Abstract
Active synapses can reduce the probability of transmitter release at neighbouring synapses. Depending on whether such heterosynaptic depression is mediated by intersynaptic diffusion of transmitter or by release of gliotransmitters, astrocytes should either hinder or promote the heterosynaptic depression. In the present study we have examined the developmental profile and astrocytic involvement in a transient heterosynaptic depression (tHeSD) in the CA1 region of the rat hippocampal slice preparation. A short stimulus burst (3 impulses at 50 Hz) to one group of synapses elicited a depression of the field EPSP evoked in another group of synapses that amounted to about 25% 0.5 s after the conditioning burst. This tHeSD was associated with an increase in the paired-pulse ratio of about 30%. The tHeSD was not present in slices from rats younger than 10 postnatal days and developed towards the adult magnitude between postnatal days 10 and 20. The tHeSD was totally prevented by the glia-specific toxin fluoroacetate (FAC), by carbenoxolone, a general blocker of connexin-based channels, and by endothelin, an endogenous peptide that has been shown to block astrocytic connexin-based channels. Antagonists to GABAB receptors and group II/III metabotropic glutamate receptors (mGluRs) abolished the tHeSD whereas antagonists to NMDA- and adenosine A1 receptors, and to group I mGluRs, did not affect the tHeSD. These results suggest that the tHeSD relies on GABAB receptors, group II/III mGluRs and on gliotransmitter release from functionally mature astrocytes.
By releasing transmitter, synapses can affect the efficacy of neighbouring synapses. Two fundamentally different routes of signalling have been proposed to account for such intersynaptic communication. In one scenario, active synapses release transmitter that activate astrocytes to release gliotransmitters that, in turn, affect the efficacy of neighbouring synapses. Gliotransmitters, such as glutamate, d-serine and ATP/adenosine, modulate release probability by activating presynaptic receptors and postsynaptic responsiveness by activating synaptic and extrasynaptic receptors (Volterra & Steinhauser, 2004; Allen & Barres, 2005; Volterra & Meldolesi, 2005; Haydon & Carmignoto, 2006). In the other scenario, synaptically released glutamate or GABA diffuses into the extracellular space to directly affect neighbouring synapses (Isaacson et al. 1993; Rusakov et al. 1999). In this latter scenario for intersynaptic communication, astrocytes counteract rather than mediate synaptic crosstalk by constituting diffusion barriers and by expressing uptake transporters for neurotransmitters. Consequently, inhibitors of transmitter uptake transporters (Isaacson et al. 1993; Rusakov et al. 1999) and retraction of astrocytes facilitate such transmitter spillover (Piet et al. 2004).
In both these scenarios for intersynaptic communication astrocytes have a critical, but opposite, role. Since astrocytes develop during a prolonged postnatal period in rodents (Konietzko & Muller, 1994; Bushong et al. 2004; Kugler & Schleyer, 2004), and since glutamate uptake is slower early in development (Fiala et al. 1998; Diamond, 2005), one would expect that intersynaptic communication mediated via astrocytes is poorly developed whereas intersynaptic communication mediated via extrasynaptic spillover is favoured. Not much is known, however, about how intersynaptic communication changes with development.
Heterosynaptic depression is a form of intersynaptic communication in which active synapses decrease the efficacy of neighbouring, inactive synapses. In the CA1 region of the hippocampus relatively intense activation (10–100 stimuli at 10–200 Hz) of Schaffer collaterals results in a heterosynaptic depression that can last for tens of minutes (Lynch et al. 1977; Wickens & Abraham, 1991; Grover & Teyler, 1993; Manzoni et al. 1994). Such synaptic activations have been shown to elicit calcium signals in the astrocytic network (Porter & McCarthy, 1996; Pasti et al. 1997; Zhang et al. 2003; Serrano et al. 2006). Astrocytic calcium signals and the release of ATP/adenosine have also been linked to heterosynaptic depression (Zhang et al. 2003; Pascual et al. 2005; Serrano et al. 2006). More modest synaptic activation (2–5 impulses at 50 Hz), resembling naturally occurring neural activity in the hippocampus (Lisman, 1997; Buhl & Buzsaki, 2005), results in a transient (lasting for seconds) heterosynaptic depression (tHeSD) (Gustafsson et al. 1989; Isaacson et al. 1993). This tHeSD was also observed when the postsynaptic neuron was voltage clamped, indicating that it relies on extracellular factors (Isaacson et al. 1993). In the present study, we show that this tHeSD is not developed before the end of the 2nd postnatal week and provide evidence that it relies on gliotransmitter release from functional astrocytes rather than relying on spillover of transmitter between synapses.
