Abstract
The original ‘lipid raft’ hypothesis proposed that lipid-platforms/rafts form in the exoplasmic plasmalemmal leaflet by tight clustering of sphingolipids and cholesterol. Their physical state, presumably similar to liquid-ordered phases in model membranes, would confer detergent resistance to rafts and enriched proteins therein. Based on this concept, detergent resistant membranes (DRMs) from solubilized cells were considered to reflect pre-existing ‘lipid rafts’ in live cells. To date, more than 200 proteins were found in DRMs including also members of the SNARE superfamily, which are small membrane proteins involved in intracellular fusion steps. Their raft association indicates that they are not uniformly distributed, and, indeed, microscopic studies revealed that SNAREs concentrate in submicrometre-sized, cholesterol-dependent clusters at which vesicles fuse. However, the idea that SNARE clusters are ‘lipid rafts’ was challenged, as they do not colocalize with raft markers, and SNAREs are excluded from liquid-ordered phases in model membranes. Independent from this disagreement, in recent years the solubilization criterion has been criticized for several reasons, calling for a more exact definition of rafts. At a recent consensus on a revised raft model, the term ‘lipid rafts’ was replaced by ‘membrane rafts’ that were defined as ‘small (10–200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes’. As a result, after dismissing the terms ‘detergent resistant’ and ‘liquid-ordered’, it now appears that SNARE clusters are bona fide ‘membrane rafts’.
What is a raft?
The eukaryotic plasma membrane is a busy place where a multitude of proteins exert numerous cellular functions. In the 1970s the plasma membrane was regarded as a two-dimensional solution of membrane proteins in a viscous phospholipid bilayer. However, after exploring dynamics and organization of many plasmalemmal components, it is now beyond controversy that plasma membranes are laterally highly organized structures. Several theories try to explain these lateral inhomogeneities, generically termed plasma membrane microdomains.
One of the most popular ones is the so-called ‘lipid raft’ hypothesis. It proposed that in the exoplasmic leaflet of the plasma membrane cholesterol and sphingolipids tightly cluster into lipid platforms/rafts (Simons & Ikonen, 1997), structured like liquid-ordered phases in model membranes. As in model membranes, such tight lipid packing would confer detergent resistance to rafts. This would allow for the isolation of ‘lipid rafts’ as detergent resistant membranes (DRMs), which contain, apart from lipids, proteins that participate preferentially in the raft phase (e.g. glycosyl-phosphatidyl-inositol (GPI)-anchored proteins). DRMs can be easily isolated from cells solubilized in the cold by non-ionic detergents. As DRMs contain proteins and lipids, they represent the fraction with the lowest buoyant density and float up during gradient density centrifugation. After centrifugation, DRMs can be collected from the top of the centrifugation tube for further analysis of their composition. Although biological membranes are quite different from model membranes with respect to their complex composition and asymmetric lipid distribution, the raft hypothesis implied that liquid-ordered phases would exist in biological membranes. Moreover, it was taken for granted that detergent resistant membranes (DRMs) would reflect pre-existing ‘lipid rafts’ in live cells. Based on this concept, DRM association was used for classifying operationally membrane proteins as ‘lipid raft’ components. Over the years, more than 200 components have been assigned to rafts (Foster et al. 2003). In some cases, highly variable or even conflicting results have been reported (see below and Table 1). One general problem is that experiments are difficult to compare. For instance, in most studies it is not stated what ratio of detergent to protein was employed during cell extraction. This experimental parameter is crucial, as at too low ratios solubilization or micellarization of membrane proteins is incomplete, and instead of DRMs, plasma membrane fragments turn up in the raft fraction. Incomplete micellarization can further be facilitated by omitting detergent from the density gradient. As has been shown recently, DRM association of some proteins can depend on the presence of detergent in the gradient steps, especially when cells are solubilized with low concentrations of detergent (Korzeniowski et al. 2003). However, others have argued that inclusion of detergent in the gradient would expose up-floating rafts to increasing ratios of detergent to protein, possibly leading to the solubilization of raft components.
Table 1.
