Abstract
Glucose deprivation (hypoglycaemia) is counterbalanced by a neuroendocrine response in order to induce fast delivery of glucose to blood. Some central neurons can sense glucose, but nevertheless the most important glucose sensors/glycaemia regulators are located outside the brain. Some recent experimental evidence obtained in carotid body (CB) slices and isolated chemoreceptor cells in culture supports a role for the CB in glucose sensing and presumably glucose homeostasis, but this role has been questioned on the basis of a lack of effect of low glucose on the carotid sinus nerve activity. This work was performed in an attempt to clarify if low glucose is or is not a stimulus for the rat CB chemoreceptors. Using freshly isolated intact CB preparations we have monitored the release of catecholamines (CAs) and ATP from chemoreceptor cells in response to several concentrations of glucose, as indices of chemoreceptor cell sensitivity to glycaemia, and the electrical activity in the carotid sinus nerve (CSN), as an index of reflex-triggering output of the CB. We have observed that basal (20% O2) and hypoxia (7 and 10% O2)-evoked release of CAs was identical in the presence of normal (5.55 mm) and low (3, 1 and 0 mm) glucose concentrations. 0 mm glucose did not activate the release of ATP from the CB, while hypoxia (5% O2) did. Basal and hypoxia (5% O2)-induced CSN action potential frequency was identical with 5.55 and 1 mm glucose. Our results indicate that low glucose is not a direct stimulus for the rat carotid body chemoreceptors.
The CB is the major peripheral chemoreceptor organ sensing changes in blood O2, CO2 and pH levels. In hypoxia, hypercapnia and acidosis, an increase in the action potential frequency of the carotid sinus nerve (CSN; the sensory nerve of the CB) is generated and its integration in the brainstem produces adaptative–homeostatic hyperventilation as well as some cardiovascular reflexes (Gonzalez et al. 1994). The first study in which the effect of low glucose on the CB was investigated dates from 1984, and it was observed that CSN activity in the freshly isolated cat CB–CSN preparation was not modified by 1 h superfusion with glucose-free solutions (Almaraz et al. 1984). After that pioneer study, experimental evidence has been obtained suggestive of a role for CBs in glucose sensing and homeostasis. Thus, Alvarez-Buylla & Alvarez-Buylla (1988) observed that intracarotid glucose infusion induced a reduction of carotid sinus nerve discharges, in vivo, in the cat. Koyama et al. (2000) showed that CSN-denervated dogs were less able to produce a counterregulatory response to insulin-induced mild hypoglycaemia than the sham-operated controls. In addition, in a CB thin-slice preparation it was found that perfusion with low or glucose-free solutions at a PO2 ≈ 150 mmHg produced a very strong and short latency (∼1 min) catecholamine (CA) release response from chemoreceptor cells of identical magnitude to that obtained while perfusing with N2-equilibrated solutions (Pardal & Lopez-Barneo, 2002) and potentiated hypoxic responses; moreover it was found that low glucose inhibited K+ currents (Pardal & Lopez-Barneo, 2002) in magnitudes that are comparable to those observed by Peers (1990) during intense hypoxia, and promoted Ca2+ entry in chemoreceptor cells (Pardal & Barneo, 2002). More recently, Zhang et al. (2007) working also at PO2 close to 150 mmHg found in cocultures of CB chemoreceptor cells and petrosal ganglion neurons that low glucose increased the spiking activity in the neurons, this increase being sensitive to purinergic (suramin) and nicotinic (mecamylamine) blockers implying that low glucose has stimulated chemoreceptor cells and promoted the release of ATP and acetylcholine. In contrast, Bin-Jaliah et al. (2004) using a freshly isolated rat CB–CSN preparation observed that lowering the glucose concentration from 10 to 2 mm did not alter the activity in the CSN; lowering the glucose further to 0 mm was equally ineffective to alter the output of the CB–CSN. In an additional study of the same group (Bin-Jaliah et al. 2005) it was observed that baseline chemoreceptor discharge obtained at 400 mmHg PO2, 40 mmHg PCO2 and 10 mm glucose was not altered by reducing the glucose to 2 or 0 mm, and additionally it was found that glucose concentration diminution blunted (low glucose) and nearly eliminated (0 glucose) the effect of hypercapnia upon chemoafferent discharge; these authors also observed that prolonging the superfusion to 20 min with 0 glucose in most cases led slowly to reversible decreases or even abolition of the CSN discharge. Thus, the findings obtained in the rat by Bin-Jaliah et al. (2004, 2005) are nearly identical to those reported by Almaraz et al. (1984).
