Abstract
TRESK (TWIK-related spinal cord K+ channel) is the most recently identified member of the two-pore-domain potassium channel (K2P) family, the molecular source of background potassium currents. Human TRESK channels are not affected by external acidification. However, the mouse orthologue displays moderate pH dependence isolated to a single histidine residue adjacent to the GYG selectivity filter. In the human protein, sequence substitution of tyrosine by histidine at this critical position generated a mutant that displays almost identical proton sensitivity compared with mouse TRESK. In contrast to human TRESK, which is specifically located in spinal cord, we detected mouse TRESK (mTRESK) mRNA in several epithelial and neuronal tissues including lung, liver, kidney, brain and spinal cord. As revealed by endpoint and quantitative RT-PCR, mTRESK channels are mainly expressed in dorsal root ganglia (DRG) and on the transcript level represent the most important background potassium channel in this tissue. DRG neurones of all sizes were labelled by in situ hybridizations with TRESK-specific probes. In DRG neurones of TRESK[G339R] functional knock-out (KO) mice the standing outward current IKso was significantly reduced compared with TRESK wild-type (WT) littermates. Different responses to K2P channel regulators such as bupivacaine, extracellular protons and quinidine corroborated the finding that approximately 20% of IKso is carried by TRESK channels. Unexpectedly, we found no difference in resting membrane potential between DRG neurones of TRESK[WT] and TRESK[G339R] functional KO mice. However, in current-clamp recordings we observed significant changes in action potential duration and amplitude of after-hyperpolarization. Most strikingly, cellular excitability of DRG neurones from functional KO mice was significantly augmented as revealed by reduced rheobase current to elicit action potentials.
Background (leak) potassium currents substantially contribute to the resting membrane potential of several excitable and non-excitable cells. This K+ conductance plays an important role in setting the cellular excitability and regulates the firing rate of neurones. Two-pore-domain potassium (K2P) channels, when expressed in heterologous systems, show constitutive activity throughout the physiological range of membrane potential and thus are classified as background K+ channels. Functional K2P channels consist of two subunits, each of which has two pore domains and four transmembrane segments. In mammals 15 different K2P channel subunits (KCNK) have been identified and, due to structural and functional characteristics, they are divided into several subfamilies, e.g. acid-sensitive TASK channels and lipid-sensitive mechano-gated TREK/TRAAK channels (reviewed by Goldstein et al. 2001; Bayliss et al. 2003; Honoré, 2007). The activity of K2P channels is regulated by various physical and chemical stimuli such as temperature (Maingret et al. 2000), membrane stress (Maingret et al. 1999), protons (Duprat et al. 1997; Rajan et al. 2000), free fatty acids, phospholipids (Fink et al. 1998; Rajan et al. 2001) and local as well as volatile anaesthetics (Patel et al. 1999; Sirois et al. 2000; Franks & Honoré, 2004). In addition, these channels are functionally expressed in many regions of the central and peripheral nervous system suggesting an important role for neuronal excitability and sensory transduction processes.
TRESK (TWIK-related spinal cord K+ channel) is the most recently identified member of the K2P channel family. Initially discovered in the human genome the mRNA of human TRESK was exclusively expressed in spinal cord accounting for the nomenclature (Sano et al. 2003). The amino acid sequence of TRESK displays less than 20% similarity to the other K2P channel subunits and therefore TRESK is grouped into a novel K2P channel subfamily. Molecular cloning of TRESK cDNA from mouse revealed only 65% amino acid identity to the human protein and therefore was considered to be a second isoform (Kang et al. 2004). However, in both human and mouse genome databases only a single TRESK gene was identified, strongly indicating that variable TRESK subunits are orthologues from the two species (Czirják et al. 2004; Keshavaprasad et al. 2005). When heterologously expressed, major functional properties of human and mouse TRESK are indistinguishable, e.g. outward rectifying current–voltage relationship, resistance to classical K+ channel blockers and sensitivity to local anaesthetics. In contrast, regulation by extracellular pH was suggested to be different between the species variants. Human TRESK exhibited weak or no pH sensitivity while substantial dependence on extracellular protons was found in the mouse orthologue (Sano et al. 2003; Kang et al. 2004; Keshavaprasad et al. 2005). Moreover, the expression pattern of mouse TRESK is less restricted with transcripts detected across tissues other than spinal cord, e.g. brain, testis, spleen and thymus (Kang et al. 2004; Keshavaprasad et al. 2005). In dorsal root ganglia of rodents at least seven different K2P channels were identified by in situ hybridization (Talley et al. 2001). Recently background K+ currents of cultured rat DRG neurones were identified at the single channel level with functional and pharmacological properties similar to TRESK channels when recombinantly expressed in COS-7 cells (Kang & Kim, 2006).
The majority of DRG cells express several proton-regulated currents; however, current characteristics with respect to charge carrier, gating kinetics and other biophysical properties vary substantially among cells (Krishtal & Pidoplichko, 1980; Bevan & Geppetti, 1994; Baumann et al. 1996). The molecular basis of some of these currents could be assigned to members of the Transient Receptor Potential-Subfamily V (TRPV) channel family (Caterina et al. 1997) or to acid-sensing ion channels (ASIC) assembled from various subunits of the epithelial sodium channel (ENaC) super-family (Waldmann & Lazdunski, 1998; Wemmie et al. 2006). Nevertheless recent studies demonstrate that background K+ channels have to be considered when membrane conductance in DRG neurones changes upon external acidification (Baumann et al. 2004; Cooper et al. 2004).
In the present study we characterized background K+ currents of DRG neurones derived from TRESK[WT] and TRESK[G339R] mice. Acid-sensitive TRESK currents in mouse were shown to depend on a single amino acid pH sensor and in situ hybridizations documented channel expression in all types of DRG neurones. The effects of the functional knock-out of TRESK channels on the excitability of DRG neurones are described in detail.
Methods
All experiments were carried out in accordance with the regional animal care committee guidelines (at the Regierung von Unterfranken, Würzburg).
Mouse tissue was obtained from adult C3H mice. The mice were killed by halothane anaesthesia (final concentration approx. 2% by volume) followed by decapitation and the organs removed for further preparation.
Oocytes were obtained by partial ovariectomy of female Xenopus laevis that were sedated in a bath consisting of 0.2% of a methansulfonate salt of 3-aminobenzoic acid ethyl ester (Sigma) and placed on ice. After surgery the frogs were allowed to slowly recover from anaesthesia while vital signs were monitored. Subsequently they were transferred to the animal facility and kept in a separate tank for 24 h before returning to the home tank. Following the final collection of oocytes the animals were humanely killed.
