca2+ release because Purkinje cells more or less express PLCβ3 in vivo (Kano et al. 1998; Nomura et al. 2007). We checked whether cultured Purkinje cells express PLCβ3 by immunohistochemistry. Cultured Purkinje cells were identified by immunoreactivity against calbindin (green in Fig. 8A1 and red in Fig. 8B1). For all the calbindin-positive cells, PLCβ3 immunoreactivity was found throughout the somata, axons and dendrites (n = 34, Fig. 8A). A similar subcellular distribution is seen in PLCβ3-positive Purkinje cells in vivo (Nomura et al. 2007). It is reported that a subpopulation of Purkinje cells in vivo express a low level of PLCβ3 while complementarily expressing a high level of PLCβ4 (Sarna et al. 2006) that is insensitive to Gβγi/o (Park et al. 1993). Such heterogeneity of PLC isoforms was absent in the culture system. All of the calbindin-positive cells displayed PLCβ4 immunoreactivity (n = 42, Fig. 8B). PLCβ4 immunoreactivity was confined to the somata and dendrites as shown in vivo (Nakamura et al. 2004). Furthermore, all of the cells showing PLCβ3 immunoreactivity also displayed PLCβ4 immunoreactivity in the culture system (n = 28, Fig. 8C). These results suggest that priming of PLCβ3 by Gβγi/o may occur in most cultured Purkinje cells.
Figure 8. Cultured Purkinje cells express both PLCβ3 and β4.
A and B, Purkinje cells (14 days old in vitro) identified by calbindin (‘CB’) immunoreactivity (green in A1 and red in B1) are immunostained with anti-PLCβ3 (red in A2) and anti-PLCβ4 (green in B2) antibodies. Arrows and arrowheads, somata and axons, respectively. Scale bar, 20 μm. The almost complete overlap of PLCβ3 and calbindin signals of the cell (superimposed images in A3) suggests that PLCβ3 is expressed throughout the soma, dendrites and axon of the Purkinje cell. By contrast, PLCβ4 immunoreactivity is found in the soma and dendrites but not in the axon (B3). C, a Purkinje cell (14 days old in vitro) displays both PLCβ3 and PLCβ4 immunoreactivities.
Co-localization of GABABR and mGluR1 in cultured Purkinje cells
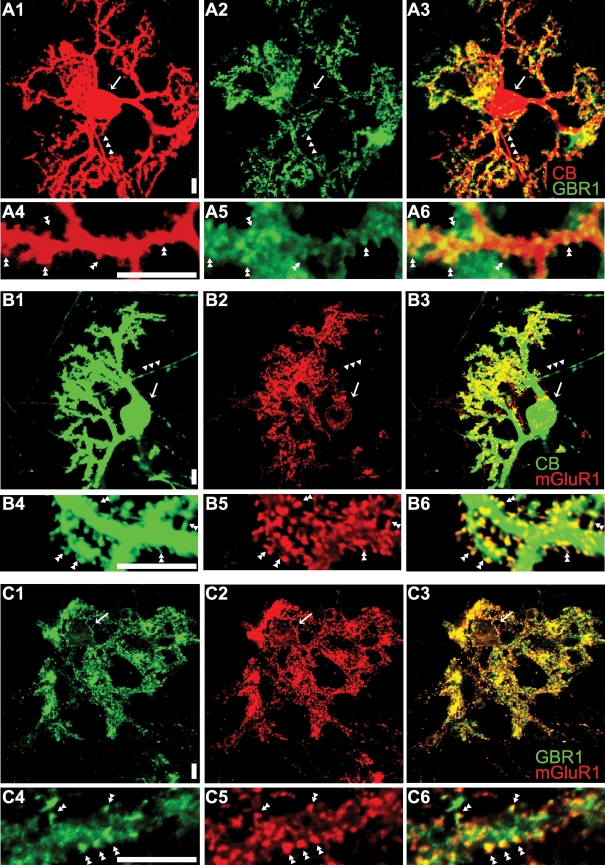
To explore the possible site for the functional interaction of GABABR and mGluR1 signalling cascades, we immunostained cultured Purkinje cells with antibodies against these receptors. Calbindin-positive cells had clear immunoreactivities for GBR1 (Fig. 9A) and mGluR1 (Fig. 9B). Higher levels of immunoreactivities were found at the dendritic spines of these cells (Fig. 9A4–6 and B4–6). Co-immunostaining with the anti-GBR1 and anti-mGluR1 antibodies shows that the subcellular distributions of GABABR and mGluR1 overlap each other (Fig. 9C). These results suggest that co-localization of these receptors at the dendritic spines seen in situ (Lujan et al. 1997; Kulik et al. 2002) is preserved in our cultured cell preparations.