We, as well as Zhang et al. (2007), have noticed that the authors who have found that the CB is not responsive to low glucose have used intact preparations and hyperoxia (Almaraz et al. 1984; Bin-Jaliah et al. 2004, 2005), while the authors who have found that low glucose is a stimulus to chemoreceptor cells (Pardal & Barneo, 2002; Zhang et al. 2007) have used preparations superfused with normoxic or hypoxic solutions. Then, the question arises of whether or not the lack of responsiveness of the intact CB preparation to low glucose is the result of the hyperoxic conditions. To define if the intact carotid body is responsive to low glucose we have measured responses specific to chemoreceptor cells (release of catecholamine and ATP) and recorded electrical activity in the carotid sinus nerve while perfusing with normoxic and hypoxic solutions containing different glucose concentrations. We have tested the effect of lowering the glucose concentration from the control (5.55 mm) to 3, 1 and 0 mm at basal PO2 of about 133 mmHg (superfusion with 20% O2, 5% CO2; balance N2; barometric pressure in Valladolid oscillates around 710 mmHg), mild hypoxia of 66 mmHg (superfusion with 10% O2, 5% CO2; balance N2) and moderate hypoxia of 46 mmHg (superfusion with 7% O2, 5% CO2; balance N2) on the release of CA from chemoreceptor cells. We also tested the effect of 1 mm glucose on the basal as well as on moderately intense hypoxic (PO2 ≈ 33 mmHg) CSN activity. The effect of prolonged superfusion with 0 mm glucose on the basal CA release and CSN activity was also studied. Lastly, in another group of experiments we compared the release of ATP from the CB in response to 0 mm glucose and a hypoxia of 33 mmHg of PO2. Glucose reduction or deprivation did not have an effect on any response when applied for short periods of time (< 15 min), but if applied for longer periods of time (up to 120 min) it caused a spontaneous increase in basal release that started to appear after 40 min of glucose deprivation, and some bursts of CSN activity with a comparable time course that culminated in a complete loss of the capacity of the CSN to respond to hypoxia. We conclude that the CB chemoreceptor cells in the intact organ are not glucose sensors and that the CB drive to respiration is not altered by short-term hypoglycaemia.
Methods
Animals and surgical procedures
Experiments were performed in adult Wistar rats of both sexes (250–350 g) obtained from the vivarium of the Faculty of Medicine of the University of Valladolid. The Institutional Committee of the University of Valladolid for Animal Care and Use approved the protocols. Rats were anaesthetized with sodium pentobarbital (60 mg kg−1i.p.) and tracheostomized, the carotid arteries were dissected, and a block of tissue including the carotid sinus area was removed and placed in a Lucite chamber.
For the release of CA and ATP experiments (5–12 CBs/experiment) the CBs were cleaned free of CSN and nearby connective tissue following procedures previously described (Vicario et al. 2000; Conde et al. 2006). For the recording of CSN activity, the CB–CSN preparation was identified under a dissecting microscope and a block of tissue, including the carotid bifurcation and the glossopharyngeal nerve, was removed and placed in a Lucite chamber in ice-cold, 100% O2-equilibrated Tyrode solution (mm: NaCl 140, KCl 5, CaCl2 2, MgCl2 1.1, Hepes 10, glucose 5.5, pH 7.40) for further dissection of tissue surrounding CB and CSN. The CB–CSN preparation was digested during 3–5 min in collagenase type I (1 mg ml−1) solution to loosen the perineurium (Rigual et al. 2002). Thereafter the CB–CSN preparation was transferred to the recording chamber. In all instances animals were killed by intracardiac overdoses of sodium pentobarbital until the beating of the heart ceased.
Labelling of catecholamine stores: release of [3H]CA
CA stores of the chemoreceptor cells were labelled by incubating the CBs for 2 h in Tyrode solution containing the natural precursor [3H]tyrosine (30 μm) (specific activity of 45 Ci mmol−1), and 6-methyl-tetrahydropterine (100 μm) and ascorbic acid (1 mm), cofactors of tyrosine hydroxylase and dopamine-β-hydroxylase, respectively (Fidone & Gonzalez, 1982). After the labelling period, individual CBs were transferred to vials containing 4 ml of precursor-free Tyrode–bicarbonate solution (composition as above except for the replacement of 24 mm of NaCl by 24 mm of NaHCO3). Solutions were continuously bubbled with 20% O2–5% CO2–75% N2 saturated with water vapour, except when hypoxic stimuli were applied. The solutions of the initial incubation periods (3 × 20 min) were discarded to wash out the precursor and the readily releasable pool of labelled CA and thereafter renewed at fixed times (every 2–10 min; see Results) and collected for subsequent analysis of their [3H]CA content. Specific stimulus protocols are provided in Results. Stimuli included hypoxias of two intensities (10 and 7% O2-equilibrated solutions) and low glucose of three concentrations (0, 1 and 3 mm of glucose) and were applied either separately or in combination (see Results). Collected solutions were acidified with glacial acetic acid to pH 3 and maintained at 4°C until analysis to prevent [3H]CA degradation. [3H]CAs present in the incubating solutions were analysed as previously described by Vicario et al. (2000) and Conde et al. (2006), in brief, by adsorption onto alumina at alkaline pH (pH 8.6), bulk elution of tritiated catechols with hydrochloric acid (1 n) and quantification by scintillation counting. CBs were homogenized (glass to glass; 4°C) in 200 μl of 0.6 m perchloric acid and centrifuged for 10 min (4°C; 12000 g) in a microfuge (Beckmann, Madrid). Tissue supernatants were analysed for their [3H]catechol content as described above for the incubating solutions. Previous HPLC analyses have shown that over 90% of the [3H]catechols present in the alumina eluates correspond to [3H]dopamine (DA) and its catabolite 3,4-[3H]dihydroxyphenylacetic acid (DOPAC) (Vicario et al. 2000). [3H]CA release data are expressed as a percentage of tissue content, i.e. dpm present in each collected incubating solution divided by dpm present in tissue supernatant multiplied by 100, or as dpm present in each collected incubating solution.