Association of SNAREs with detergent resistant membranes
| SNARE | Association with DRMs (%) |
|---|---|
| Syntaxin 1 | 0% in P2 from homogenized rat brain (Hering et al. 2003); 0% in pancreatic β cells (Ohara-Imaizumi et al. 2004); 0% and 22% in neuroendocrine cells (Lang et al. 2001; Chamberlain et al. 2001), < 10% in synaptosomes from rat brain (Gil et al. 2005) |
| Syntaxin 2 | 0% in mast cells (Pombo et al. 2003); 15% in alveolar epithelial cells (Chintagari et al. 2006) |
| Syntaxin 3 | > 50% in mast cells (Pombo et al. 2003; for epithelial cells see also Lafont et al. 1999) |
| Syntaxin 4 | < 10% in endothelial cell membranes and macrophages (Predescu et al. 2005; Kay et al. 2006); approx. 35% in adipocytes (Chamberlain & Gould, 2002); less than 20% and 50% in mast cells (Puri & Roche, 2006; Pombo et al. 2003) |
| SNAP-25 | 0%, 20% and 24% in neuroendocrine cells (Lang et al. 2001; Chamberlain et al. 2001; Salaun et al. 2005b); 20% in synaptosomes from rat brain (Gil et al. 2005) |
| SNAP-23 | < 10% in endothelial cell membranes (Predescu et al. 2005); 31% in alveolar epithelial cells (Chintagari et al. 2006); approx. 50% in macrophages (Kay et al. 2006); 54% in neuroendocrine cells (Salaun et al. 2005b); 50 and 70% in mast cells (Pombo et al. 2003; Puri & Roche, 2006); > 70% in adipocytes (Chamberlain & Gould, 2002) |
| Synaptobrevin/VAMP2 | 5–24% in neuroendocrine cells (Chamberlain et al. 2001); < 10% in synaptosomes from rat brain (Gil et al. 2005); approx. 20% in mast cells (Puri & Roche, 2006); 22% in alveolar epithelial cells (Chintagari et al. 2006); 36% in adipocytes (Chamberlain & Gould, 2002); 70–80% in macrophages (Kay et al. 2006) |
| Cellubrevin/VAMP3 | 20–30% in macrophages (Kay et al. 2006) |
Cell solubilization was performed using Triton X-100 at concentrations ranging from 0.1 to 1%. In the studies by Lang et al. (2001) and Hering et al. (2003) detergent was included in the density gradient steps.
Another widely used approach for the study of rafts is cholesterol depletion experiments. To support the idea of functional raft association of a specific membrane protein, it is typically shown that depletion of cellular cholesterol is accompanied with its loss of function and DRM association. However, this interpretation has been largely criticized as cholesterol is such an important cellular component that its depletion may not be directly related to the loss of a specific function. Unfortunately, so far no biochemical tools are available for sphingolipid extraction that would allow for raft disruption with less dramatic side-effects.
In summary, in spite of major efforts the further development of the raft model has been unsatisfactory, as no clear picture of rafts has emerged regarding their molecular composition, dynamics and size. In addition, in recent years the application of DRM isolation for raft characterization has been challenged (Shogomori & Brown, 2003; Lichtenberg et al. 2005), as several findings strongly indicate that DRMs are not identical with pre-existing rafts. Another unresolved question is why isolation of DRMs from cells requires low temperatures. As a result, their very existence has been questioned, and also attempts at a major revision of the raft model have been undertaken. The recent ‘Keystone Symposium on Lipid Rafts and Cell Function’ brought together leading scientists working from different angles in the raft field, including biophysicists, biochemists and cell biologists (Pike, 2006). At this meeting it was well recognized that the major problem in the raft field is the lack of a clear definition that would allow membrane domains to be classified as rafts. As a result, exact criteria have been defined. First, the term ‘lipid raft’ was replaced by ‘membrane raft’, as it is now widely acknowledged that rafts do not form solely by lipid-driven interactions but involve also proteins. It was debated whether ‘detergent resistance’ should be considered as a raft criterion. However, it was generally appreciated that this approach can induce artificially the formation of lipid phases and therefore does not provide information of physiological relevance. Also the term ‘liquid-ordered’ was dismissed, as there is so far no solid evidence that such phases exist in biological membranes. Finally, an agreement has been reached according to which membrane rafts are ‘small (10–200 nm), heterogenous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes. Small rafts can sometimes be stabilized to form larger platforms through protein–protein and protein–lipid interactions’ (Pike, 2006).