Figure 9. Co-localization of GABABR and mGluR1 in cultured Purkinje cells.
A and B, Purkinje cells (20 days old in vitro) identified by calbindin (‘CB’) immunoreactivity (A1 and B1) are immunostained with anti-GBR1 (A2) and anti-mGluR1 (B2) antibodies (A3 and B3, superimposed images). Arrows and arrowheads, somata and axons, respectively. GBR1 and mGluR1 immunoreactivities are detected at most dendritic spines (double arrow heads in the close-up images, A5 and 6 and B5 and 6). C, co-immunostaining of Purkinje cells (20 days old in vitro) with the anti-GBR1 (C1) and anti-mGluR1 (C2) antibodies shows the overlapping subcellular distribution of these antigens (C3, superimposed image). Co-localization of these antigens is found at dendritic spines (double arrow heads in the close-up images, C4–C6). Scale bars, 10 μm.
GABABR enhances LTD in situ
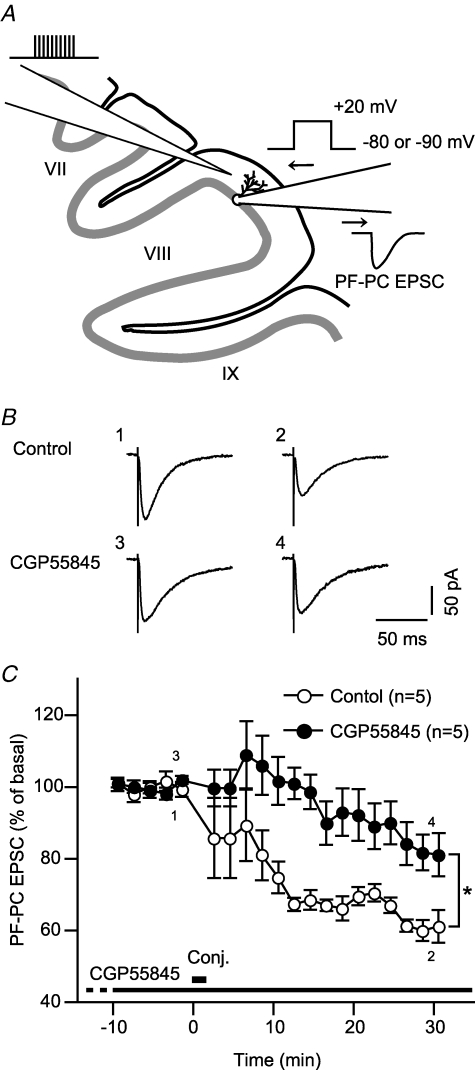
Lastly, we tested whether GABABR indeed enhances LTD of parallel fibre–Purkinje cell synaptic transmission, using cerebellar slices. The extracellular fluid in the cerebellar slices might contain GABA because the cerebrospinal fluid contains GABA and cerebellar interneurons synaptically release GABA (see Discussion). We examined the effect of CGP55845, a GABABR-selective antagonist that is expected to interfere with GABA action on GABABR. We induced LTD with conjunctive stimuli (30 sets at 0.5 Hz) each of which consisted of a train of electrical pulses to the parallel fibres and a depolarizing voltage step to the Purkinje cell (Fig. 10A). The electrical pulses were given in relatively high-frequency bursts (100 Hz) to facilitate GABA release from interneurons activated by the parallel fibre stimuli (Dittman & Regehr, 1997; Hirono et al. 2001). Under the control condition, parallel fibre–Purkinje cell EPSC was depressed over 30 min after the conjunctive stimuli (Fig. 10B). The continuous presence of CGP55845 (2 μm) abrogated initial depression after the conjunctive stimuli and slowed down the development of LTD (Fig. 10B). For both conditions, LTD appeared to develop to the maximal extents at 17–21 min after the conjunctive stimuli (mean amplitudes of parallel fibre–Purkinje cell EPSC at this period were not significantly different those at 27–31 min after the conjunctive stimuli, P > 0.1, paired Student's t test). Over 30–31 min after the conjunctive stimuli, the mean relative amplitude of parallel fibre–Purkinje cell EPSC with CGP55845 (81.1 ± 5.9% of the basal level, n = 5) was significantly larger than that of the control (61.1 ± 4.4%, n = 5) (Fig. 10B). This result indicates that GABABR-mediated enhancement may occur for LTD in situ.