Methods
Slice preparation and solutions
Experiments were performed on hippocampal slices from 5- to 50-day-old Wistar rats. The animals were killed in accordance with the guidelines of the local ethical committee for animal research. Rats were anaesthetized with isoflurane (Abbott) prior to decapitation. The brain was removed and placed in an ice-cold solution containing (mm): 140 choline chloride, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, 1.3 ascorbic acid and 7 dextrose. Transverse hippocampal slices (300–400 μm thick) were cut with a vibratome (HM 650V Microm, Germany) in the same ice-cold solution and they were subsequently stored in artificial cerebrospinal fluid (ACSF) containing (mm): 124 NaCl, 3 KCl, 2 CaCl2, 4 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 0.5 ascorbic acid, 3 myo-inositol, 4 d,l-lactic acid, and 10 d-glucose. After at least 1 h of storage at 25°C, a single slice was transferred to a recording chamber where it was kept submerged in a constant flow (∼2 ml min−1) at 30–32°C. The perfusion fluid contained (mm); 124 NaCl, 3 KCl, 4 CaCl2, 4 MgCl2, 26 NaHCO3, 1.25 NaH2PO4,and 10 d-glucose. Picrotoxin (100 μm) was always present in the perfusion fluid to block GABAA receptor-mediated activity. All solutions were continuously bubbled with 95% O2 and 5% CO2 (pH ∼7.4). The higher than normal Ca2+ and Mg2+ concentrations were used to block network activity.
Recording and analysis
Electrical stimulation of Schaffer collateral–commissural axons and recordings of synaptic responses were carried out in the stratum radiatum of the CA1 region. Stimuli consisted of biphasic constant current pulses (15–80 μA, 200 μs, STG 1002 Multi Channel Systems MCS Gmbh, Reutlingen, Germany) delivered through tungsten wires (resistance ∼0.1 MΩ) or glass electrodes (resistance ∼1 MΩ). Two stimulation electrodes were positioned in the stratum radiatum with a distance of about 500 μm from each other. The two synaptic inputs were activated every 10 s. Field EPSPs were recorded with a glass micropipette (filled with perfusion fluid or 1 m NaCl, resistance 1–2 MΩ) placed between the two stimulation electrodes (Fig. 1A). Field EPSPs were sampled at 10 kHz with an EPC-9 amplifier (HEKA Elektronik, Lambrecht, Germany) and filtered at 1 kHz. Evoked responses were analysed off-line using custom-made IGOR Pro (WaveMetrics, Lake Oswego, OR, USA) software. Field EPSP magnitude was estimated by linear regression over the first 0.8 ms of the initial slope. The presynaptic volley was measured as the slope of the initial positive–negative deflection, and experiments in which the volley changed more than 10% during the experiment were discarded.
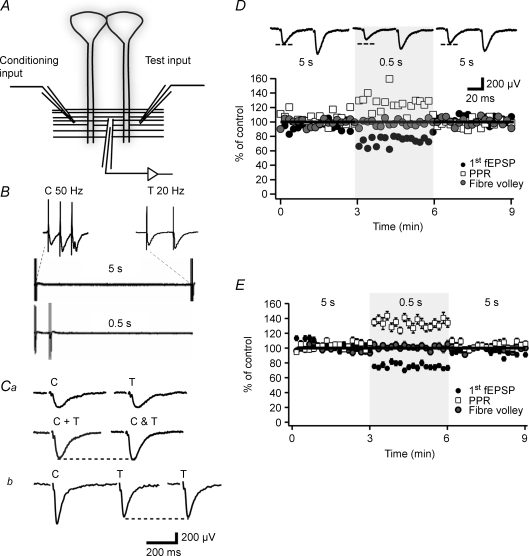
Figure 1. The transient heterosynaptic depression is associated with increased paired-pulse ratio.
A, schematic illustration of the placement of stimulation and recording electrodes in the stratum radiatum of the CA1 region. B, sweeps illustrating the long-interval conditioning (5 s, upper) used as control and the short-interval conditioning (0.5 s, lower) used to elicit the tHeSD. The conditioning input is activated with 3 impulses at 50 Hz as shown expanded in the left inset. The test input is activated with 2 impulses at 20 Hz as shown expanded in the right inset. Ca, summation test for synaptic pathway independence. The sum (C + T, left) of the fEPSP evoked from the conditioning input (C, upper left sweep) and the fEPSP evoked from the test input (T, upper right sweep) is compared to the fEPSP obtained when activating the two inputs simultaneously (C & T). Since the sum of the two fEPSPs (C + T) is not larger than the simultaneously evoked fEPSP (C & T) this result indicates that the two synaptic pathways do not share common synapses. Cb, paired-pulse test for synaptic pathway independence. The fEPSPs evoked from the test input (T) are shown when activated with (T, left) and without (T, right) the activation of the conditioning input (C) 50 ms earlier. The absence of a larger (facilitated) test fEPSP with a preceding conditioning stimulation indicates that the two synaptic pathways do not share common synapses. D, an experiment illustrating the reversible depression of the fEPSP (filled circles) and the associated increase in paired-pulse ratio (open squares). Note that fibre volley remains stable (grey circles). Shaded area indicates short-interval conditioning (0.5 s). Values of fEPSP initial slope and paired-pulse ratio are normalized to the average value during the long-interval conditioning. Average fEPSPs taken from the long-, short- and long-interval conditioning, respectively, are shown on top (n = 18 sweeps for each interval). E, graph summarizing 56 experiments such as that shown in A from 21- to 50-day-old-rats.