As mentioned above, for many different proteins raft association has been suggested, including members of the SNARE family involved in exocytosis. In the following, the SNARE proteins will be introduced and the pros and cons for their raft association will be discussed.
SNARE proteins involved in exocytosis
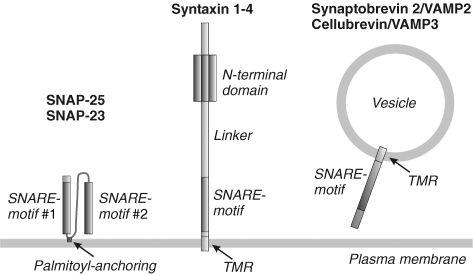
SNAREs are a superfamily of small, mostly membrane associated proteins, essential for all intracellular membrane fusion steps (except for mitochondrial fusion). They share as a common feature a conserved stretch of 60–70 aa, the so called SNARE motif (Jahn & Scheller, 2006). For each fusion step, a specific set of SNARE proteins is required. SNAREs associated with the plasma membrane are involved in regulated and constitutive exocytosis (Fig. 1). For instance, in regulated exocytosis the neuronal SNAREs syntaxin 1A and SNAP-25 at the plasma membrane form a complex with synaptobrevin 2/VAMP2 at the vesicle membrane, mediating fusion of the opposed membranes. The corresponding SNAREs involved in constitutive exocytosis are represented by syntaxin 2–4, SNAP-23 and cellubrevin/VAMP3. Three groups of exocytotic SNAREs can be distinguished on the basis of structure and location (Fig. 1): first, SNAREs with two SNARE motifs that are plasma membrane associated via palmitoyl anchors (SNAP-25 and SNAP-23); second, SNAREs with an N-terminal domain, SNARE motif and TMR located at the plasma membrane (syntaxins); and third, vesicle associated SNAREs with one SNARE motif and a TMR (synaptobrevin 2/VAMP2 and cellubrevin/VAMP3).
Figure 1. Structure and localization of SNAREs involved in exocytosis.
SNAP-25 and SNAP-23 possess two SNARE motifs connected by a linker region which is attached to the membrane via 4 (SNAP-25) or 5 (SNAP-23) palmitoyl anchors. All other exocytotic SNAREs are associated with the membrane via a TMR that is C-terminally attached to their SNARE motif, and only syntaxins have in addition an independently folded N-terminal domain connected to the SNARE motif via a linker region. According to the SNARE hypothesis, membrane fusion is mediated by complex formation between the SNARE motifs of the SNAREs localized on the opposed membranes destined to fuse.
SNAREs concentrate in cholesterol-dependent clusters
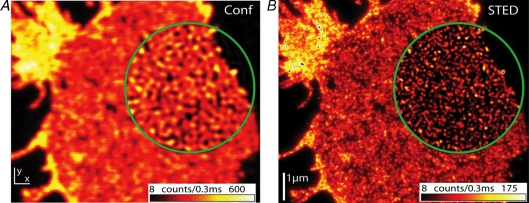
Whenever investigated, the plasma membrane associated SNAREs syntaxin 1–4, SNAP-25 and SNAP-23 have been found to concentrate in spotty structures called clusters by most groups. Partial overlap between syntaxin 1 and SNAP-25 clusters has been observed (Lang et al. 2001), indicating that different subpopulations of clusters exist with regard to composition. The size of syntaxin clusters has been analysed resulting in values from 170 to 256 nm (for syntaxin 1 in neuroendocrine and pancreatic β cells see Lang et al. (2001) and Ohara-Imaizumi et al. (2004), respectively; for syntaxin 3 and 4 clusters in epithelial cells see Low et al. (2006)). For SNAP-25 clusters a value of 277 nm has been reported (Ohara-Imaizumi et al. 2004). In a more recent study, nanoscale resolution STED microscopy has been applied to better resolve syntaxin 1 clusters (Sieber et al. 2006), revealing that syntaxin spots visualized by conventional microscopy often are composed of several individual clusters. Hence, due to technical limitations previous reports may have largely overestimated the size of SNARE clusters which are probably smaller than 100 nm (for SNAP-25 clusters see Fig. 2).