Figure 10. GABABR-mediated LTD enhancement may occur in situ.
A, as schematically shown, we measured LTD of parallel fibre–Purkinje cell (PF-PC) EPSC in cerebellar slices. Whole-cell voltage-clamp recording was made from a Purkinje cell in the lamina VII or VIII. Parallel fibres were stimulated by electrical pulses delivered through a glass pipette positioned within a 1/3-part amid the molecular layer. LTD was induced with 30 sets of conjunctive stimuli (repeated at 0.5 Hz) each of which consisted of 10 pulses to the parallel fibres (100 Hz) and a depolarizing voltage step (+20 mV, 100 ms) to the Purkinje cell through the recording pipette. B, relative amplitude of parallel fibre–Purkinje cell (PF-PC) EPSC in the absence (control, n = 5) or continuous presence (n = 5) of CGP55845, a GABABR-selective antagonist (2 μm) plotted against time after the conjunctive stimuli (‘Conj.’). CGP55845 was included in the bath solution from at least 30 min before the conjunctive stimuli. Dot and error bar, the mean and s.e.m. of the data for each 2 min period. Mean value over 29–30 min after the conjunctive stimuli significantly differs between the two conditions (*P < 0.05, Mann–Whitney's U test).
Discussion
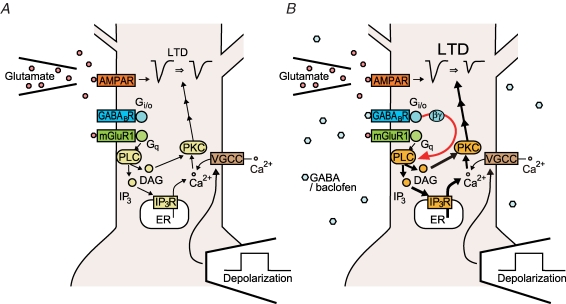
We found that GABABR activation enhanced LTDglu, increasing the magnitude of depression (Fig. 1B and C). GABABR-mediated LTDglu enhancement emerged immediately after the induction phase of LTDglu (Fig. 1C). GABABR agonist application within the period of the induction stimuli was sufficient to enhance LTDglu (Fig. 4). These results suggest that GABABR signalling modulates mechanisms underlying LTDglu induction (Fig. 11). In cultured Purkinje cell preparations, glutamate-evoked mGluR1 signalling and depolarization-evoked Ca2+ influx are known as the key factors for triggering LTD induction (Fig. 11A) (Linden et al. 1991; Ito, 2002). GABABR activation augmented DHPG-evoked mGluR1 signalling (Fig. 5A and D; for details, see below) while reduced K+-evoked Ca2+ influx (Fig. 6A and C). A previous study (Mintz & Bean, 1993) shows that GABABR negatively regulates the P/Q-type channels in Purkinje cells. These results suggest that GABABR signalling augments mGluR1 signalling in Purkinje cells and thereby facilitate LTDglu induction (Fig. 11B).