Molecular cloning
Human TRESK cDNA (GenBank Accession No. AB087138; Sano et al. 2003) was amplified from genomic DNA by a three-step PCR strategy. Individual exons were PCR amplified (primer sequences for exon 1: forward 5′-ATGGAGGTCTCGGGGCAC-3′, reverse 5′-GTTT-TCTGTCTTCCACCACTGTTTCACTGCAGTTCAAG-3′; exon 2: forward 5′-TGGTGGAAGACAGAAAACAGG-3′, reverse 5′-GGTAGATGTAGCCATAGCCCACGGTGCTG-AACACCGTG-3′; exon 3: forward 5′-GGCTATGGCTA-CATCTACCCCG-3′, reverse 5′-TCACTTTTTAACAAG-GTGG-3′) and assembled by splicing overlap extension to result in a cDNA that codes for the entire open reading frame of human TRESK. The mouse orthologue of TRESK was identified by screening the NCBI genome database with the human protein sequence. Specific primers covering the entire open reading frame (forward 5′-AAGAGGATGGAGGCTGAGGAG-3′; reverse 5′-TTACCAAGGTAGCGAAACTTC-3′) were chosen to isolate a cDNA of mouse TRESK by reverse transcriptase PCR from total RNA of DRGs. For functional expression in Xenopus oocytes the cDNAs of human and mouse TRESK were cloned into polyadenylating transcription vector pSGEM. Point mutations were introduced by QuikChange Mutagenesis Kit from Stratagene (La Jolla, CA, USA) according to the manufacturer's instructions. All PCR products and mutants were sequenced on both strands on an ABI Prism 310 Genetic Analyser (Applied Biosystems, Weiterstadt, Germany). DNA analysis and sequence alignments were performed with Lasergene software (DNAstar, Madison, WI, USA).
PCR
Total RNA extraction
Tissues of individual wild-type male C3H mice (8–10 weeks old) were dissected. Following the manufacturer's protocol total RNA of each preparation was isolated with RNeasy mini kit and digested with RNase-free DNase (Qiagen) to remove contaminations of genomic DNA. The quantity and quality (optical density ratio 260/280 > 2.0) of RNA was assessed using a NanoDrop UV spectrophotometer (Peqlab Biotechnologie, Erlangen, Germany). As resolved by agarose gel electrophoresis, distinct bands of 28S and 18S ribosomal RNA indicated good quality nucleic acid. Only intact RNA samples were used for gene expression analysis.
Conventional RT-PCR (endpoint PCR)
To verify the expression profile of TRESK channels 1 μg total RNA from different mouse tissues was reverse transcribed in a final volume of 20 μl with oligo-dT primers and SuperscriptII (Invitrogen Groningen, the Netherlands). Gene-specific and intron-spanning primers (forward 5′-CCGA-GCTGCAATGTGGAGCTGTTTGAGA-3′; reverse 5′-GA-AGTAGAAAGCATCCTCGAAGCCTA-3′) were generated to selectively amplify a 288 base-pair (bp) fragment of mTRESK cDNA. Using 1 μl cDNA and Taq polymerase (Qiagen) PCRs were run on a standard thermo cycler (model T3; Biometra, Göttingen, Germany) with the following conditions (total volume 25 μl): initial denaturation step 4 min 94°C; 36 cycles: 1 min 94°C, 1.5 min 54°C, 1.5 min 72°C; final elongation step 4 min 72°C. Samples were tested for GAPDH expression by PCR (30 cycles) with gene-specific primers (forward 5′-CGGCAAATTCAACGGCACAGTCAA-3′; reverse 5′-CTTTCCAGAGGGGCCATCCA CAG-3′) resulting a cDNA fragment of 424 bp. Complete PCR samples or a fraction (GAPDH: 2.5 μl) were analysed on 2% agarose gels.
Prior to quantitative real-time RT-PCR the presence of different K2P channels in mouse DRGs were assayed by conventional PCR using QuantiTect SYBRGreen PCR kit (Qiagen) and QuantiTect Primer Assay (Qiagen; TRESK, Cat. no. QT00168189; TREK, QT00250229; TRAAK, QT00102445; TALK-1, QT01163953; TASK-2, QT01047739; TASK-1, QT01665020; TASK-3, QT01037323). PCR was started with an initial denaturation step of 95°C for 15 min and then subjected to 40 cycles of denaturation (15 s at 94°C), annealing (30 s at 55°C), and extension (30 s at 72°C). PCR products (TRESK, 103 bp; TREK, 73 bp; TRAAK, 90 bp; TALK-1, 104 bp, TASK-2, 87 bp, TASK-1, 91 bp, TASK-3, 75 bp) were analysed on 2.5% agarose gels.
Quantitative real-time RT-PCR
In order to quantify the relative expression of diverse K2P channels (see above) in mouse DRGs or between different tissues (brain and DRGs) we applied two-step quantitative real-time RT-PCR (qRT-PCR). Total RNA (0.5 μg) of individual animals was reverse transcribed with random hexamer and oligo-dT primers using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). Reaction mixtures were supplemented with ddH2O to a final volume of 100 μl. Real-time PCR and data analysis were performed in a total volume of 25 μl using 96-well plates and an iCycler IQ system (Bio-Rad Laboratory). To each microwell was added 1 μl cDNA, 12.5 μl QuantiTect SYBRGreen PCR kit (Qiagen), 2.5 μl QuantiTect Primer Assay (Qiagen) and 10 μl ddH2O. All PCRs were run in duplicate and conditions were the same as applied in endpoint PCR (see above). Amplicons were quantified with the comparative threshold cycle (CT) method computed by iCyler IQ software (Bio-Rad Laboratories). To evaluate the efficiency of amplification, a standard curve was constructed using the CT values versus 10-fold dilutions (10−1 ng μl−1 to 10−8 ng μl−1) of each cDNA fragment which was purified from agarose gels with Quantum Prep gel extraction kit (Bio-Rad Laboratories). qRT-PCR with identical efficiencies were taken to quantify the relative expression of different K2P channel genes in DRGs. To normalize these qRT-PCR data the expression of several housekeeping genes was analysed in cDNA samples of different animals: β-2-myoglobin (B2M, QuantiTect Primer Assay), βactin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPD), 18S ribosomal RNA (18S; forward 5′-GAAA-CTGCGAATGGCTCATTAAA-3′, reverse 5′-CCACAGT-TATCCAAGTAGGAGAGGA-3′). Using the geNorm algorithm (http://medgen.ugent.be/~jvdesomp/genorm/index.php) developed by Vandesompele et al. (2002) the stability of each housekeeping gene was tested and a normalization factor for each cDNA sample was calculated from at least three of the most stable housekeeping genes. Relative expression of housekeeping genes (in arbitrary units: ACTB, 0.052 ± 0.005; B2M, 0.038 ± 0.004; GAP, 0.847 ± 0122) was between one and two orders of magnitude higher than the expression of the most prominant K2P channel gene (TRESK, 0.0049 ± 0.001). Data were analysed with Excel software (Microsoft Corporation) and presented as mean ± s.d. Student's t test was chosen to test for significant differences.