Figure 2. Nanoscale resolution STED microscopic analysis of SNAP-25 organization.
Confocal and STED micrograph of a plasma membrane sheet immunostained for SNAP-25. Encircled regions show linearly deconvolved data. Conventional resolution provided by confocal microscopy (A) is not sufficient to resolve individual SNAP-25 clusters present at high density. STED microscopic resolution (B) reveals that individual clusters are smaller than 60 nm average size (published with permission from Willig et al. 2006; Copyright Institute of Physics Publishing).
Cluster integrity is dependent on cholesterol as major changes in syntaxin 1 (Lang et al. 2001; Ohara-Imaizumi et al. 2004), syntaxin 3 (Low et al. 2006) and SNAP-23 (Chamberlain & Gould, 2002) redistribution can be observed upon depletion of cholesterol from the plasma membrane. In contrast, syntaxin 4 clusters are stable in the absence of cholesterol (Low et al. 2006). Moreover, photoactivatable cholesterol has been crosslinked to syntaxin 1, suggesting close proximity of both molecules in the membrane, most likely because cholesterol is present inside of the syntaxin cluster (Lang et al. 2001). Cholesterol and therefore cluster integrity seems to be of functional importance, as depletion of cholesterol also results in inhibition of secretion in neuroendocrine cells (Chamberlain et al. 2001; Lang et al. 2001), pancreatic β cells (Ohara-Imaizumi et al. 2004) and alveolar epithelial type II cells (Chintagari et al. 2006). In hippocampal cultures, cholesterol depletion leads to an augmentation of spontaneous neurotransmission and severe impairment of evoked responses (Wasser et al. 2007). However, it cannot be ruled out that cholesterol depletion and effects on exocytosis are not directly related. Though cluster integrity depends on cholesterol, it is not sufficient for proper syntaxin clustering. In addition, homophilic interactions involving the SNARE motif of syntaxin are essential (Sieber et al. 2006). Moreover, it has been reported that the integrity of syntaxin 3 and syntaxin 4 clusters depends on microtubule and actin filaments, respectively (Low et al. 2006). Purely lipid–TMR interactions could probably not differentiate between syntaxins that are remarkably similar on the level of amino acid sequence, but highly specific protein interactions mediating cluster formation can explain why syntaxin 1and 4 (Sieber et al. 2006) or syntaxin 3 and 4 (Low et al. 2006) are strictly segregated in different clusters. Provided docking and fusion would occur at syntaxin clusters, segregation of different syntaxins by specific cluster formation would separate spatially the different types of exocytotic events in the plasma membrane and hence compartmentalize cellular processes. In fact, it has been shown that syntaxin 1 and 4 clusters define docking and fusion sites for corresponding secretory organelles. In neuroendocrine and pancreatic β cells, secretory organelles dock and fuse at syntaxin 1 clusters (Lang et al. 2001; Ohara-Imaizumi et al. 2004; Aoyagi et al. 2005) and in epithelial cells syntaxin 4 clusters represent sites for caveolae docking and fusion (Predescu et al. 2005).