Figure 11. Possible mechanisms underlying GABABR-mediated LTDglu enhancement.
A, in the cultured Purkinje cells, glutamate-evoked mGluR1 signalling including Ca2+ release from the intracellular stores and depolarization-evoked Ca2+ influx are key factors for inducing LTDglu. B, GABA and baclofen promotes activation of Gi/o protein by GABABR. Activated Gi/o protein is cleaved into the α and βγ subunits; the latter may be more important for LTDglu enhancement than the former. The βγ subunits may augment mGluR1-mediated signalling presumably by acting on PLC, and this may result in LTDglu enhancement. For further explanation, see Discussion. AMPAR, AMPA-type ionotoropic glutamate receptor; DAG, diacylglycerol; ER, endoplasmic reticulum; IP3, inositol trisphosphate; IP3R, IP3 receptor; PKC, protein kinase C; PLC, phospholipase C; VGCC, voltage-gated calcium channel.
Inhibition of Gi/o protein by PTX abolished GABABR-mediated LTDglu enhancement (Fig. 2A and B). PTX did not appear to exert an inhibitory effect directly on glutamate-evoked currents in Purkinje cells (see online supplemental material, Supplemental Fig. 1). Thus, the effect of PTX on LTDglu enhancement cannot be ascribed to a PTX-induced reduction of glutamate-evoked currents which would occlude LTDglu. Activation of Gi/o protein by mastoparan mimicked LTDglu enhancement (Fig. 2C and D). Activation of adenylyl cyclase by forskolin did not abolish GABABR-mediated LTDglu enhancement (Fig. 3). Forskolin at the dose used is thought to increase cAMP level regardless of the presence or absence of micromolar levels of baclofen (Onali et al. 2003). Thus, this manipulation should cancel the inhibitory action of Gαi/o on adenylyl cyclase. These results raise the possibility that Gβγi/o primarily mediates GABABR-mediated LTDglu enhancement (Fig. 11B). There are several possible targets for Gβγi/o. In various cell types, Gβγi/o are known to exert a synergistic action with Gq/11 protein on some PLC isoforms including PLCβ3 (Park et al. 1993). All of the cultured Purkinje cells expressed PLCβ3 (Fig. 8). Thus, in most of the cases in this study, such a synergistic action on PLC should occur, and this might augment downstream mGluR1 signalling mediated by PLC (Fig. 11B). Moreover, a study (Zeng et al. 2003) has reported a possibility that Gβγi binds to and exerts a direct agonistic action on type-1 IP3R, which is important for cerebellar LTD induction (Inoue et al. 1998).
Depletion of Ca2+ in the intracellular stores by thapsigargin abolished mGluR1-mediated [Ca2+]i rises (Fig. 7C). In the presence of this agent, the conjunctive depolarization/glutamate stimuli did not induce LTDglu (Fig. 7A and B). These results suggest that mGluR1-coupled Ca2+ release from the intracellular stores is essential for inducing LTDglu in our cultured cell preparations (Fig. 11A) as reported in cerebellar slice preparations (Inoue et al. 1998). Thus, the GABABR-mediated augmentation of mGluR1-coupled Ca2+ release may contribute to LTDglu enhancement (Fig. 11B). A resultant elevation of the cytoplasmic [Ca2+] may promote activation of PKC (Ito, 2002), which in turn facilitates endocytosis of AMPA-type glutamate receptors (Matsuda et al. 2000; Chung et al. 2003). A recent study (Hansel et al. 2006) shows that genetic or pharmacological suppression of α-calcium/calmodulin-dependent kinase II, which is activated at a high [Ca2+]i, switches the polarity of plasticity from depression to potentiation at parallel fibre–Purkinje cell synapses. mGluR1-coupled Ca2+ release might ensure induction of LTDglu by boosting the activity of this kinase.