In situ hybridization
Adult wild-type C3H mice were decapitated under halothane anaesthesia and DRGs were removed, embedded in tissue-Tek® (Sakura Finetek, Zeterwoude, the Netherlands) and frozen at −20°C until required. Sections, 15 μm thick, were cut on a cryostat, thaw-mounted on saline-coated slides and air-dried. After fixation for 10 min in 4% paraformaldehyde dissolved in PBS, slides were washed in PBS, dehydrated, and stored in ethanol until hybridization. Digoxigenin-labelled sense and antisense cRNA probes were in vitro transcribed from cDNA fragments subcloned into pCR-TOPO (Invitrogen) according to the manufacturer's protocol (Roche Diagnostics, Mannheim, Germany). The fragments corresponded to nucleotides 905–1502 of mTRESK and 37–544 of mTRPV1 (base position on coding strand). Hybridizations were carried out following the protocol used by Bartsch et al. (1992) and the label was detected by alkaline phosphatase-coupled antibodies directed to digoxigenin (Nucleic Acid Detection Kit, Roche Diagnostics). To identify the complete cellular fraction of the tissue, sections were Nissl-counterstained with cresyl violet. Control sections were hybridized with sense cRNA probes or digested with RNase (50 ng ml−1) for 30 min at 37°C before hybridization. These control experiments resulted in a complete loss of specific hybridization signal.
Cell culture
With some modifications DRG cells were isolated as has been previously described (Petersen et al. 1996). Briefly, adult mice anaesthetized with halothane were decapitated and DRGs from all levels were dissected. The ganglia were incubated in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) containing collagenase Type 4 (10 U ml−1) for 90 min at 37°C with three changes of medium. Individual cells were obtained by treatment with trypsin (10 000 U ml−1 PBS) for 10 min and subsequently triturated with a fire-polished siliconized glass pipette. The cell suspension was centrifuged through a layer of 80% Percoll (Amersham Bioscience, Freiburg, Germany) in PBS to obtain the neuronal cell fraction in the precipitate. Dispersed cells were plated on poly-l-lysine-coated cover slips and cultured in DMEM supplemented with 10% horse serum and 100 units ml−1 penicillin–streptavidin at 37°C with 5% CO2.
Generation of TRESK mutant mouse line G339R
A DNA archive from > 16 000 N-ethyl-N-nitrosourea (ENU)-mutagenized C3HeB/FeJ male mice (Augustin et al. 2005) was screened for point mutations in TRESK exon 3 by PCR amplification of the respective chromosomal regions followed by heteroduplex analysis of the generated fragments using a temperature gradient capillary electrophoresis system (Li et al. 2002). Fragments displaying typical heteroduplex migration patterns were sequenced to determine the nature of the nucleotide substitution. In one animal a G–A transition at position 630 of TRESK exon 3 was detected, which leads to the replacement of a glycine by arginine at position 339 in the TRESK polypeptide (G339R). This mutation led to a functional knock-out of the protein, as has been proven upon expression of the mutant gene in Xenopus oocytes. Sperms from the heterozygous mutant carrier animal (G1 animal), identified in the archive screening, were used for an in vitro fertilization of C3HeB/FeJ wild-type oocytes to generate G2 animals. The colony was expanded by breeding heterozygous G2 animals with wild-types and the resulting heterozygous offspring (G3) were used to raise the homozygous TRESK[G339R] mutants (G4).
Electrophysiology
For expression of TRESK channels in Xenopus laevis oocytes capped run-off poly(A+) cRNA transcripts from linearized cDNA of TRESK were synthesized and injected into defolliculated oocytes. Oocytes were incubated at 19°C in ND96 solution (96 mm NaCl, 2 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 5 mm 4-(2-hydroxyethyl)-1-piperazine-N′-2-ethanesulphonic acid (Hepes), pH 7.4–7.5), supplemented with 100 μg ml−1 gentamicin and 2.5 mm sodium pyruvate, and assayed 48 h post-injection. Two-electrode voltage-clamp measurements were performed with a TURBO TEC-10 C amplifier (npi, Tamm, Germany) and sampled through an EPC9 (Heka Electronics, Lamprecht, Germany) interface using PULSE/PULSEFIT software (Heka). For rapid exchange of external solutions, oocytes were placed in a small-volume perfusion chamber with a constant flow of ND96 or ‘high K+ solution’ (96 mm KCl, 2 mm NaCl, 1 mm MgCl2, 1 mm CaCl2, 5 mm Hepes, pH 7.4–7.5).
In addition, primary cultures from DRG neurones were grown on glass cover slips. Whole-cell recordings (Hamill et al. 1981) were performed 11–14 days after isolation at room temperature in a bath solution consisting of 135 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 10 mm glucose, 5 mm Hepes, pH 7.4. Patch pipettes were pulled from borosilicate glass capillaries (Kimble Products, UK), and heat-polished to give input resistances of 3–7 MΩ (whole-cell). The pipette recording solution contained 140 mm KCl, 2 mm MgCl2, 1 mm ethylene-bis(oxyethylenenitrilo) tetraacetate (EGTA), 1 mm Na2ATP, 100 μm cyclic AMP, 100 μm GTP and 5 mm Hepes (pH 7.3). Currents were recorded with an EPC9 (Heka) patch-clamp amplifier and low-pass-filtered at 1–2 kHz. Stimulation and data acquisition were controlled by the PULSE/PULSEFIT software package (Heka) on a Macintosh computer, and data analysis was performed with IGOR software (WaveMetrics, Lake Oswego, OR, USA). Data are presented as mean ± s.d. (number of cells). Statistical analysis was performed with the Stat/Statview software program. Student's t test was chosen to test for significant differences.
Results
Differential pH sensitivity of human and mouse TRESK channels
The coding regions of human and mouse TRESK cDNAs were cloned from human genomic DNA and mouse total RNA of dorsal root ganglia, respectively. The amino acid (AA) sequence of mouse TRESK (mTRESK, 394 AAs) is only 65% identical to the human TRESK (hTRESK) sequence (405 AAs). Despite their low sequence similarity the channel subunits are definitely orthologues and not different isoforms because searches in the genome databases of mouse and human discovered only a single TRESK gene in each species.