Data are expressed as means ± s.e.m. Statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001) for paired and independent samples was evaluated using Student's t test.
Drugs
Drugs were from Tocris Cookson (Bristol, UK) except picrotoxin from Sigma-Aldrich (Stockholm, Sweden) and endothelin-1 from Neosystem (Illkirch, France).
Results
To examine transient heterosynaptic depression (tHeSD), in the CA1 stratum radiatum, two independent synaptic inputs were activated alternately (Fig. 1A). One input was used as test input and was activated using paired-pulse stimulation (50 ms between stimuli) to disclose possible changes in release probability associated with tHeSD (Fig. 1B). The stimulation intensity was adjusted such that the second (facilitated) fEPSP was just below threshold for population spike activity. The other input was used as conditioning input and was activated with a 3-impulse train (50 Hz) and the stimulus intensity was adjusted such that a small population spike was evident on the second and/or the third (facilitated) fEPSP. The two inputs were activated every 10 s and the interval between the activation of the two inputs was either 5 s (‘long interval’) or 0.5 s (‘short interval’) (Fig. 1B). Two different tests were performed to ascertain that the two stimulation electrodes activated different axons. First, the initial slope of the sum of the two fEPSPs evoked separately was not larger than the initial slope of the fEPSP evoked simultaneously from the two electrodes (Wigström & Gustafsson, 1985) (Fig. 1Ca). Second, there was no facilitation of the test fEPSP when preceded (50 ms) by a single stimulation of the conditioning pathway (Sastry et al. 1986) (Fig. 1Cb).
The tHeSD is associated with increased paired-pulse ratio
The experimental protocol consisted of an initial control period using a long interval with 18 stimulations (3 min), a test period using the short interval with 18 stimulations (3 min), and a final control period using the long interval with 18 stimulations. Before starting the experimental protocol, the synaptic inputs were stimulated for variable periods to obtain stable responses. Nevertheless, during the experimental sequence there was often a slight decay (< 10%) of the synaptic responses (cf. Fig. 1E). To deal with this decay and not overestimate any tHeSD, the effect on the test synapses by the short interval was compared to the average effect on the test synapses by the first and the last long interval control period.
Figure 1D shows the result from one typical experiment from a 21-day-old rat. The short interval conditioning resulted in a clear depression of the test fEPSP slope which was associated with an increased paired-pulse ratio (PPR). Figure 1E summarizes the results from 56 such experiments from 21- to 50-day-old rats. The test fEPSP was depressed to 76 ± 1.3% of control (n = 56, P < 0.0001). This tHeSD was accompanied by a 29 ± 2.4% (n = 56, P < 0.0001) increase of the PPR. The presynaptic volley was unchanged following a shift to the 0.5 s interval (102 ± 4%, n = 52, P = 0.52) (Fig. 1E). Prolongation of the conditioning–test interval to 10 s did not result in any significant increase in the test fEPSP (107 ± 9%, n = 6, P = 0.5, data not shown), showing that the presently examined heterosynaptic depression is transient on a 5 s scale.
tHeSD is absent in young animals
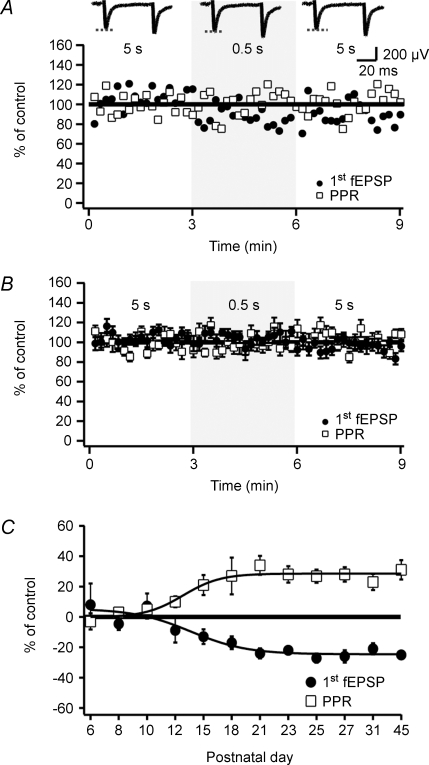
We next examined the magnitude of the tHeSD in slices from 5- to 10-day-old rats. Figure 2A shows an experiment from a 6-day-old rat and in which the short-interval conditioning produced no depression of the test fEPSP, or any change of the PPR. Figure 2B summarizes 26 experiments from rats aged between 5 and 10 postnatal days. In striking contrast to the findings from the older rats, the tHeSD was absent in this younger group. The test fEPSP following the short-interval conditioning was 104 ± 4% (n = 26, P = 0.508) of control and the PPR was also not altered (99 ± 2%, n = 26, P = 0.261). Figure 2C illustrates the developmental profile of the tHeSD. This plot shows that the tHeSD emerges by the end of second postnatal week and is fully developed within the third postnatal week.
Figure 2. tHeSD is absent in young animals.