Endogenous release of ATP from carotid body
The CBs were incubated in 500 μl of Tyrode–bicarbonate solution. The incubating solutions were maintained at 37°C and continuously bubbled with 20% O2–5% CO2–75% N2 except when the hypoxic stimuli were applied; in all instance the gas mixtures were saturated with water vapour. The solution of the initial incubation period (1 × 15 min) was discarded. After, the solutions were renewed every 10 min and collected. The stimuli include: between minute 25 and 35, incubation with low glucose (0 mm glucose), and between minute 55 and 65, intense hypoxia (33 mmHg of PO2). The collected fractions were acidified with 300 μl of 3 m PCA. At the end of the experiment, the CBs were immersed in 100 μl of 3 m PCA and weighed. The collected fractions were maintained for 10 min at 0°C and then centrifuged 12 000 g for 10 min (4°C). ATP was extracted from the supernatant as described by Cunha et al. (1994). ATP release was quantified by a bioluminescence luciferin–luciferase assay. Briefly, for ATP quantification 100 μl of the samples was added to 100 μl of luciferin–luciferase (FLE50, Sigma) and to 2 ml of buffer (mm: Hepes 20, MgCl2 25, Na2HPO4 5). The reaction begins when the enzyme is added to the mixture. The samples were analysed in triplicate during 1 min by bioluminescence using a luminescence counter (Beckham). Quantification of endogenous ATP released by CB was done against external standards.
Recording of CSN activity
The CB–CSN preparation was transferred to a recording chamber mounted on a dissection microscope (Nikon Corporation, Tokyo, Japan) and superfused (37°C) with bicarbonate/CO2 buffered Tyrode solution (mm: NaCl 120, NaHCO3 24, KCl 3, CaCl2 2, MgCl2 1.1, glucose 5, pH 7.40). Recordings of single or few fibres of CSN were made using a suction electrode. The pipette potential was amplified (NeuroLog, Digitimer, Welwyn Garden City, UK), displayed on an oscilloscope and stored in a PC (200 Hz acquisition rate, Axonscope, Axon Instruments, Union City, CA, USA). Chemoreceptor activity was identified (spontaneous generation of action potentials at irregular intervals) and confirmed by its increase in response to hypoxia (normoxia: 20% O2, 5% CO2; balance N2; hypoxia: 0% O2, 5% CO2; balance N2). CSN activity was digitalized, summed every second and converted in a voltage proportional to the sum. The effects of low glucose (1 mm of glucose) on the control and hypoxic CSN activity were studied while perfusing the preparations with normoxic (20% O2-equilibrated) and hypoxic (5% O2-equilibrated) solutions; both responses were obtained from the same CB and 1 mm of glucose was present in the superfusing solution 5 min prior to stimulus application (20% O2-equilibrated solutions) and the 3 min of hypoxic stimulation.
Data analysis
Data were evaluated using GraphPad Prism v. 4 (GraphPad Software Inc., San Diego, CA, USA) and are presented as means ± s.e.m. The significance of the differences between the means was calculated by unpaired Student's t test and by one- and two-way analysis of variance (ANOVA) with Dunnett's and Bonferroni's multiple comparison tests, respectively. P values of 0.05 or less were considered to represent significant differences.
Results
Low glucose has no effect on the basal or hypoxic induced release of [3H]catecholamines from rat carotid body
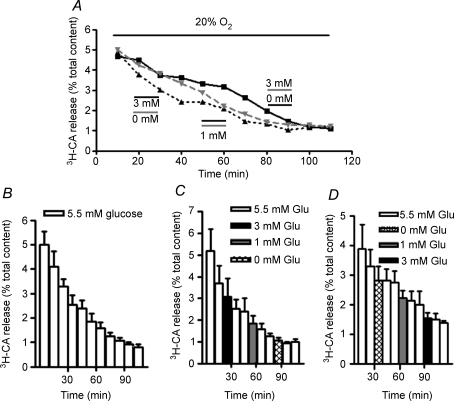
Figure 1A shows three single experiments carried out with individual CBs in normoxic conditions (20% O2-equilibrated solutions; PO2 ≈ 133 mmHg). Control CB was continuously superfused with a solution containing 5.5 mm glucose. Experimental CBs were similarly superfused with a solution containing 5.5 mm glucose except for the periods indicated. In one of the experimental CBs, we tested sequentially decreasing (0, 1 and 3 mm), and in the other increasing levels of hypoglycaemia (3, 1 and 0 mm) as potential stimuli for the CB. The ascending and descending patterns of low glucose application were aimed to avoid potential processes of sensitization or desensitization of the responses that might blur or mask the genuine effect of low glucose. As can be observed in Fig. 1C the mean basal (normoxic) release of [3H]CA from the CB was not modified by the graded reduction of glucose (3, 1 and 0 mm) maintaining the standard monotonic slow decay observed in control CB (Fig. 1B; see Almaraz et al. 1986 and Conde et al. 2006). In the same manner, the graded increase of glucose levels (0, 1 and 3 mm) did not modify the mean basal release of [3H]CA from the rat CB (Fig. 1D).
Figure 1. Effect of low glucose on the basal release of [3H]CA from rat carotid body.
A, the time course of the release of [3H]CA in three individual CBs incubated with solutions equilibrated with 20% O2. Control CB (continuous line) was continuously incubated with solutions containing 5.5 mm glucose and experimental CBs (discontinuous lines) were similarly incubated except for the marked periods where the glucose concentration was diminished. In one of the experimental CBs (dashed line) the concentration of glucose was reduced to 3 mm between minutes 20 and 30, to 1 mm between minutes 50 and 60 and to 0 mm between minutes 80 and 90. In the other experimental CB the sequence of low glucose incubation was reversed. In B, C and D each column represents the [3H]CA present in each 10 min collected fraction corresponding to the mean (± s.e.m.; n = 5–6) release in control and experimental CBs. To calculate the release as the percentage of the total tissue content, the dpm [3H]CA in each collected fraction was divided by the dpm of [3H]CA present in the tissue at any given moment in the experiment (i.e. dpm present in the tissue at the end of the experiment plus dpm present in the fractions collected after the one under consideration).