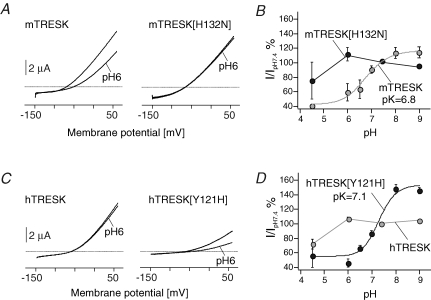
Interestingly a histidine immediately downstream of the selectivity filter sequence (GYG) in the first pore domain of mTRESK (His-132) is substituted by a tyrosine in the human sequence (His-121). The residue at this position was shown to be critical for extracellular pH sensitivity in TASK-1 and TASK-3 (Rajan et al. 2000; Lopes et al. 2000). When expressed in Xenopus laevis oocytes, currents of hTRESK were not affected by changes in extracellular pH whereas mTRESK channels displayed substantial sensitivity to external proton concentrations. Channel activity of mTRESK was reduced by 40% when extracellular pH was changed from 7.4 to 6.0 with an IC50 of 6.8. Further acidification to pH 4.5 resulted in additional current decrease by 20%. Assuming that pH sensitivity of mTRESK also depends on the conserved histidine adjacent to the GYG selectivity filter (His-132) we substituted this residue by asparagine and discovered that the mutant (mTRESK[H132N]) was insensitive to extracellular protons (Fig. 1A and B). Likewise, we tested whether the exchange of tyrosine with histidine in hTRESK is able to transfer pH dependence to the proton-insensitive channel. When expressed in Xenopus oocytes the mutants (hTRESK[Y121H]) generated functional channels that were sensitive to external acidification in the same range as channels assembled from mTRESK subunits (Fig. 1C and D).
Figure 1. Extracellular proton sensitivity of wild-type and mutant TRESK channels from mouse and human.
A and C, two-electrode voltage-clamp recordings from Xenopus oocytes injected with cRNA of mouse (A) and human (C) TRESK. Current–voltage relations are shown from oocytes expressing wild-type (left panels) and mutant (right panels) TRESK channels in response to voltage ramps from −150 mV to +60 mV at physiological and acidic extracellular pH as indicated. B and D, pH–current response curves of mouse (B) and human (D) TRESK channels expressed in Xenopus oocytes. Relative amplitudes (I/IpH7.4) of wild-type and mutant TRESK currents are plotted against pH.
Dominant expression of TRESK channels in dorsal root ganglia of the mouse
The tissue distribution of TRESK in mouse was monitored by endpoint PCR with gene-specific primers. Strong expression of TRESK was detected in DRGs whereas lower amounts of transcript were detected in spinal cord and brain. As revealed by qRT-PCR there was a 6-fold difference in the level of mRNA expression between DRG and brain (0.80 ± 0.17 and 0.15 ± 0.16, respectively; P < 0.01) In non-neuronal tissues substantial expression of TRESK was found in lung and heart; however, only weak signals were observed in liver, testis, kidney, small intestine and spleen. As a positive control the expression of the housekeeping enzyme GAPDH was determined (Fig. 2A).
Figure 2. Relative expression profile of mouse TRESK channels.
A, using endpoint PCR, an amplicon of 288 bp indicates expression of TRESK in different mouse tissues as indicated (upper panel). To monitor sample integrity a 424 bp fragment of GAPDH was PCR-amplified in parallel (lower panel). Bar graph in B shows normalized gene expression in DRGs of diverse K2P channels as indicated. Data are presented in arbitrary units as mean ± s.d. calculated from four individual tissue samples. General presence of various K2P channels in DRGs was monitored by endpoint PCR as depicted in the agarose gel of the inset.
To explore the presence of other K2P channel transcripts (TRESK, TREK, TRAAK, TALK-1, TASK-2, TASK-1, TASK-3) in DRG neurons we applied endpoint PCR with specific and validated primer sets (QuantiTect Primer Assay) which were subsequently used in qRT-PCR. With the exception of TASK-3 all other tested K2P channels were unambiguously detected in DRGs. As a control the expected PCR product of TASK-3 was amplified from mouse brain cDNA (Fig. 2B, inset) indicating proper function of the primer set. Relative gene expression of the identified K2P channels was analysed by SYBRGreen qRT-PCR. Data collected from four individual DRG preparations were used to calculate normalized gene expression (abitrary units) of TRESK (0.87 ± 0.185), TREK (0.31 ± 0.06), TRAAK (0.09 ± 0.03), TALK-1 (0.03 ± 0.025), TASK-2 (0.08 ± 0.02), and TASK-1 (0.11 ± 0.02). As depicted in Fig. 2B TRESK is the most abundant K2P channel with a 2.8-fold higher expression than the second most abundant K2P channel TREK. Interestingly, the mRNA level of the acid-sensitive channel TASK-1 is 8-fold lower compared with proton-sensitive mTRESK (see also section: ‘Comparison of IKso of TRESK[WT] and TRESK[G339R] mice’). The expression of all other tested genes was nearly one order of magnitude lower. All differences in gene expression between TRESK and the other K2P channels were highly significant (P < 0.001). In summary our data from endpoint and quantitative RT-PCR demonstrate that TRESK channels in mouse are mainly expressed in DRGs and at the transcript level represent the most important background potassium channel in this tissue.
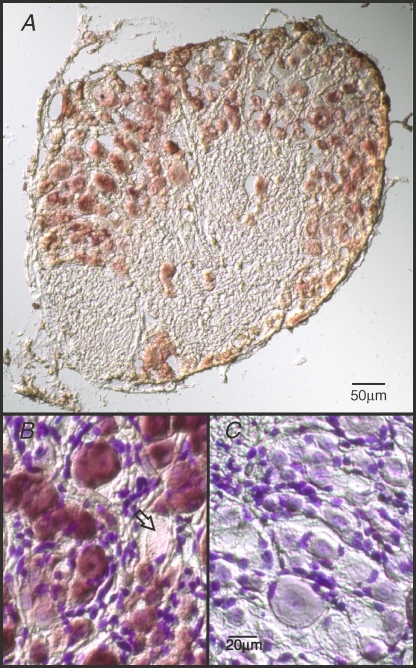
To specify in detail the DRG distribution of TRESK we analysed cryo-sections of DRGs by in situ hybridization with digoxigenin-labelled cRNA probes. Transcripts of TRESK were detected in most of the DRG neurones (Fig. 3). Small-, medium- and large-diameter neurones were specifically labelled with different intensity without any correlation with cell size. Transcripts of TRPV1 receptors as a marker of nociceptive cells were only detected in small sized DRG neurones (data not shown). In contrast only a marginal fraction of neurones (less than 10%) was found to be negative for TRESK expression (Fig. 3B) indicating that TRESK distribution does not correlate with pain-sensing cells.
Figure 3. In situ hybridization of TRESK mRNA in mouse dorsal root ganglia.