PTX pretreatment by itself attenuated the magnitude of LTDglu (Fig. 2A and B) as previously reported (Ito & Karachot, 1990). A small amount of G proteins may be active in a constitutive manner due to the ligand-independent basal activity of G protein-coupled receptors (Smit et al. 2007). Thus, Gi/o protein could always enhance LTDglu slightly unless inhibited by PTX.
Previous studies (Hirono et al. 2001; Tabata et al. 2004) show that in Purkinje cells, GABABR activation augments an mGluR1-mediated inward cation current (slow EPSC at parallel fibre–Purkinje cell synapses) by raising the ligand sensitivity of mGluR1 in a Gi/o protein-independent manner and by potentiating the mGluR1 signalling cascade in a Gi/o protein-dependent manner. GABABR-mediated LTDglu enhancement resembles the latter action of GABABR in terms of Gi/o protein dependence. However, activation of Gi/o-coupled receptors other than GABABR (e.g. A1 adenosine receptor; Tabata et al. 2007) fails to augment the slow EPSC (Hirono et al. 2001), suggesting that Gi/o protein activation is not sufficient to enhance this response. This contrasts with Gi/o protein activation being sufficient to enhance LTDglu (Fig. 2C and D). LTDglu enhancement and cation current augmentation may involve different mechanisms.
Co-localization of GABABR and mGluR1 at the dendritic spines of Purkinje cells is seen commonly in culture (Fig. 9) and in situ (Lujan et al. 1997; Kulik et al. 2002). Therefore, GABABR-mediated enhancement described above could possibly occur for mGluR1-mediated LTD at real parallel fibre–Purkinje cell synapses. We confirmed this possibility, using cerebellar slices. A GABABR-selective antagonist reduced the magnitude of LTD induced by conjunctive parallel fibre stimulation and postsynaptic depolarization (Fig. 10). In mammals, the cerebrospinal fluid contains a few tens of nanomolar of GABA in vivo (Bohlen et al. 1979). Some fraction of this GABA could remain in the cerebellar slices. Parallel fibre stimulation may further increase ambient GABA concentration in the cerebellar slices by facilitating GABA release from interneurons activated by the parallel fibre inputs. Released GABA may spill over from the synaptic clefts and act on neighbouring parallel fibre–Purkinje cell synapses (Dittman & Regehr, 1997; Hirono et al. 2001). CGP55845 might interfere with GABA action on GABABR in the Purkinje cells (Fig. 11). The effect of CGP55845 on LTD is not attributable to its action on presynaptic GABABR. GABABR mediates presynaptic inhibition of parallel fibre–Purkinje cell synapses (Dittman & Regehr, 1996). A GABABR antagonist is shown to relieve this inhibition (Hirono et al. 2001), and this would rather enhance LTD.
The concentration of GABA contained in the cerebrospinal fluid of healthy animals (see above) is high enough to activate GABABR considerably (GABABR's affinity for GABA, ∼1 μm; Sodickson & Bean, 1996). GABA spilt over from the interneurons' synapses might further facilitate GABABR activation in Purkinje cells. Therefore, GABABR-mediated enhancement of cerebellar LTD could occur under physiological conditions in vivo. Our findings demonstrate a novel mechanism that would facilitate cerebellar motor learning.
Acknowledgments
We thank Dr R. Shigemoto for his gift of the antibodies against GABABR and Dr T. Yoshida for his advice on immunohistochemistry. This work was supported by Grants-in-Aid for Scientific Research (18019022 and 17700305 to T.T., 17023021 and 17100004 to M.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Y.K. was a recipient of a Research Fellowship for Young Scientists and a graduate student fellowship from the Japan Society for the Promotion of Science (18·08750).