A, an experiment from a 6-day-old rat illustrating the absence of transient heterosynaptic depression. Shaded area indicates short-interval conditioning (0.5 s). Values of fEPSP initial slope and paired-pulse ratio are normalized to the average value during the long-interval conditioning. Average fEPSPs taken from the long-, short- and long-interval conditioning, respectively, are shown on top (n = 18 sweeps from each interval). B, graph summarizing 26 experiments such as that shown in A from 5- to 10-day-old rats. C, developmental profile of the transient heterosynaptic depression. Average change in fEPSP initial slope (filled circles) and paired-pulse ratio (open squares) during the short-interval conditioning are plotted versus postnatal age (n = 5–15 slices per group). Sigmoidal fits (dashed lines) are included for visual guidance.
Astrocytes are necessary for the tHeSD
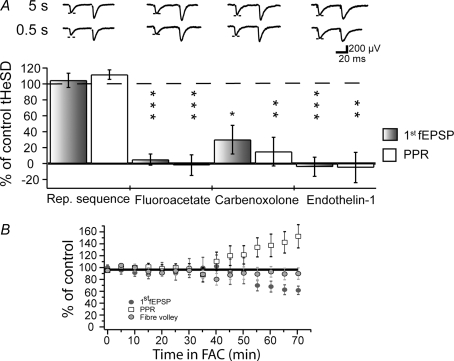
The developmental profile of the tHeSD is consistent with the view that astrocytes mediate, rather than restrict, the tHeSD. To test the possible involvement of astrocytes in the tHeSD, we used a glia-specific toxin (FAC) and two different blockers of connexin-based channels (carbenoxolone and endothelin-1). To evaluate the pharmacological effects on tHeSD, the experimental sequence was performed twice, first in the absence then in the presence of the drug. In these matched pharmacological experiments, the magnitude of the second test tHeSD was expressed as a percentage of the first control tHeSD. Experiments in which the first control tHeSDs did not reach 10% depression (3 of 56) were excluded from further analysis. To ascertain that repetition of the experimental sequence did not affect the amount of tHeSD, we performed the double sequence in the absence of any drugs. As shown in Fig. 3A (‘Rep. sequence’), the second tHeSD was not different from the first tHeSD.
Figure 3. Astrocytes are necessary for the tHeSD.
A, the graph illustrates the results of matched comparisons of tHeSD before and after pharmacological interventions. The filled bars compare the effect on the fEPSP slope and the open bars compare the effect on the paired-pulse ratio. 100% means the same tHeSD as in the control situation and 0% means absence of tHeSD. Typical control (‘5 s’, upper) and test (‘0.5 s’, lower) sweeps are shown above the bars. Dashed line indicates the amplitude of the control fEPSP. ‘Rep. sequence’ experiments showing no change in the tHeSD when the experimental protocol is repeated in the absence of any drug. ‘Fluoroacetate’ shows effect of the astrocyte-specific metabolic inhibitor fluoroacetate (10 mm) on the tHeSD (n = 6). ‘Carbenoxolone’ shows effect of the connexin-based channel inhibitor carbenoxolone (100 μm) on the tHeSD (n = 6). ‘Endothelin-1’ shows the effect of the connexin-based channel inhibitor endothelin-1 (1 μm) on the tHeSD (n = 6). B, average (5 min binned) 1st fEPSP initial slope (dark grey circles), paired-pulse ratio (open squares) and volley (light grey circles) after application of fluoroacetate (FAC) expressed as percentage of 1st fEPSP initial slope, paired-pulse ratio and volley before application and plotted against minutes in FAC (n = 8).
FAC is exclusively taken up by astrocytes and is converted in the astrocyte to fluorocitrate, which is an inhibiting substrate of the Krebs cycle enzyme aconitase and has been shown to specifically depress astrocytic function (Fonnum et al. 1997). Following application of FAC (10 mm), the fEPSP was stable for about 40 min (Fig. 3B). Thereafter we observed a progressive decay of the fEPSP that was associated with an increase of the PPR (Fig. 3B). Moreover, in 3 of 6 experiments in the prolonged presence (> 80 min) of FAC we observed spontaneous large long-lasting (300–1200 ms) negative potentials, possibly akin to the previously described astrocytes-mediated slow inward currents (Angulo et al. 2004; Fellin et al. 2004; Fiacco et al. 2007). To examine whether FAC affected the tHeSD, we compared the tHeSD 30 min after the application (when FAC still had not produced any change of the fEPSP, Table 1) with the control tHeSD before the application of FAC. As shown in Fig. 3A, 30 min exposure to FAC completely inhibited the tHeSD and the associated increase in PPR.
Table 1.