Neuronal expression of TRESK was detected with digoxigenin-labelled cRNA probes. A, bright-field photomicrograph using Nomarski optics shows stained sections of ganglia with transcripts of TRESK located in DRG neurones of all sizes. Different signal intensities do not correlate with the size of the neurones. Scale bar, 50 μm. B and C, high-power magnification of Nissl-stained section with TRESK mRNA detected in most DRG neurones (B) and signal specificity documented by unlabelled neurones hybridized with sense cRNA probes (C). As indicated by the arrow, a single DRG neurone in B does not express TRESK mRNA. Small-diameter satellite glial cells surrounding DRG neurones are visualized by dark staining. Scale bar, 20 μm.
Pharmacological profile of IKso from dorsal root ganglia
In order to investigate the contribution of TRESK currents to the standing outward potassium current, IKso, we performed whole-cell patch-clamp recordings from primary cultured DRG neurones. The pulse protocol was designed to minimize activation of voltage-gated transient K+ outward currents. Initially, the membrane potential was depolarized from a holding potential of –70 mV to −25 mV for 500 ms and subsequently hyperpolarized to −135 mV. Current amplitude of IKso was measured after 500 ms at the end of the depolarizing step and averaged 1.40 ± 0.7 nA (n = 71) (Fig. 4). Application of the local anaesthetic bupivacaine reduced IKso amplitude by 90.1 ± 4.8% (n = 10). In 5.4 mm external K+ bath solution bupivacaine-sensitive subtraction currents reversed at –84 ± 9.9 mV (n = 10) which is close to the calculated Nernst potential (–85.4 mV) for potassium-selective currents.
Figure 4. Whole-cell voltage-clamp recordings from cultured DRG neurones.
Left panel, depolarizing step from −70 mV holding potential to −25 mV elicits a standing outward current that is inhibited by bupivacaine, acidification and quinidine. Bar graph to right depicts the normalized remaining current after application of inhibitors as indicated.
Although data obtained by quantitative PCR suggest that other K2P channels play a minor role in maintaining IKso we could not rule out the contribution of, e.g., TREK or TASK channels to this current component. Therefore, we tried to discriminate between subfamilies by using more specific blockers.
As TRESK currents in mice are inhibited by extracellular protons we calculated the fraction of acid-sensitive IKso in DRG neurones from current recordings at pH 6.0. The subtraction current of IKso at pH 7.4 and IKso at pH 6.0 reversed at −84.7 ± 13.9 mV, indicating again that the pH-sensitive current is carried by potassium. Upon acidification of the bath solution current amplitude of IKso was attenuated by 56 ± 13% (n = 25, Fig. 4).
Again, the acid-sensitive current component could be contributed by TRESK and TASK channels. The antiarrhythmic drug quinidine is a potential tool to discriminate between both current components, because TRESK channels are more sensitive to quinidine compared with TASK channels (Leonoudakis et al. 1998; Kim et al. 2000; Sano et al. 2003; Kang et al. 2004). Application of quinidine (100 μm) to DRG neurones reduced IKso by 29.2 ± 15.5% (n = 13, Fig. 4). TREK-1 has been shown to be completely insensitive to 100 μm quinidine (Fink et al. 1996).
Mercury ions have been shown to selectively block mTRESK expressed in Xenopus oocytes, whereas TASK-1 and TASK-3 currents are highly up-regulated by mercury (Czirják & Enyedi, 2006). Application of mercury (1 μm) reduced IKso by 9.1 ± 4.7% (n = 9) in DRG neurones, showing that the net effect in these cells is nevertheless inhibitory and suggests a considerable contribution of TRESK. However, externally applied mercury seems to be inappropriate to selectively discriminate TRESK current from TASK conductances present in native cells.
The overall pharmacological properties of IKso in DRG neurones favour the hypothesis that a substantial fraction of this current is carried by TRESK, although substances tested are not highly selective and the effects are heterogeneous within the cell population.
Comparison of IKso of TRESK[WT] and TRESK[G339R] mice
Following ENU-induced random mutagenesis three mouse strains with mutations in the TRESK gene (I306V, G339R, F349L) were identified. Mutation G339R located in the second K+ selectivity filter of TRESK was chosen to prove channel function in heterologous systems. When expressed in Xenopus oocytes, wild-type TRESK evoked K+-selective currents with amplitudes of 9.48 ± 4.4 μA (Vh=+30 mV; n = 13), whereas virtually no current could be recorded from oocytes injected with cRNA of the G339R mutant (0.78 ± 0.14 μA, n = 9, data not shown). From this experiment we conclude that most probably homozygous TRESK[G339R] mice do not express any functional TRESK channels.
To estimate the contribution of TRESK to IKso, DRG neurones from TRESK-deficient mice were taken into culture and K+ currents were recorded with the same pulse protocol as described above. The current amplitude at the end of a 500 ms step to −25 mV amounted to 1.02 ± 0.6 nA (n = 51) indicating a reduction of 27% compared with IKso in neurones from TRESK[WT] (n = 72, Fig. 5). Again we could show that the elicited current was mainly carried by K+ because subtraction currents from bupivacaine inhibition and acidification to pH 6.0 reversed at −82 ± 22.8 mV (n = 12) and −82.1 ± 15.9 mV (n = 4), respectively.
Figure 5. Comparison of IKso in DRG neurones from TRESK[WT] and TRESK[G339R] mice.
A, upper traces show standing outward currents from TRESK[WT] and TRESK[G339R] mice upon an initial depolarizing step to −25 mV. Bupivacaine-derived subtraction currents (lower traces) reversed at −84 ± 9.9 mV (TRESK[WT], black trace) and −82 ± 23 mV (TRESK[G339R], grey trace), respectively. Bar graph in B quantifies IKso amplitude at a depolarizing step to −25 mV for TRESK[WT] and TRESK[G339R] mice. Bar graph in C compares inhibition of IKso in both genotypes as indicated.
Next we asked the question whether differences in IKso between the two genotypes occurred when substances known to regulate TRESK channels were applied. Saturating concentrations of bupivacaine (1 mm) reduced IKso by 85.5 ± 18.8% (TRESK[WT]: 90.1 ± 4.8%). This difference was not significant and demonstrated again that bupivacaine is a blocker of K2P channels without discriminating between subfamilies. The more selective agents instead should generate a significant difference in blocking IKso in neurones of mutant animals. This significant difference in IKso between both genotypes was observed upon acidification of the extracellular bath solution. Lowering pH to 6.0 reduced IKso in neurones from TRESK[G339R] mice only by 31.2 ± 14.7% (n = 11), whereas IKso from TRESK[WT] neurones was reduced by 56.4 ± 13% (P < 0.01) (Fig. 5). In addition, a significant difference in IKso reduction was found upon application of quinidine (100 μm). In neurones of TRESK[G339R] mice IKso was significantly less reduced (18.6 ± 13.3%, n = 11) compared with neurones from TRESK[WT] mice (29.2 ± 15.5%, n = 13, Fig. 5).