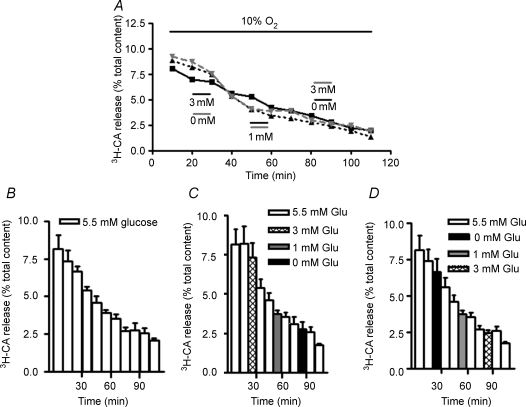
In the next series of experiments we tested low glucose for its effect on the release of [3H]CA elicited by the natural hypoxic stimulus of mild and moderate intensities, 10% and 7% O2, respectively. Figure 2A shows single experiments performed with individual CBs incubated in mild hypoxic conditions (PO2 ≈ 66 mmHg applied during the entire experiment). Incubating solutions for control CB contained 5.5 mm glucose for the entire experiment. For the experimental CBs, incubating solutions also contained 5.5 mm glucose except for the indicated 10 min periods; in one of the experimental CB the incubating solution contained 0, 1 and 3 mm glucose, respectively, for minutes 20–30, 50–60 and 80–90 of the experiment, while in the other experimental CB an increasing level of hypoglycaemia (3, 1 and 0 mm glucose) was tested at the same intervals of the experiment. It can be observed that glucose does not modify the release of [3H]CA evoked by mild hypoxia. Notice also that the same monotonic decay of the CA release, but with a higher CA output, was observed at 66 (Fig. 2A) and at 133 (Fig. 2A) mmHg of PO2. Figure 2B–D shows mean time courses of the mild hypoxic release in control conditions (5.5 mm glucose) and with intercalated episodes of hypoglycaemia. It can be observed that none of the low glucose levels tested modified (inhibited or potentiated) the time course for the [3H]CA evoked by mild hypoxia.
Figure 2. Effect of hypoglycaemia on the [3H]CA released from rat carotid body evoked by mild (10% O2) hypoxia.
A, the time course of the release of [3H]CA in three individual CBs incubated with solutions equilibrated with 10% O2. Control CB (continuous line) was continuously incubated with solutions containing 5.5 mm glucose and experimental CBs (dashed lines) were similarly incubated except for the marked periods where the glucose concentration was diminished. In one of the experimental CBs (dashed line) the concentration of glucose was reduced to 3 mm between minutes 20 and 30, to 1 mm between minutes 50 and 60 and to 0 mm between minutes 80 and 90. In the other experimental CB the sequence of low glucose incubation was reversed. In B–D each column represents the [3H]CA present in each 10 min collected fraction corresponding to the mean (+s.e.m.; n = 6) release in control and experimental CBs.
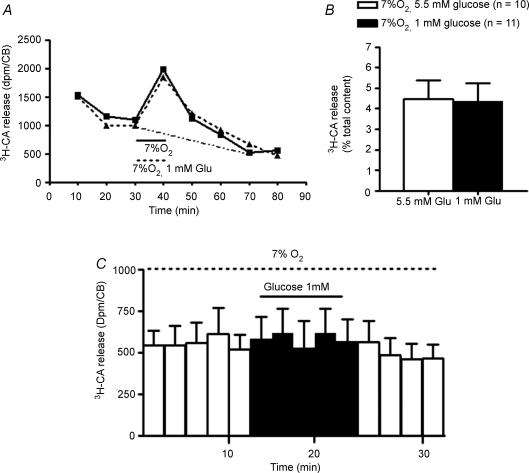
Figure 3A shows a single experiment carried out with two individual CBs. In the control CB hypoxia (superfusion with 7% O2) was applied from minutes 30 to 40 of the experiment and solutions contained 5.5 mm glucose throughout the experiment. The experimental CB was similarly superfused except for the coapplication of hypoxia and hypoglycaemia (1 mm of glucose) between minutes 30 and 40 as indicated in the drawing. It is evident from Fig. 3A that no interaction existed between hypoxia and hypoglycaemia as the time courses of [3H]CA release induced by hypoxia were nearly identical. The magnitude of the hypoxic release was similarly unaffected by concurrent hypoglycaemia as the mean release of [3H]CA evoked by hypoxia in 10 control CBs was identical to that obtained in 11 CBs in which hypoxia and hypoglycaemia were applied simultaneously: thus, hypoxia itself released [3H]CA amounting to 4.45 ± 0.91% of the CB content and hypoxia + low glucose released 4.37 ± 0.85% (P = 0.95). The absence of effects of low glucose on basal and hypoxia-induced release of [3H]CA seen with our experimental protocol contrasts with the observations made by Pardal & Barneo (2002), and differs from the experiments of the just mentioned authors in the protocol time course: they used short periods of superfusion (typically 2 min) with hypoglycaemic solutions and the possibility exists that a short-lived burst of CA release has passed unnoticed in our experiments where the superfusates were collected every 10 min. To exclude this possibility, we performed a new group of experiments testing the effect of 1 mm glucose on the release of [3H]CA evoked by 7% O2 collecting the fractions every 2 min (Fig. 3C). No effect of low glucose was seen in these conditions.
Figure 3. Effect of hypoglycaemia on the [3H]CA released from rat carotid body evoked by moderate (7% O2) hypoxia.