Effects of various pharmacological manipulations on facilitated (f)EPSP slope and paired-pulse ratio (PPR)
| Drug | 1st fEPSP | P | PPR | P | n |
|---|---|---|---|---|---|
| Carbenoxolone (10 min) | 78 ± 2% | ** | 25 ± 7% | ** | 6 |
| Endothelin-1 | 107 ± 16% | P = 0.71 | 107 ± 6% | P = 0.27 | 6 |
| Fluoroacetate (10 min) | 100 ± 6% | P = 0.85 | 104 ± 3% | P = 0.22 | 9 |
| Fluoroacetate (30 min) | 92 ± 7% | P = 0.87 | 101 ± 9% | P = 0.92 | 9 |
| CGP52432 | 95 ± 5% | P = 0.34 | 105 ± 4% | P = 0.18 | 4 |
| DPCPX | 132 ± 7% | * | 85 ± 4% | * | 4 |
| LY 341495 | 102 ± 5% | P = 0.40 | 106 ± 5% | P = 1.13 | 6 |
| APCD | 83 ± 3% | * | 112 ± 4% | * | 8 |
| l-AP4 (50 μm) | 97 ± 5% | P = 0.58 | 108 ± 5% | P = 0.17 | 4 |
| l-AP4 (1.2 mm) | 59 ± 8% | * | 118 ± 6% | * | 8 |
The values are expressed as a percentage of the control before the application of the drug.
P < 0.05
P < 0.01.
Application of carbenoxolone (100 μm) resulted in a progressive decrease of the fEPSP (Table 1). This depressive effect of carbenoxolone was associated with an increase in PPR (from 1.4 to 1.75 ± 0.07, n = 6, P < 0.001 (paired t test)) and with no change in the presynaptic volley (103 ± 6%; P = 0.55). Carbenoxolone is known to have other effects than blocking connexin-based channels, one of these being to inhibit voltage-gated calcium channels (Vessey et al. 2004), which would explain the carbenoxolone-mediated depression. Nevertheless, carbenoxolone prevented the tHeSD and the associated increase in PPR (Fig. 3A).
We next tested endothelin-1 (1 μm), an endogenous peptide that has been shown to block coupling between astrocytes by de-phosphorylating the major astrocytic connexin, connexin43 (Blomstrand et al. 2004). In contrast to carbenoxolone, endothelin-1 did not cause any reduction in the magnitude of the synaptic response (Table 1). In common with carbenoxolone, however, endothelin-1 prevented the tHeSD and the associated increase in PPR (Fig. 3A). These results show that functional connexin-based channels are necessary for the manifestation of the tHeSD.
The tHeSD relies on GABAB receptors, but not on NMDA or adenosine A1 receptors
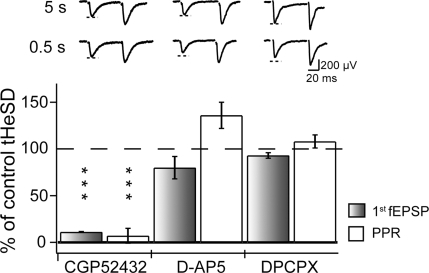
Previous reports on tHeSD have shown that this form of depression requires GABAB, but not NMDA receptor activation (Gustafsson et al. 1989; Isaacson et al. 1993). In agreement with these results we found that 4 μm of the GABAB receptor (GABABR) antagonist CGP52432 abolished the tHeSD and the increase in PPR (Fig. 4). Application of CGP52432 did not cause any increase of the fEPSP (Table 1), indicating that there is no tonic GABABR-mediated depression of glutamate release. In line with the previous studies (Gustafsson et al. 1989; Isaacson et al. 1993) we also found that the NMDA receptor antagonist d-(-2)-amino-5-phosphopentanoic acid (d-AP5) (50 μm) did not significantly reduce the tHeSD or the associated increase in PPR (Fig. 4).
Figure 4. GABAB receptors, but not NMDA and adenosine A1 receptors, are involved in the transient heterosynaptic depression.
The graph illustrates the results of matched comparisons of tHeSD before and after pharmacological interventions. The filled bars compare the effect on the fEPSP slope and the open bars compare the effect on the paired-pulse ratio. 100% means the same tHeSD as in the control situation and 0% means absence of tHeSD. Typical control (‘5 s’, upper) and test (‘0.5 s’, lower) example sweeps are shown above the bars. Dashed line indicates the amplitude of the control fEPSP. ‘CGP52432’ shows the effect of GABAB receptor antagonist CGP52432 (4 μm) on the tHeSD (n = 4). B, ‘d-AP5’ shows the effect of the NMDA receptor antagonist d-AP5 (50 μm) on the tHeSD (n = 6). C, ‘DPCPX’ shows the effect of adenosine A1 receptor antagonist DPCPX (0.2 μm) on the tHeSD (n = 4).
The developmental profile, requirement for intact astrocytic metabolism and connexin-based channels indicate that astrocytes are actively involved in the generation of the tHeSD. Previous reports on astrocyte-mediated heterosynaptic depression have shown that astrocyte-derived adenosine mediates the presynaptic inhibition (Manzoni et al. 1994; Zhang et al. 2003; Pascual et al. 2005; Serrano et al. 2006). We therefore tested the effect of the adenosine A1 receptor antagonist DPCPX (0.2 μm) on the tHeSD. DPCPX caused a marked increase of the fEPSP that was associated with a decrease in the PPR (Table 1). Despite this effect of DPCPX on basal excitatory synaptic transmission, blockade of adenosine A1 receptors did not inhibit the tHeSD and the associated increase in PPR (Fig. 4).