Comparison of IKso from TRESK[WT] and TRESK[G339R] mice together with differences of IKso between genotypes induced by pharmacological substances corroborated our finding that TRESK current contributes approximately by 20% to IKso.
DRG neurones from TRESK[WT] and TRESK[G339R] display different excitability and characteristics of the action potential
In order to investigate the physiological role of TRESK currents in DRG neurones, we conducted current-clamp recordings. First, we measured the resting membrane potential of neurones of both genotypes. TRESK[WT] cells exhibit a resting membrane potential of −60.83 ± 5.75 mV (n = 9) that was not different from the membrane potential of TRESK[G339R] cells (−60.82 ± 5.45 mV; n = 16). Hence, the functional knock-out of the TRESK gene has no implication with regard to the maintenance of the resting membrane potential in DRG neurones.
Second, we analysed the characteristics of the first action potential that occurred upon application of a family of depolarizing step pulses with an increment of 50 pA. DRG neurones respond only with a single action potential even to 500 ms depolarizing stimuli. The overall amplitude was not significantly different in neurones of TRESK[WT] mice (111.45 ± 14.86 mV, n = 9) compared with neurones of TRESK[G339R] mice (122.13 ± 14.06 mV, n = 16), respectively (Fig. 6B).
Figure 6. Current-clamp recordings in DRG neurones from TRESK[WT] and TRESK[G339R] mice.
A, current injections of 50 pA steps depolarized DRG neurones from their normal resting potential to the threshold of action potential (AP) induction. Neurones from TRESK[WT] mice (left recording) were less excitable than neurones from TRESK[G339R] mice (right recording). B, bar graph shows significant difference between genotypes (TRESK[WT], filled columns; TRESK[G339R], open columns) in rheobase current, but not in overall amplitude of APs and resting membrane potential. C, current-clamp whole-cell recordings display differences in AP duration and amplitude of after-hyperpolarization between genotypes (left, TRESK[WT]; right, TRESK[G339R]). Bar graph in D compares AP duration and amplitude of after-hyperpolarization in both genotypes.
Some characteristics of the action potential, however, displayed significant differences. The injected current needed for depolarizing the cell to elicit an action potential was 350 ± 111.8 pA (n = 9) for TRESK[WT] cells and 212.5 ± 104.08 pA (n = 16) for TRESK[G339R] cells (P < 0.05), respectively (Fig. 6A and B). This difference documents a higher sensitivity of cells from TRESK[G339R] mice to depolarizing stimuli.
Significant differences also occurred in the amplitude of the after-hyperpolarization measured from the peak of repolarization to the value of the sustained depolarization (23.23 ± 4.36 mV (n = 9) for TRESK[WT] and 31.28 ± 2.06 mV (n = 16) for TRESK[G339R], respectively; P < 0.01). The duration of the action potential measured at half-maximal amplitude also displayed significant differences (6.05 ± 5.23 ms (n = 9) for TRESK[WT] and 2.43 ± 0.85 ms (n = 16) for TRESK[G339R], P < 0.05) (Fig. 6A and B). These data seem to contradict the fact that in mutant mice a potassium leak conductance is missing. The lack of a repolarizing current component would argue for an increase in action potential duration instead of a decrease (see also Discussion).
Discussion
Protein sequences of TRESK subunits are markedly different between orthologues of human and mouse (65% identity). As a functional consequence, channels from both species display distinct sensitivity to changes in extracellular proton concentrations (Keshavaprasad et al. 2005). Mouse channels are inhibited by external acidification with a pK around 7.0, whereas channels assembled from human TRESK subunits are pH insensitive. Structural elements sensing extracellular pH are heterogeneous among K2P channels. Within the TASK-2/TALK subfamily a conserved arginine (or lysine in hTALK-2) close to the second K+ selectivity filter (mTASK-2[R224]) was found to act as the pH sensor (Niemeyer et al. 2007). In contrast, proton sensitivity of TASK-1 and -3 depends on a histidine adjacent to the first GYG selectivity filter (Rajan et al. 2000; Lopes et al. 2000). Our data unequivocally demonstrate that the homologous histidine in mTRESK (His-132) is responsible for its proton-dependent activity. Moreover, the exchange of tyrosine for histidine in hTRESK demonstrates that a single histidine at this critical position is necessary and sufficient to confer pH dependence.
It is remarkable that mTRESK is regulated by extracellular protons whereas the human orthologue is not. Due to the fact that most physiological processes are strictly pH dependent this discrepancy may be a basic mechanism causing variations between species.
As another feature of TRESK the expression pattern of human and mouse channels was found to be different. Whereas transcripts of hTRESK were detected only in spinal cord and brain (Sano et al. 2003; Liu et al. 2004), we and others (Kang et al. 2004) discovered TRESK mRNA of rodents in neuronal (spinal cord, brain) as well as in non-neuronal tissues (e.g. liver, testis, spleen, heart, lung). Endpoint PCR analysis revealed the highest expression of mTRESK in dorsal root ganglia which is consistent with data from qRT-PCR that detects 5.4-fold more TRESK mRNA in DRGs than in brain. In addition we demonstrate that several other K2P channels such as TASK-1 and -2, TREK-1 and TRAAK are also expressed whereas transcripts of TASK-3 are almost not detectable in DRGs. These findings are in good agreement with previous studies by qRT-PCR and in situ hybridization (Medhurst et al. 2001; Talley et al. 2001). However, the relative expression of different K2P channel genes in DRGs has not been analysed so far. In the present study we compare the amounts of transcripts from different K2P channel genes by qRT-PCR and discovered TRESK as the most prominent representative. The quantification of different K2P channel transcripts is an important indication of the contribution of these channels to total background K+ current, although additional electrophysiological data are necessary to estimate their individual contribution to this current component (see below).
As revealed by in situ hybridizations TRESK is localized in more than 90% of the DRG neurones. Transcripts of mTRESK were detected in small-, medium- and large-diameter DRG neurones. Similarly, single channel recordings of primary cells from rat DRG have also shown a TRESK-like K+ current in neurones of different diameter (Kang & Kim, 2006). As a marker for pain-sensing cells TRPV1 receptors were detected only in small-diameter neurones, indicating that the TRESK expression pattern does not overlap with the nociceptive neuronal circuit.