A, results obtained from two individual CBs. Control CB (continuous line) was incubated with solutions containing 5.5 mm glucose and equilibrated with 20% O2 except between minutes 30 and 40 where the hypoxic stimulus (7% O2-equilibrated solutions) was applied. Experimental CB was similarly treated, but the hypoxic incubating solution contained 1 mm glucose. Fractions collected every 10 min. B, mean evoked release (n = 10–11) elicited by 7% O2 and by 7% O2 with the reduction of glucose from 5.5 mm to 1 mm. Evoked release corresponds graphically to the area under the curves and the dashed–dotted line in Fig. 2A, which represents the interpolated basal release; mathematically it is calculated by the sum of dpm in samples 30–40 min, 40–50 min, 50–60 min and 60–70 min minus dpm in samples [(20–30 min + 70–80 min)/2]× 4. The resulting dpm is expressed as percentage tissue content. C, effect of low glucose (1 mm) on the mean (n = 11) time course of the release of [3H]CA evoked by 7% O2 (stimuli, both 7% O2 and 1 mm glucose, were applied during 10 min between minutes 12 and 22 of the experimental protocol; for the rest of the experiment the incubating solution contained 5.5 mm glucose). Each column represents the [3H]CA collected each 2 min. Data represent means +s.e.m.
Low glucose does not alter the endogenous release of ATP from rat carotid body chemoreceptor cells
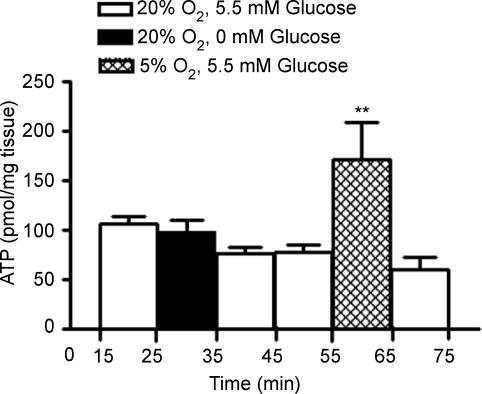
Recently it was published by Zhang et al. (2007) that low glucose stimulates ATP secretion in fresh tissue slices of rat CB, suggesting that low glucose utilizes similar neurotransmitter and neuromodulatory mechanisms to hypoxia. Since our data on the effect of low glucose on the release of CA are the opposite of previously published findings (Pardal & Barneo, 2002), we also wanted to investigate if low glucose modifies the release of ATP from the intact rat CB. As can be observed in Fig. 4, incubation of CB with a solution without glucose (0 mm) did not alter the ATP levels released from whole rat CB (98.04 ± 11.04 pmol (mg tissue)−1, n = 5) when compared with basal values (105.5 ± 7.54 pmol (mg tissue)−1, n = 5). In order to check the CB functionality, we tested in the same experiments the effect of hypoxia (5% O2) on the release of ATP and we observed that this stimulus increased by 122.1% the release of ATP from whole rat CB (Fig. 4), from 76.98 ± 7.83 pmol (mg tissue)−1 (ATP release from minutes 45–55 of the experiment; n = 5) to 170.6 ± 34.11 pmol (mg tissue)−1 (ATP release from minutes 55–65 of the experiment n = 5) (P = 0.008). Since the total ATP content in the CBs is in the vicinity of 4 nmol (mg tissue)−1, the fractional release of ATP in normoxia (ATP release × 10 min−1/ATP tissue content) amounts to 1.98%/10 min (Conde & Monteiro, 2006).
Figure 4. Effect of low glucose (0 mM glucose) and hypoxia (5% O2) on ATP release from rat CB chemoreceptor cells (n = 5).
0 mm glucose was applied between minutes 25 and 35 of the protocol and 5% O2 between minutes 55 and 65. The incubating solutions contained 5.5 mm glucose except when hypoglycaemic stimulus was applied. Data represent means ± s.e.m.**P < 0.01; one way ANOVA with Bonferroni's multicomparison test.
Low glucose has no effect on spontaneous and hypoxic chemosensory activity of carotid sinus nerve
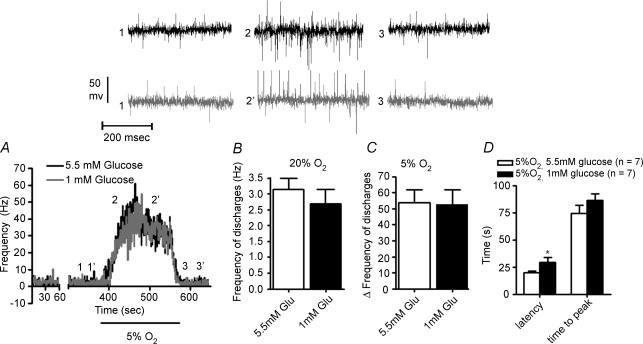
The direct effect of low glucose on the output of the CB was tested on the in vitro CB–CSN preparation by monitoring the action potential frequency in the CSN. In Fig. 5A a single experiment is presented. The preparation was initially superfused with solutions containing 5.5 mm glucose, and after a period of recording in normoxic conditions (superfusion with 20% O2-equilibrated solution) a hypoxic stimulus was applied (3 min superfuson with solutions equilibrated with 5% O2). The superfusion with normoxic normoglycaemic solution continued for 10 min and thereafter we started a superfusion with normoxic hypoglycaemic (1 mm glucose) that lasted 5 min followed by a 3 min hypoxic test while maintaining hypoglycaemia. To facilitate the comparison of the responses the records are superimposed, and at the top of the figure sample neurograms are shown. Reduction of glucose in the superfusion from 5.55 mm to 1 mm did not modify the basal normoxic activity of CSN (Fig. 5A and B) (5.55 mm glucose: 3.133 ± 3.6 Hz versus 1 mm glucose: 2.70 ± 0.4 Hz; P = 0.455), nor the frequency of discharges evoked by hypoxia in the CSN (Fig. 5C): 53.81 ± 7.8 (5% O2+ 5.55 mm glucose, n = 7) versus 52.69 ± 9.1 (5% O2+ 1 mm glucose, n = 7) (P = 0.93). Superfusion with 1 mm glucose delayed significantly the onset of the hypoxic response (latency of the response) (P < 0.05, two-way ANOVA with Bonferroni's multicomparison test) but not the time to reach the maximal activity (time to peak) (Fig. 5D).