SNAREs in detergent resistant membranes (DRMs)
DRM association of exocytotic SNAREs has been investigated in many cell types (see Table 1) using highly variable conditions for solubilization and inclusion or exclusion of detergent in the gradient steps. For this reason, studies are difficult to compare (see also above), and in some cases conflicting results have been obtained. For instance, when DRMs are isolated from neuroendocrine PC12 cells with detergent present in the gradient steps, both syntaxin 1 and SNAP-25 were absent from the ‘lipid raft’ fraction (Lang et al. 2001; for syntaxin 1 see also Hering et al. 2003). In contrast, when detergent was omitted from the gradient, syntaxin 1 and SNAP-25 were associated with the raft fraction to 22% and 24%, respectively (Chamberlain et al. 2001). In other studies, syntaxin association with rafts varied from 0 to 50% (Table 1), and SNAP-25 and SNAP-23 have been found mostly in the DRM fraction, in some cases up to 70%. It appears as if palmitoyl anchoring directly affects the affinity for rafts or for DRMs. In fact, modified affinities for rafts were observed when cysteines that are used for palmitoylation were mutated (Salaun et al. 2005a). A correlation of these effects with changes in secretion suggested that the association of SNAP-25 and SNAP-23 with rafts directly regulates exocytosis. Also synaptobrevin 2/VAMP2 and cellubrevin/VAMP3 are found in the DRM fraction. At this point it is unclear what the significance of this finding is, as secretory granules are not known to have raft phases. From a functional point of view, segregation of proteins on the surface of a secretory organelle may be less important then in the plasma membrane.
At this point it is unclear what the percentage of ‘lipid raft’ association actually means, as it is unknown what percentage of the plasma membrane is composed of rafts. For instance, provided 20% of the total plasma membrane surface would be covered by rafts, 10% association with DRMs would be no enrichment in rafts but a 2-fold depletion.
Evidence against SNARE enrichment in ‘lipid rafts’
Applying other approaches, evidence against enrichment of SNAREs in ‘classical lipid rafts’ has been obtained. It has been shown on membrane sheets from neuroendocrine cells that syntaxin 1 clusters did not overlap with the raft marker Thy-1 (Lang et al. 2001), and in pancreatic β cells no overlap between syntaxin 1 clusters and the raft marker flotilin could be detected (Ohara-Imaizumi et al. 2004). This indicates that SNAREs are not enriched in ‘classical’ Thy-1 and flotilin rafts, although it cannot be excluded that they are enriched in a subtype of rafts. When SNAREs were studied in artificial model membranes as giant unilammelar vesicles, in which liquid-ordered and liquid-disordered phases form, syntaxin 1 and synaptobrevin 2/VAMP2 preferred the non-raft phase (Bacia et al. 2004). Here it should be noted that unlike recombinant synaptobrevin 2/VAMP2 that has been used in these experiments, native synaptobrevin 2/VAMP2 is palmitoylated during development and palmitoylation may change the affinity of the protein for liquid-ordered phases. In another study, it was shown by atomic force microscopy that syntaxin 1 was excluded from raised lipid domains that contain sphingomyelin in supported bilayers (Saslowsky et al. 2003). In summary, apart from the above discussed DRM association, so far no strong evidence for the enrichment of SNAREs in classical ‘lipid rafts’ has been obtained.
Are SNARE clusters ‘membrane rafts’?
As outlined above, it is generally accepted that SNAREs are concentrated in cholesterol-dependent microdomains that are of functional importance for exocytosis. However, so far there has been conflicting evidence for whether these microdomains are ‘lipid rafts’. The recent definition of ‘membrane rafts’ may now allow for a more exact classification. Accordingly, SNARE clusters fulfil most of the criteria: they probably contain cholesterol, compartmentalize cellular dynamics, and their size is in the required range. So far it remains to be established if they contain also sphingolipids, if they are dynamic structures and to what degree they are heterogeneous. Hence, in the future it may well turn out that SNAREs are present bona fide membrane rafts.
Acknowledgments
The author would like to thank Drs Jahn and Rizzoli for helpful comments on the manuscript. T.L. was supported by a grant from the Deutsche Forschungsgemainschaft (LA1272/2-1).