The generation of a pharmacological profile helps to identify the contribution of TRESK to the standing outward current IKso. Electrophysiological studies (Kang & Kim, 2006) identified a TRESK-like 14 pS channel in rat DRG neurones that is significantly inhibited upon acidification to pH 6.3, application of bupivacaine (100 μm) and quinidine (100 μm). From the frequency of occurrence of this channel type in cell-attached patches, which was found in small-, medium- and large-diameter neurones, the authors conclude that TRESK contributes to 80% of the background K+ current.
In our experiments comparison of TRESK[WT] and TRESK[G339R] currents yielded a 27% difference in IKso, supporting the notion that TRESK substantially contributes to IKso in DRG neurones but seemed to be overestimated in the above-mentioned study. Acidification to pH 6.0 reduces TRESK currents to ∼50%, whereas TASK-1 currents (IC50∼7.3) and TASK-2 currents (IC50 ranges from 7.5 to 7.8) are completely blocked (Morton et al. 2003). The difference in current reduction upon acidification between genotypes supports our assumption that IKso consists approximately of 20% of TRESK currents.
In addition, TASK currents are moderately reduced upon application of 100 μm quinidine (Kim et al. 2000), whereas TRESK currents should be blocked completely (Sano et al. 2003). When we expressed mTRESK in Xenopus oocytes 100 μm quinidine reduced the current amplitude only by 70% (S. Tovornik unpublished observations). Presuming that this concentration of quinidine does not block TRESK current in DRG neurones completely, the difference in amplitude reduction of IKso in cells from TRESK[G339R] mice compared with cells from TRESK[WT] mice corroborated our estimation of a ∼20% contribution of TRESK to IKso. This value is significantly smaller than that reported by Kang & Kim (2006); nevertheless we identified TRESK as a major component of IKso.
The nearly complete block by bupivacaine only demonstrates that IKso is mainly carried by several different K2P channels, all of them known to be sensitive to bupivacaine. Mercury ions have been suggested to act as a selective blocker for TRESK currents when expressed in Xenopus oocytes (Czirják & Enyedi, 2006). Unfortunately, other K2P channels like TASK-1 and TASK-3 are significantly augmented upon application of mercury ions. Therefore, in primary cultured cells with the expression profile of several different K2P channels mercury cannot serve as a selective marker of TRESK currents.
The resting membrane potential of DRG neurones is about −60 mV (Pluteanu et al. 2002). The fact that it is not different in neurones from TRESK[WT] and TRESK[G339R] mice implies that TRESK contributes only to a minor extent to the stabilization of the resting membrane potential. In DRG neurones from chick embryos a background current was identified with similar characteristics compared with IKso in murine DRG neurones (Fioretti et al. 2004). Single channel recordings demonstrated in this study that the open probability of the background current was negligibly low at resting potentials but could be activated upon application of histamine. Histamine H1 receptors couple to Gq-type G-proteins which in turn regulate the activity of the observed background potassium channel. In Xenopus oocytes we could show that TRESK currents also couple to H1 receptors. Application of 1 μm histamine led to an augmentation of TRESK currents up to 100% (S. Tovornik, unpublished observations). Additionally, we could verify the existence of H1 receptors in cultured murine DRG neurones (T. Dobler, unpublished observations), suggesting that TRESK currents are activated via histamine H1 receptors. So far we have not been able to demonstrate this coupling in native cells. Application of histamine to DRG neurones does not seem to influence the amplitude of IKso, perhaps due to a lack of an intracellular signalling pathway component that is washed out in whole-cell recordings. Low open probability at resting membrane potential and activation by histamine favour the hypothesis that the physiological role of this background current seems to be assessed only under certain physiological conditions, e.g. in inflammatory responses when histamine or other modulators of inflammatory responses like bradykinin are released into the surrounding tissue.
The TRESK[G33R] mouse model is derived from a random chemical mutagenesis project and sometimes concerns arise that the observed phenotype might be influenced by co-segregating mutations in genes different from TRESK. However, it is known from extensive genomic archive screens that the average mutation load in each G1 animal is one nucleotide exchange in every 2.69 Mbp genomic DNA (Augustin et al. 2005). This translates to about 20 functional mutations in coding sequences in each G1 animal or to approximately one mutation per chromosome. During in vitro fertilization using wild-type oocytes the mutation load is reduced to 10 mutations in coding sequences in the G2 generation with one mutation being the TRESK mutation and 9 out of 19 freely segregating passenger mutations. The mutation load is further reduced during colony expansion to 5 mutations in coding sequences in the G3 animals with one TRESK mutation and 4 out of 19 passenger mutations. With the given mutation load, the probability is therefore extremely low that the observed phenotype in all analysed homozygous G4 animals is influenced by a co-segregating mutation in genes different from TRESK (for a more detailed discussion see Augustin et al. 2005).
It is well known that potassium channels are crucial for shaping the onset, duration and frequency of action potentials (Grigg et al. 2000). Our results support the notion that the function of TRESK is not to stabilize the resting membrane potential, but instead dampen cellular excitability upon slight depolarizations. On the other hand we measured reduced action potential durations and higher amplitudes of after-hyperpolarization in TRESK[G339R] mice. In rat DRG neurones it has been shown that blockade of Ca2+-activated K+ channels (BKCa) leads to an increase of action potential duration (Zhang et al. 2003). In TASK-3 knock-out animals neurones from cerebellar granule cells display the same effect of broader action potentials (Brickley et al. 2007). Thus, faster repolarization of action potentials in neurones from TRESK[G339R] mice may result from an up-regulation of other potassium channels which would also explain the increase of after-hyperpolarization. Previously it has been demonstrated that loss of Cl− currents in granule cells by targeted knock-out of GABAA receptors was compensated by elevated expression of TASK-3 channels leading to unaltered neuronal excitability (Brickley et al. 2001). As a mechanism to control excitability via the background K+ conductance (IKso), changes in the resting membrane potential were found to regulate the transcription of TASK-3 channels (Zanzouri et al. 2006).
Accordingly it will be of interest to elucidate whether normal resting membrane potenial and shorter action potentials in DRG neurones of functional TRESK knock-out mice result from changes in the expression profile of ion channels especially K+ channels. In addition, DRG neurones of TRESK wild-type and knock-out mice are a good model for discovering new mechanisms for the transfer of electrical properties of the cell-to-gene expression.
Acknowledgments
We thank Tanja Martini, Maria Oppmann, Brigitte Trost and Gabi Ortega for excellent technical assistance and Brigitte Egenberger for her contribution in cloning the human TRESK ortholog. We are also grateful to Dr Corey Smith and Christine Schmeisser for critically reading the manuscript.