Figure 5. Effect of low glucose (1 mM) on the spontaneous (20% O2) carotid sinus nerve (CSN) activity and on the activity elicited by hypoxia (superfusion with solutions equilibrated with 5% O2).
A, typical recording of the effect of low glucose (1 mm) on the frequency of action potentials of CSN during superfusion with a solution equilibrated with 5% O2. In black is shown the control response to hypoxia (5.5 mm glucose) and in grey the effect of hypoglycaemia (1 mm) on the hypoxic response. Both responses were obtained from the same CB and 1 mm of glucose was present in the superfusing solution the 5 min prior to and the 3 min of hypoxic stimulation; the rest of the experiment the superfusing solution contained 5.5 mm glucose. At the top of the figure are shown sample neurograms obtained at the times indicated in A. B–D show, respectively, mean basal frequencies, mean peak frequencies and latencies and times to peak in control (open bars) and in low glucose-treated preparations (filled bars). Data represent means +s.e.m. (n = 7; P < 0.05, two-way ANOVA with Bonferroni's multicomparison test.
Effects of long-term application of low glucose on CA release and CSN chemosensory activity
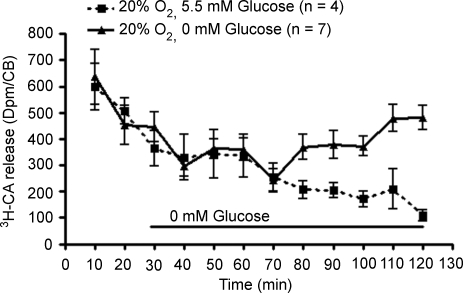
We also searched for the effects of longer applications (100 and 120 min) of 0 mm glucose on the basal release of [3H]CA from CB chemoreceptor cells and CSN chemosensory activity. Figure 6 shows that low glucose did not alter basal [3H]CA release for the initial 40 min (minutes 30–70 of the experiment). Thereafter, the release of the [3H]CA started to increase and continued to increase until the end of the experiment. Contrary to that, the release from control CBs exhibited the normal monotonic decay usually observed (see also Figs 1 and 2). In the same manner, and as previously observed by Almaraz et al. (1984) in the cat, long-term superfusion with 0 glucose did not modify the spontaneous normoxic CSN chemosensory activity in the rat in the first hour of application, but prolonging the 0 glucose superfusion to 100 min resulted in a decrease in the spontaneous CSN activity and a loss of capacity to respond to hypoxia (data not shown; see Almaraz et al. 1984).
Figure 6. Effect of long-term application of 0 mM glucose on the basal [3H]CA release from rat carotid body.
Mean (± s.e.m.) time course of [3H]CA basal release from rat CB incubated with 5.5 mm glucose (controls, continuous line; n = 4) and from rat CBs incubated with 5.5 mm glucose for the initial 30 min and with 0 mm glucose between minutes 30 and 120 (experimental, discontinuous line; n = 7). Incubated solutions were collected every 10 min, each point representing the [3H]CA present in the solutions collected.
Discussion
Using two different types of approach, the release of [3H]CA and ATP directed to testing the functionality of chemoreceptor cells, and the action potential frequency in the CSN directed to measure the output of the entire CB complex, we have demonstrated that low glucose is not a direct stimulus in freshly isolated intact CB preparations. Low glucose, of concentrations from 3 to 0 mm,per se did not activate chemoreceptor cells nor the chemoreceptor cell–CSN complex and was equally ineffective in altering the activity elicited by hypoxic stimulus.
The present results confirm previous findings from our laboratory using the intact cat CB–CSN preparation (Almaraz et al. 1984). It should be stated that those old experiments were beyond the scope of the present study, and on no occasion did we detect an activation of CSN discharges. The present findings also confirm those recently reported by Bin-Jaliah et al. (2004, 2005) in the freshly isolated rat CB–CSN preparation: low and 0 glucose do not activate the arterial chemoreceptors. As mentioned in the Introduction, the studies just mentioned used preparations superfused with solutions equilibrated at PO2 > 400 mmHg. Although, conceptually, probably high PO2 represents the ideal conditions to test for the ability of the CB to sense any given stimulus (different from hypoxia itself), the fact that Pardal & Lopez-Barneo (2002; see also Zhang et al. 2007) described sensitivity of chemoreceptor cells to low glucose at near physiological PO2 prompted the study at different PO2. As shown throughout our study, at none PO2 tested (∼133, 66, 46 and 33 mmHg) did low glucose or zero glucose activates either chemoreceptor cells or the CB–CSN complex. Thus, differences in the PO2 used in the experiments in intact preparations versus slices or cocultures is not the factor determining divergent findings as suggested by Zhang et al. (2007) as we originally thought in designing the study.