References
- Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- Bacia K, Schuette CG, Kahya N, Jahn R, Schwille P. SNAREs prefer liquid-disordered over ‘raft’ (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J Biol Chem. 2004;279:37951–37955. doi: 10.1074/jbc.M407020200. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH, Gould GW. The vesicle- and target-SNARE proteins that mediate Glut4 vesicle fusion are localized in detergent-insoluble lipid rafts present on distinct intracellular membranes. J Biol Chem. 2002;277:49750–49754. doi: 10.1074/jbc.M206936200. [DOI] [PubMed] [Google Scholar]
- Chintagari NR, Jin N, Wang P, Narasaraju TA, Chen J, Liu L. Effect of cholesterol depletion on exocytosis of alveolar type II cells. Am J Respir Cell Mol Biol. 2006;34:677–687. doi: 10.1165/rcmb.2005-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil C, Soler-Jover A, Blasi J, Aguilera J. Synaptic proteins and SNARE complexes are localized in lipid rafts from rat brain synaptosomes. Biochem Biophys Res Commun. 2005;329:117–124. doi: 10.1016/j.bbrc.2005.01.111. [DOI] [PubMed] [Google Scholar]
- Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Kay JG, Murray RZ, Pagan JK, Stow JL. Cytokine secretion via cholesterol-rich lipid raft-associated SNAREs at the phagocytic cup. J Biol Chem. 2006;281:11949–11954. doi: 10.1074/jbc.M600857200. [DOI] [PubMed] [Google Scholar]
- Korzeniowski M, Kwiatkowska K, Sobota A. Insights into the association of FcγRII and TCR with detergent-resistant membrane domains: isolation of the domains in detergent-free density gradients facilitates membrane fragment reconstitution. Biochemistry. 2003;42:5358–5367. doi: 10.1021/bi027135x. [DOI] [PubMed] [Google Scholar]
- Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci U S A. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Low SH, Vasanji A, Nanduri J, He M, Sharma N, Koo M, Drazba J, Weimbs T. Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol Biol Cell. 2006;17:977–989. doi: 10.1091/mbc.E05-05-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nishiwaki C, Kikuta T, Kumakura K, Nakamichi Y, Nagamatsu S. Site of docking and fusion of insulin secretory granules in live MIN6 β cells analyzed by TAT-conjugated anti-syntaxin 1 antibody and total internal reflection fluorescence microscopy. J Biol Chem. 2004;279:8403–8408. doi: 10.1074/jbc.M308954200. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Pombo I, Rivera J, Blank U. Munc18–2/syntaxin3 complexes are spatially separated from syntaxin3-containing SNARE complexes. FEBS Lett. 2003;550:144–148. doi: 10.1016/s0014-5793(03)00864-0. [DOI] [PubMed] [Google Scholar]
- Predescu SA, Predescu DN, Shimizu K, Klein IK, Malik AB. Cholesterol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane. J Biol Chem. 2005;280:37130–37138. doi: 10.1074/jbc.M505659200. [DOI] [PubMed] [Google Scholar]
- Puri N, Roche PA. Ternary SNARE complexes are enriched in lipid rafts during mast cell exocytosis. Traffic. 2006;7:1482–1494. doi: 10.1111/j.1600-0854.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Salaun C, Gould GW, Chamberlain LH. Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J Biol Chem. 2005a;280:19449–19453. doi: 10.1074/jbc.M501923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, Gould GW, Chamberlain LH. The SNARE proteins SNAP-25 and. SNAP-23 display different affinities for lipid rafts in PC12 cells. Regulation by distinct cysteine-rich domains. J Biol Chem. 2005b;280:1236–1240. doi: 10.1074/jbc.M410674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslowsky DE, Lawrence JC, Henderson RM, Edwardson JM. Syntaxin is efficiently excluded from sphingomyelin-enriched domains in supported lipid bilayers containing cholesterol. J Membr Biol. 2003;194:153–164. doi: 10.1007/s00232-003-2035-7. [DOI] [PubMed] [Google Scholar]
- Shogomori H, Brown DA. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem. 2003;384:1259–1263. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- Sieber JJ, Willig KI, Heintzmann R, Hell SW, Lang T. The SNARE motif is essential for the formation of syntaxin clusters in the plasma membrane. Biophys J. 2006;90:2843–2851. doi: 10.1529/biophysj.105.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig KI, Keller J, Bossi M, Hell SW. STED microscopy resolves nanoparticle assemblies. New J Phys. 2006;8:106. [Google Scholar]