References
- Augustin M, Sedlmeier R, Peters T, Huffstadt U, Kochmann E, Simon D, Schoniger M, Garke-Mayerthaler S, Laufs J, Mayhaus M, Franke S, Klose M, Graupner A, Kurzmann M, Zinser C, Wolf A, Voelkel M, Kellner M, Kilian M, Seelig S, Koppius A, Teubner A, Korthaus D, Nehls M, Wattler S. Efficient and fast targeted production of murine models based on ENU mutagenesis. Mamm Genome. 2005;16:405–413. doi: 10.1007/s00335-004-3028-2. [DOI] [PubMed] [Google Scholar]
- Bartsch S, Bartsch U, Dörries U, Fraissner A, Weller A, Ekblom P, Schachner M. Expression of tension in the developing and adult cerebellar cortex. J Neurosci. 1992;12:736–749. doi: 10.1523/JNEUROSCI.12-03-00736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann TK, Burchiel KJ, Ingram SL, Martenson ME. Responses of adult human dorsal root ganglion neurones in culture to capsaicin and low pH. Pain. 1996;65:31–38. doi: 10.1016/0304-3959(95)00145-X. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Chaudhary P, Martenson ME. Background potassium channel block and TRPV1 activation contribute to proton depolarization of sensory neurones from humans with neuropathic pain. Eur J Neurosci. 2004;19:1343–1351. doi: 10.1111/j.1460-9568.2004.03097.x. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3:205–219. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- Bevan SJ, Geppetti P. Protons: small stimulants of casaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Aller MI, Sandu C, Veale EL, Alder FG, Sambi H, Mathie A, Wisden W. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. J Neurosci. 2007;27:9329–9340. [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor – a heat activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cooper BY, Johnson RD, Rau KK. Characterization and function of TWIK-related acid sensing K+ channels in a rat nociceptive cell. Neuroscience. 2004;129:209–224. doi: 10.1016/j.neuroscience.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Czirják G, Enyedi P. Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK. J Biol Chem. 2006;281:14677–14682. doi: 10.1074/jbc.M602495200. [DOI] [PubMed] [Google Scholar]
- Czirják G, Enyedi P. Zinc and mercury ions distinguish TRESK from the other two-pore-domain K+ channels. Mol Pharmacol. 2006;69:1024–1032. doi: 10.1124/mol.105.018556. [DOI] [PubMed] [Google Scholar]
- Czirják G, Tóth ZE, Enyedi P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J Biol Chem. 2004;279:18550–18558. doi: 10.1074/jbc.M312229200. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external variations near physiological pH. EMBO J. 1997;17:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel activated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioretti B, Catacuzzeno L, Tata AM, Franciolini F. Histamine activates a background, arachidonic acid-sensitive K channel in embryonic chick dorsal root ganglion neurons. Neuroscience. 2004;125:119–127. doi: 10.1016/j.neuroscience.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Franks NP, Honoré E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25:601–608. doi: 10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Grigg JJ, Brew HM, Tempel BL. Differential expression of voltage-gated potassium channel genes in auditory nuclei of the mouse brainstem. Hearing Res. 2000;140:77–90. doi: 10.1016/s0378-5955(99)00187-2. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp technique for high resolution current recordings from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Honoré E. The neuronal K2P channel: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurones. Am J Physiol Cell Physiol. 2006;291:C138–C146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- Kang D, Mariash E, Kim D. Functional expression of TRESK-2, a new member of the tandem-pore K+ channel family. J Biol Chem. 2004;279:28063–28070. doi: 10.1074/jbc.M402940200. [DOI] [PubMed] [Google Scholar]
- Keshavaprasad B, Liu C, Au JD, Kindler CH, Cotten JF, Yost CS. Species-specific differences in response to anesthetics and other modulators by the K2P channel TRESK. Anesth Analg. 2005;101:1042–1049. doi: 10.1213/01.ane.0000168447.87557.5a. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3 a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. A receptor for proton in the nerve cell membrane. Neuroscience. 1980;5:2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Liu Z, Monroe H, Culiat CT. Integrated platform for detection of DNA sequence variants using capillary array electrophoresis. Electrophoresis. 2002;23:1499–1511. doi: 10.1002/1522-2683(200205)23:10<1499::AID-ELPS1499>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Liu C, Au JD, Zou HL, Cotton JF, Yost CS. Potent activation of the human tandem pore domain K channel TRESK with clinical concentrations of volatile anesthetics. Anesth Analg. 2004;99:715–1722. doi: 10.1213/01.ANE.0000136849.07384.44. [DOI] [PubMed] [Google Scholar]
- Lopes CMB, Gallagher PG, Buck ME, Butler MH, Goldstein SAN. Proton block and voltage gating are potassium-dependent in the cardiac leak channel Kcnk3. J Biol Chem. 2000;275:16969–16978. doi: 10.1074/jbc.M001948200. [DOI] [PubMed] [Google Scholar]
- Maingret F, Fosset M, Lesage F, Lazdunski M, Honoré E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honoré E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honoré E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2000;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol Brain Res. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- Morton MJ, O'Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K+ channels TASK-1 and -2. Pflugers Arch. 2003;445:577–583. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- Niemeyer MI, González-Nilo FD, Zúniga L, Gonzalez W, Cid LP, Sepúlveda FV. Neutralization of a single arginine residue gates open a two-pore domain, alkali-activated K+ channel. Proc Natl Acad Sci U S A. 2007;104:666–671. doi: 10.1073/pnas.0606173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Petersen M, Lamotte RH, Klusch A, Kniffki KD. Multiple capsaicin-evoked currents in isolated rat sensory neurones. Neuroscience. 1996;75:495–505. doi: 10.1016/0306-4522(96)00259-x. [DOI] [PubMed] [Google Scholar]
- Pluteanu F, Ristoiu V, Flonta ML, Reid G. α1-Adrenoceptor-mediated depolarization and β-mediated hyperpolarization in cultured rat dorsal root ganglion neurones. Neurosci Lett. 2002;329:277–280. doi: 10.1016/s0304-3940(02)00665-1. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Karschin C, Preisig-Müller R, Grzeschik K-H, Daut J, Karschin A, Derst C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem. 2001;276:7302–7311. doi: 10.1074/jbc.M008985200. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Müller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. J Biol Chem. 2000;275:16650–16657.. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003;278:27406–27412. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lei Q, Talley EM, Lynch C, 3rd, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research 0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Zanzouri M, Lauritzen I, Duprat F, Mazzuca M, Lesage F, Lazdunski M, Patel A. Membrane potential-regulated transcription of the resting K+ conductance TASK-3 via the calcineurin pathway. J Biol Chem. 2006;281:28910–28918. doi: 10.1074/jbc.M606092200. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience. 2003;122:1003–1011. doi: 10.1016/j.neuroscience.2003.08.035. [DOI] [PubMed] [Google Scholar]