We do not have a satisfactory explanation for the discrepancies in findings, but it might be that differences in preparations can be responsible for them. For example, Pardal & Lopez-Barneo (2002) used rat CB slices (the intact rat CB weighs as an average 50 μg, i.e. if considered spherical it will be less than 250 μm in diameter) cultured for 48–72 h and therefore the possibility exists that either the preparation of the slices itself (temperature shock, attachment with cyanoacrylate of the blocks) or the culture conditions modifies the phenotype of chemoreceptor cells. These considerations hold also for part of the study of Zhang et al. (2007). In fact several studies support our contentions. For example, Gauda (2002) and Gauda et al. (2004) in semiquantitative in situ hybridization histochemistry and immunohistochemistry did not detect cholinergic traits in chemoreceptor cells, traits that were patent in microganglion cells and nerve fibres innervating the rat CB; on the contray, Zhang et al. (2000) provided evidences for cholinergic markers in chemoreceptor cells in their chemoreceptor cell–petrosal ganglion neuron cocultures. The recent studies of Reyes et al. (2007a,b), although performed in different species, point in the same direction: these authors found in in vivo and in vitro preparations that a combination of nicotinic and purinergic blockers only partially reduced the activity elicited by hypoxia in the CSN or reflexly in the phrenic nerve in cat preparations; Zhang et al. (2000) in their coculture preparation found that the same mixture of blockers completely abrogates the hypoxic responses. Recent data of He et al. (2005, 2006) obtained in the intact rat CB–CSN preparation would also be consistent with the observations of Reyes et al. (2007a,b).
More intriguing are the findings on the release of ATP by Zhang et al. (2007); in slices prepared and studied in the course of 1 h, i.e. when the phenotypic changes are unlikely to have appeared, they found that hypoglycaemia promotes the release of ATP from CB slices. However, we are puzzled by the data of these authors on the release of ATP. In a previous publication of the same laboratory there was reported a release of ATP from CB slices that was extremely high (Buttigieg & Nurse, 2004), and in the more recent publication (Zhang et al. 2007) they also report amounts of ATP released that still appear very high if they are compared with data obtained in bovine chromaffin cells stimulated with nicotine (Kuijpers et al. 1989) and in hippocampal synaptosomes (Cunha et al. 2000). In these two last preparations, as well as in the intact CB (present study), the ATP release per 3 min approaches 0.6% of the tissue content, nearly 15 times lower than the release obtained in the slice preparation by Zhang et al. (2007).
In any case it should be mentioned that there are also discrepancies between the findings of Pardal & Lopez-Barneo (2002) and those of Zhang et al. (2007). The first authors found that input resistance measured in patch-clamped cells (−80 mV) was not altered by removal of glucose; on the contrary, glucose deficiency produced a reversible reduction of outward K+ current amplitude in the entire range of membrane potentials (−40 to +60 mV) implying that chemoreceptor cell depolarization and Ca2+ entry was associated with an increase in cell input resistance (see Kumar, 2007). On the contrary, Zhang et al. (2007) found that hypoglycaemia produced chemoreceptor cell depolarization, which was associated with a decrease in input resistance in the cell. A very recent publication of Lopez-Barneo's laboratory (Garcia-Fernandez et al. 2007) using the same slice preparation as the previous study (Pardal & Lopez-Barneo, 2002) confirms most of their previous observations. However, contrary to previous findings, they report now that low glucose induces a transient receptor potential channel-mediated inward current (i.e. a decrease in input resistance) that would be responsible for cell depolarization and triggering of the CA release response. In the most recent publication (Garcia-Fernandez et al. 2007) they also observed that inhibition of K+ currents with TEA or iberiotoxin elicits a minimal secretory response while in a previous publication (Pardal et al. 2000), using the same slice preparation, they reported that TEA and iberiotoxin elicited a powerful secretory response that nearly doubled that elicited by intense hypoxia, which in turn was comparable to that elicited by 0 glucose (Pardal & Lopez-Barneo, 2002).
In conclusion, the present data do not support the role of chemoreceptor cells as glucose sensors, and certainly do not confirm the notion that the CB forms part of a group of extracranial glucose sensors like pancreas, liver and hepatic portal vein, thereby, initiating reflex responses when the levels of glucose fall below the normal range. On the contrary, our data fit the notions advanced by Bin-Jaliah et al. (2004) indicating that the carotid body-dependent increase in ventilation observed in hypoglycaemia, for example in hypoglycaemia induced by insulin injection, would be produced through the increased systemic metabolic rate and modifications in arterial blood gas tensions produced by insulin, and not by hypoglycaemia per se.
Acknowledgments
We want to thank Ma de los Llanos Bravo for technical assistance. The work was supported by grants BFU2004-06394 (DGICYT), PI042462 and CIBER CB06/06/0050 (FISS-ICiii), VA045/04 and VA011C05 (JCyL) and CEPR/Fundação para a Ciência e Tecnologia (Portugal). S.V.C. is funded by a PhD grant from Fundação para a Ciência e Tecnologia (SFRH/BD/14178/2003).