The tHeSD relies on activation of group II/III mGluRs
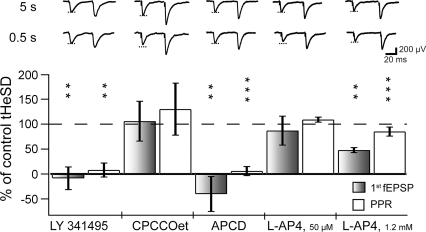
It has previously been shown that astrocytes can release glutamate (Haydon & Carmignoto, 2006) and that activation of GABAB receptors on astrocytes can trigger such release (Kang et al. 1998; Liu et al. 2004a,b). Moreover, in a mixed neuronal–glial culture, it has been reported that astrocytic stimulation can result in mGluR-dependent reduction of excitatory synaptic transmission (Araque et al. 1998). To explore this possibility we used the broad spectrum mGluR antagonist LY 341495. As shown in Fig. 5, LY 341495 (20 μm) prevented the tHeSD and the associated increase in PPR. In contrast to glutamatergic transmission onto some interneurons in the CA1 region (Losonczy et al. 2003), LY 341495 did not affect the fEPSPs (Table 1) indicating glutamatergic synaptic transmission onto pyramidal neurons is not tonically inhibited by active mGluRs.
Figure 5. Metabotropic glutamate receptors are necessary for the transient heterosynaptic depression.
The graph illustrates the results of matched comparisons of tHeSD before and after pharmacological interventions. The filled bars compare the effect on the fEPSP slope and the open bars compare the effect on the paired-pulse ratio. 100% means the same tHeSD as in the control situation and 0% means absence of tHeSD. Typical control (‘5 s’, upper) and test (‘0.5 s’, lower) example sweeps are shown above the bars. Dashed line indicates the amplitude of the control fEPSP. ‘LY 341495’ shows the effect of the group I, II and III metabotropic glutamate receptor antagonist LY 341495 (50 μm) on the tHeSD (n = 6). ‘CPCCOet’ shows the effect of the group I metabotropic glutamate receptor antagonist CPCOOet (150 μm) on the tHeSD (n = 4). ‘APCD’ shows the effect of the group II metabotropic glutamate agonist APCD (50 μm) on the tHeSD (n = 8). ‘l-AP4, 50 μm’ shows the effect of the group III metabotropic glutamate agonist l-AP4 (50 μm; mGluR4, 6, 8) on the tHeSD (n = 4). ‘l-AP4, 1.2 (mm)’ shows the effect of the group III metabotropic glutamate agonist l-AP4 (1.2 mm; mGluR4, 6, 7, 8) on the tHeSD (n = 8).
Activation of mGluRs is thus necessary for the generation of the tHeSD, but mGluRs might be involved in the generation of the tHeSD in several different ways. Group I mGluRs might be involved by raising the calcium concentration in astrocytes (Pasti et al. 1997; Bezzi et al. 2004). However, the group I mGluR antagonist CPCCOet (50–150 μm) did not affect the tHeSD or the increase in PPR (Fig. 5). Application of the group II mGluR agonist APCD (50 μm) resulted in a depression of the fEPSP and an increase in the PPR (Table 1) and fully prevented the tHeSD and the associated increase in PPR (Fig. 5). A low concentration of the group III mGluR agonist l-2-amino-phosphonobutyric acid (l-AP4) (50 μm), thought to activate mGluR4, 6 and 8 (Conn & Pin, 1997), did not affect the fEPSP (Table 1) (Baskys & Malenka, 1991) or the tHeSD (Fig. 5). On the other hand, a high concentration of l-AP4 (1.2 mm), thought to, in addition, activate mGluR7, caused a marked reduction of the fEPSP (Table 1). This higher concentration of l-AP4 also reduced the tHeSD to about half compared to the control tHeSD (Fig. 5). Thus, activation of group II and III mGluRs occluded and partially occluded the tHeSD, respectively.
Discussion
For CA3–CA1 synapses it has been previously described that a brief stimulus burst (3 impulses at 50 Hz) produces a heterosynaptic depression, lasting a few seconds (Gustafsson et al. 1989; Isaacson et al. 1993). In the present study we report that this transient heterosynaptic depression (tHeSD) becomes manifest only towards the end of the second postnatal week, paralleling the astrocytic development (Konietzko & Muller, 1994; Bushong et al. 2004; Schools et al. 2006). Moreover, the findings that the tHeSD was prevented by blockers of connexin-based channels and by an inhibitor of astrocytic metabolism, suggest that the tHeSD is mediated by active release of gliotransmitter from astrocytes, rather than being mediated by direct spillover of transmitter released from active synapses. Our results further suggest that astrocytes are activated by synaptically released GABA acting on GABABRs and that the gliotransmitter is glutamate, which in turn acts on presynaptic mGluRs causing a transient reduction in release probability at the neighbouring, non-activated, synapses (Fig. 6).