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.141010/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.141010
References
- Bohlen P, Huot S, Palfreyman MG. The relationship between GABA concentrations in brain and cerebrospinal fluid. Brain Res. 1979;167:297–305. doi: 10.1016/0006-8993(79)90824-2. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crepel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Mechanism and kinetics of heterosynaptic depression at a cerebellar synapse. J Neurosci. 1997;17:9084–9059. doi: 10.1523/JNEUROSCI.17-23-09048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, de Jeu M, Belmeguenai A, Houtman SH, Buitendijk GH, Andreev D, De Zeeuw CI, Elgersma Y. αCaMKII is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Higashijima T, Uzu S, Nakajima T, Ross EM. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins) J Biol Chem. 1988;263:6491–6494. [PubMed] [Google Scholar]
- Hirono M, Yoshioka T, Konishi S. GABAB receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat Neurosci. 2001;4:1207–1216. doi: 10.1038/nn764. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Ige AO, Bolam JP, Billinton A, White JH, Marshall FH, Emson PC. Cellular and sub-cellular localisation of GABAB1 and GABAB2 receptor proteins in the rat cerebellum. Brain Res Mol Brain Res. 2000;83:72–80. doi: 10.1016/s0169-328x(00)00199-6. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kato K, Kohda K, Mikoshiba K. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J Neurosci. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. The molecular organization of cerebellar long-term depression. Nat Rev Neurosci. 2002;3:896–902. doi: 10.1038/nrn962. [DOI] [PubMed] [Google Scholar]
- Ito M, Karachot L. Messengers mediating long-term desensitization in cerebellar Purkinje cells. Neuroreport. 1990;1:129–132. doi: 10.1097/00001756-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Kakizawa S, Kishimoto Y, Hashimoto K, Miyazaki T, Furutani K, Shimizu H, Fukaya M, Nishi M, Sakagami H, Ikeda A, Kondo H, Kano M, Watanabe M, Iino M, Takeshima H. Junctophilin-mediated channel crosstalk essential for cerebellar synaptic plasticity. EMBO J. 2007;26:1924–1933. doi: 10.1038/sj.emboj.7601639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Miyazaki T, Yanagihara D, Iino M, Watanabe M, Kano M. Maintenance of presynaptic function by AMPA receptor-mediated excitatory postsynaptic activity in adult brain. Proc Natl Acad Sci U S A. 2005;102:19180–19185. doi: 10.1073/pnas.0504359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikubo Y, Egashira Y, Tanaka T, Shinoda Y, Tominaga-Yoshino K, Ogura A. Long-lasting synaptic loss after repeated induction of LTD: independence to the means of LTD induction. Eur J Neurosci. 2006;24:1606–1616. doi: 10.1111/j.1460-9568.2006.05032.x. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Watanabe M, Kurihara H, Offermanns S, Jiang H, Wu Y, Jun K, Shin HS, Inoue Y, Simon MI, Wu D. Phospholipase Cβ4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc Natl Acad Sci U S A. 1998;95:15724–15729. doi: 10.1073/pnas.95.26.15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Nyiri G, Notomi T, Malitschek B, Bettler B, Shigemoto R. Distinct localization of GABAB receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J Neurosci. 2002;15:291–307. doi: 10.1046/j.0953-816x.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–89. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- Llano I, DiPolo R, Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994;12:663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Walton KD, Lang EJ. Cerebellum. In: Shepherd GM, editor. The Synaptic Organization of the Brain. 5. New York: Oxford University Press; 2003. pp. 271–309. [Google Scholar]
- Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 α, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cβ4 with metabotropic glutamate receptor type 1α and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- Narasimhan K, Pessah IN, Linden DJ. Inositol-1,4,5-trisphosphate receptor-mediated Ca mobilization is not required for cerebellar long-term depression in reduced preparations. J Neurophysiol. 1998;80:2963–2974. doi: 10.1152/jn.1998.80.6.2963. [DOI] [PubMed] [Google Scholar]
- Nomura S, Fukaya M, Tsujioka T, Wu D, Watanabe M. Phospholipase Cβ3 is distributed in both somatodendritic and axonal compartments and localized around perisynapse and smooth endoplasmic reticulum in mouse Purkinje cell subsets. Eur J Neurosci. 2007;25:659–672. doi: 10.1111/j.1460-9568.2007.05334.x. [DOI] [PubMed] [Google Scholar]
- Onali P, Mascia FM, Olianas MC. Positive regulation of GABAB receptors dually coupled to cyclic AMP by the allosteric agent CGP7930. Eur J Pharmacol. 2003;471:77–84. doi: 10.1016/s0014-2999(03)01823-5. [DOI] [PubMed] [Google Scholar]
- Park D, Jhon DY, Lee CW, Lee KH, Rhee SG. Activation of phospholipase C isozymes by G protein βγ subunits. J Biol Chem. 1993;268:4573–4576. [PubMed] [Google Scholar]
- Sarna JR, Marzban H, Watanabe M, Hawkes R. Complementary stripes of phospholipase Cβ3 and Cβ4 expression by Purkinje cell subsets in the mouse cerebellum. J Comp Neurol. 2006;496:303–313. doi: 10.1002/cne.20912. [DOI] [PubMed] [Google Scholar]
- Sato M, Tabata T, Hashimoto K, Nakamura K, Nakao K, Katsuki M, Kitano J, Moriyoshi K, Kano M, Nakanishi S. Altered agonist sensitivity and desensitization of neuronal mGluR1 responses in knock-in mice by a single amino acid substitution at the PKC phosphorylation site. Eur J Neurosci. 2004;20:947–955. doi: 10.1111/j.1460-9568.2004.03552.x. [DOI] [PubMed] [Google Scholar]
- Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Abe T, Nomura S, Nakanishi S, Hirano T. Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron. 1994;12:1245–1255. doi: 10.1016/0896-6273(94)90441-3. [DOI] [PubMed] [Google Scholar]
- Shpakov AO, Pertseva MN. Molecular mechanisms for the effect of mastoparan on G proteins in tissues of vertebrates and invertebrates. Bull Exp Biol Med. 2006;141:302–306. doi: 10.1007/s10517-006-0156-6. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, Pardo L, Leurs R. Pharmacogenomic and structural analysis of constitutive G protein-coupled receptor activity. Annu Rev Pharmacol Toxicol. 2007;47:53–87. doi: 10.1146/annurev.pharmtox.47.120505.105126. [DOI] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, Van der Putten H, Bettler B, Kano M. Ca2+ activity at GABAB receptor constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc Natl Acad Sci U S A. 2004;101:16952–16957. doi: 10.1073/pnas.0405387101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Haruki S, Nakayama, Kano M. GABAergic activation of an inwardly rectifying K+ current in mouse cerebellar Purkinje cells. J Physiol. 2005;563:443–457. doi: 10.1113/jphysiol.2004.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Kawakami D, Hashimoto K, Kassai H, Yoshida T, Hashimotodani Y, Fredholm BB, Sekino Y, Aiba A, Kano M. G protein-independent neuromodulatory action of adenosine on metabotropic glutamate signalling in mouse cerebellar Purkinje cells. J Physiol. 2007;581:693–708. doi: 10.1113/jphysiol.2007.129866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Sawada S, Araki K, Bono Y, Furuya S, Kano M. A reliable method for culture of dissociated mouse cerebellar cells enriched for Purkinje neurons. J Neurosci Methods. 2000;104:45–53. doi: 10.1016/s0165-0270(00)00323-x. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Nakagawa S, Kushiya E, Yamasaki M, Fukaya M, Iwanaga T, Simon MI, Sakimura K, Kano M, Watanabe M. Gq protein α subunits Gαq and Gα11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur J Neurosci. 2000;12:781–792. doi: 10.1046/j.1460-9568.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Zeng W, Mak DO, Li Q, Shin DM, Foskett JK, Muallem S. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gβγ. Curr Biol. 2003;13:872–876. doi: 10.1016/s0960-9822(03)00330-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.