References
- Almaraz L, Gonzalez C, Obeso A. Effects of high potassium on the release of [3H]dopamine from the cat carotid body in vitro. J Physiol. 1986;379:293–307. doi: 10.1113/jphysiol.1986.sp016254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaraz L, Obeso A, Gonzalez C. Metabolic dissociation of carotid body chemoreceptors responses to different types of stimulation: preliminary findings. In: Pallot DJ, editor. The Peripheral Arterial Chemoreceptors. New York: Oxford University Press; 1984. pp. 141–151. [Google Scholar]
- Alvarez-Buylla R, Alvarez-Buylla ER. Carotid sinus receptors participate in glucose homeostasis. Respir Physiol. 1988;723:347–359. doi: 10.1016/0034-5687(88)90093-x. [DOI] [PubMed] [Google Scholar]
- Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physol. 2004;556:225–266. doi: 10.1113/jphysiol.2003.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin-Jaliah I, Maskell PD, Kumar P. Carbon dioxide sensitivity during hypoglycaemia-induced, elevated metabolism in the anaesthetized rat. J Physiol. 2005;563:883–893. doi: 10.1113/jphysiol.2004.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophy Res Com. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Conde SV, Monteiro EC. Profiles for ATP and adenosine release at the rat carotid body in response to O2 concentrations. Adv Exp Med Biol. 2006;580:179–184. doi: 10.1007/0-387-31311-7_27. [DOI] [PubMed] [Google Scholar]
- Conde SV, Obeso A, Vicario I, Rigual R, Rocher R, Gonzalez C. Caffeine inhibition of rat carotid body chemoreceptors is mediated by A2A and A2B adenosine receptors. J Neurochem. 2006;98:616–628. doi: 10.1111/j.1471-4159.2006.03912.x. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Almeida T, Ribeiro JA. Modification by arachidonic acid of extracellular adenosine metabolism and neuromodulatory action in the rat hippocampus. J Biol Chem. 2000;275:37572–37581. doi: 10.1074/jbc.M003011200. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Milusheva E, Vizi ES, Ribeiro JA, Sebastião AM. Excitatory and inhibitory effects of A1 and A2 adenosine receptor activation on the electrically evoked [3H]acetylcholine release from different areas of the rat hippocampus. J Neurochem. 1994;63:207–214. doi: 10.1046/j.1471-4159.1994.63010207.x. [DOI] [PubMed] [Google Scholar]
- Fidone SJ, Gonzalez C. Catecholamine synthesis in rabbit carotid body in vitro. J Physiol. 1982;333:69–79. doi: 10.1113/jphysiol.1982.sp014439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fernandez M, Ortega-Saenz P, Castellano A, Lopez-Barneo J. Mechanisms of low-glucose sensitivity in carotid body glomus cells. Diabetes. 2007 doi: 10.2337/db07-0122. in press. [DOI] [PubMed] [Google Scholar]
- Gauda EB. Gene expression in peripheral arterial chemoreceptors. Micro Res Tech. 2002;59:153–167. doi: 10.1002/jemt.10190. [DOI] [PubMed] [Google Scholar]
- Gauda EB, Cooper R, Johnson SM, McLemore GL, Marshall C. Autonomic microganglion cells: a source of acetylcholine in the rat carotid body. J Appl Physiol. 2004;96:384–391. doi: 10.1152/japplphysiol.00897.2003. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- He L, Chen J, Dinger B, Stensaas L, Fidone S. Effect of chronic hypoxia on purinergic synaptic transmission in rat carotid body. J Appl Physiol. 2006;100:157–162. doi: 10.1152/japplphysiol.00859.2005. [DOI] [PubMed] [Google Scholar]
- He L, Dinger B, Fidone S. Effect of chronic hypoxia on cholinergic chemotransmission in rat carotid body. J Appl Physiol. 2005;98:614–619. doi: 10.1152/japplphysiol.00714.2004. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49:1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- Kuijpers GA, Rosario LM, Ornberg RL. Role of intracellular pH in secretion from adrenal medulla chromaffin cells. J Biol Chem. 1989;264:698–705. [PubMed] [Google Scholar]
- Kumar P. How sweet it is: sensing low glucose in the carotid body. J Physiol. 2007;578:627. doi: 10.1113/jphysiol.2006.126250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Lopez-Barneo L. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Pardal R, Ludewig U, Garcia-Hirschfeld J, Lopez-Barneo J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc Natl Acad Sci U S A. 2000;97:2361–2366. doi: 10.1073/pnas.030522297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2+-activated K+ current. Neurosci Lett. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Reyes EP, Fernandez R, Larrain C, Zapata P. Effects of combined cholinergic-purinergic block upon cat carotid body chemoreceptors in vitro. Respir Physiol Neurobiol. 2007a;156:17–22. doi: 10.1016/j.resp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Reyes EP, Fernandez R, Larrain C, Zapata P. Carotid body chemosensory activity and ventilatory chemoreflexes in cats persist after combined cholinergic-purinergic block. Respir Physiol Neurobiol. 2007b;156:23–32. doi: 10.1016/j.resp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Rigual R, Rico AJ, Prieto-Lloret J, de Felipe C, Gonzalez C. Chemoreceptor activity is normal in mice lacking the NK1 receptor. Eur J Neurosci. 2002;16:2078–2084. doi: 10.1046/j.1460-9568.2002.02293.x. [DOI] [PubMed] [Google Scholar]
- Vicario I, Rigual R, Obeso A, Gonzalez C. Characterization of the synthesis and release of catecholamine in the rat carotid body in vitro. Am J Physiol Cell Physiol. 2000;278:C490–C499. doi: 10.1152/ajpcell.2000.278.3.C490. [DOI] [PubMed] [Google Scholar]
- Zhang M, Buttigieg J, Nurse C. Neurotransmitter mechanisms mediating low-glucose signalling in co-cultures and fresh tissue slices of rat carotid body. J Physiol. 2007;578:735–750. doi: 10.1113/jphysiol.2006.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signaling at rat carotid body chemoreceptors. J Physiol. 2000;525:143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]