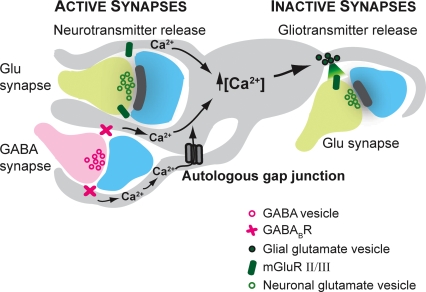
Figure 6. Working model for the transient heterosynaptic depression.
Schematic illustration of two active (left) synapses (one glutamatergic and one GABAergic) and one inactive (right) glutamatergic synapse with part of an astrocyte in between. The astrocyte is equipped with GABABRs and group II/III mGluRs, whose activation can increase intracellular calcium which can spread via autologous gap junctions. The activated astrocyte releases the gliotransmitter glutamate, which activates presynaptic group II/III mGluRs and inhibits release probability at glutamatergic terminals.
It has become increasingly evident that astrocytes have an important role in modulating synaptic transmission and promoting synaptic plasticity (Bains & Oliet, 2007). For example, astrocyte-derived TNFα (Stellwagen & Malenka, 2006) and uncaging of astrocytic calcium (Perea & Araque, 2007) can enhance excitatory synaptic transmission in the hippocampal CA1 region. Regarding heterosynaptic depression, astrocytes, with their fine processes embracing the synapses, are perfectly positioned to mediate this form of plasticity by reacting to neurotransmitters and releasing gliotransmitters (Fig. 6) (Pascual et al. 2005; Serrano et al. 2006). The emerging picture is, however, that astrocyte-mediated heterosynaptic depression is not a uniform form of synaptic plasticity. Thus, the time course and the mediating mechanisms of the heterosynaptic depression depend on the nature of the conditioning stimuli. At CA3–CA1 synapses, relatively intense stimulation, of the kind typically used for the induction of long-lasting forms of long-term potentiation (hundreds of stimuli at ≥ 100 Hz), results in a long-lasting (tens of minutes to hours) heterosynaptic depression, or heterosynaptic long-term depression (Lynch et al. 1977; Scanziani et al. 1996; Serrano et al. 2006). More moderate stimulation (10–100 stimuli at 10–100 Hz) results in HeSD that lasts seconds to minutes (Manzoni et al. 1994; Zhang et al. 2003). Typical for these relatively long-lasting forms of heterosynaptic depression is the requirement for NMDA receptor activation (Manzoni et al. 1994; Scanziani et al. 1996; Serrano et al. 2006), adenosine A1 receptor activation (Manzoni et al. 1994; Zhang et al. 2003; Pascual et al. 2005; Serrano et al. 2006) and calcium signals in the astrocytic network (Pascual et al. 2005; Serrano et al. 2006). The tHeSD, which can amount to 25–40%, is induced by a very brief high-frequency burst and it lasts no more than a few seconds (present study; Gustafsson et al. 1989; Isaacson et al. 1993). In addition to distinct conditioning stimuli and duration, tHeSD is also separate from longer-lasting forms of heterosynaptic depression in that it is not dependent on activation of NMDA- or adenosine A1 receptors (Fig. 4). The involvement of GABABR activation appears to be in common with more longer-lasting forms of heterosynaptic depression (Scanziani et al. 1996; Serrano et al. 2006) and the requirement for mGluRs (Fig. 5) has previously not been implicated in heterosynaptic depressions.
Three findings from the present study indicate that astrocytes mediate, rather than restrict, the tHeSD: the distinct developmental profile of the tHeSD, the total inhibition of the tHeSD by the astrocytic metabolic inhibitor FAC and by the connexin-based channel blockers endothelin-1 and carbenoxolone. FAC selectively inhibits astrocyte metabolism since astrocytes, in contrast to neurons, have uptake mechanisms for acetate (Fonnum et al. 1997). Carbenoxolone is a general blocker of connexin-based channels that has previously been used to implicate astrocytes in heterosynaptic depression (Zhang et al. 2003). Endothelin-1, an endogenous 21-amino acid peptide, potently blocks astrocyte gap junction channels via dephosphorylation of connexin-43 (Blomstrand et al. 2004). Since endothelin receptors are very weakly expressed by neurons at postnatal day 16 and heavily expressed by astrocytes from postnatal day 1–30 in cortical tissue (J. Cahoy and B. Barres, personal communication) the critical connexin-based channels should be located in astrocytes. Connexin-based channels in astrocytes can be found either in intercellular gap junctions, in autologous (intradomain) gap junctions or in unapposed hemichannels. It is difficult to determine to what extent these different functions of connexin-based channels are important for the tHeSD. It is, however, interesting to note that autologous junctions have been suggested to involve as much as 75% of an astrocyte's gap junctions channels (Nagy & Rash, 2003) and that a single hippocampal astrocyte might cover a domain that includes approximately 140 000 synapses (Bushong et al. 2002). Such intradomain channels might then very well be critically involved in mediating intersynaptic communication, such as the tHeSD.
Our results show that both glutamate and GABA, via activation of mGluR and GABABR, respectively, are critically involved in the tHeSD. Since mGluRs and GABABRs are expressed on GABAergic and glutamatergic terminals (Conn & Pin, 1997) as well as on astrocytes (Winder et al. 1996; Pasti et al. 1997; Kimelberg et al. 2000; Bezzi et al. 2004), glutamate and GABA may be involved at various locations in the generation of the tHeSD. Previous results, however, suggest that GABABR activation on astrocytes and group II/III mGluR activation on the glutamatergic terminals, might be critical steps in mediating the tHeSD. It has been shown that astrocytic GABABR activation leads to an increase in astrocytic calcium and potentiation of mIPSCs in hippocampal pyramidal neurons (Kang et al. 1998; Liu et al. 2004a). Moreover, calcium uncaging in astrocytes inhibits IPSCs in hippocampal interneurons by mechanisms involving group II/III mGluR activation (Liu et al. 2004b). The blockade of the tHeSD by the general mGluR antagonist LY 341495, together with the lack of effect by the group I antagonist CPCCOet (Fig. 5), links the tHeSD to group II/III mGluR activation. The findings that that group II agonist APCD totally occluded the generation of the tHeSD and that group III agonist l-AP4 prevented about half of the tHeSD further support this conclusion. This involvement of group II/III mGluRs in the tHeSD demonstrates a previously unrecognized role for mGluRs for the CA3–CA1 synapses.
The possible adaptive purpose of this synaptic depression depends to a large extent on whether the active synapses are also depressed, or whether they are protected from the depression. If the active synapses are protected, this depression would contribute to increase the contrast between active and inactive synapses. On the other hand, if the active synapses are also affected, it would seem natural to view such a transient synaptic depression as a negative feedback restricting the spatial and temporal extension of neural activity. Presynaptic mGluRs are natural candidates to mediate such synaptic feedback at excitatory synapses.
Acknowledgments
This project was supported by the Swedish Research Council (project numbers 01580 and 12600).
References
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Bains JS, Oliet SH. Glia: they make your memories stick! Trends Neurosci. 2007;30:417–424. doi: 10.1016/j.tins.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Baskys A, Malenka RC. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol. 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Blomstrand F, Venance L, Siren AL, Ezan P, Hanse E, Glowinski J, Ehrenreich H, Giaume C. Endothelins regulate astrocyte gap junctions in rat hippocampal slices. Eur J Neurosci. 2004;19:1005–1015. doi: 10.1111/j.0953-816x.2004.03197.x. [DOI] [PubMed] [Google Scholar]
- Buhl DL, Buzsaki G. Developmental emergence of hippocampal fast-field ‘ripple’ oscillations in the behaving rat pups. Neuroscience. 2005;134:1423–1430. doi: 10.1016/j.neuroscience.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Diamond JS. Deriving the glutamate clearance time course from transporter currents in CA1 hippocampal astrocytes: transmitter uptake gets faster during development. J Neurosci. 2005;25:2906–2916. doi: 10.1523/JNEUROSCI.5125-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 1997;21:106–113. [PubMed] [Google Scholar]
- Grover LM, Teyler TJ. Presynaptic mechanism for heterosynaptic, posttetanic depression in area CA1 of rat hippocampus. Synapse. 1993;15:149–157. doi: 10.1002/syn.890150207. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Asztely F, Hanse E, Wigström H. Onset characteristics of long-term potentiation in the guinea-pig hippocampal CA1 region in vitro. Eur J Neurosci. 1989;1:382–394. doi: 10.1111/j.1460-9568.1989.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Schools GP, Cai Z, Zhou M. Freshly isolated astrocyte (FIA) preparations: a useful single cell system for studying astrocyte properties. J Neurosci Res. 2000;61:577–587. doi: 10.1002/1097-4547(20000915)61:6<577::AID-JNR1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Konietzko U, Muller CM. Astrocytic dye coupling in rat hippocampus: topography, developmental onset, and modulation by protein kinase C. Hippocampus. 1994;4:297–306. doi: 10.1002/hipo.450040313. [DOI] [PubMed] [Google Scholar]
- Kugler P, Schleyer V. Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus. 2004;14:975–985. doi: 10.1002/hipo.20015. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci U S A. 2004a;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Kang J, Nedergaard M. Astrocyte activation of presynaptic metabotropic glutamate receptors modulates hippocampal inhibitory synaptic transmission. Neuron Glia Biol. 2004b;1:307–316. doi: 10.1017/S1740925X05000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Somogyi P, Nusser Z. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J Neurophysiol. 2003;89:1910–1919. doi: 10.1152/jn.00842.2002. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Astrocyte and oligodendrocyte connexins of the glial syncytium in relation to astrocyte anatomical domains and spatial buffering. Cell Comm Adhesion. 2003;10:401–406. doi: 10.1080/15419060390263191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM, Stewart MG. Hippocampal synapses: do they talk to their neighbours? Trends Neurosci. 1999;22:382–388. doi: 10.1016/s0166-2236(99)01425-3. [DOI] [PubMed] [Google Scholar]
- Sastry BR, Goh JW, Auyeung A. Associative induction of posttetanic and long-term potentiation in CA1 neurons of rat hippocampus. Science. 1986;232:988–990. doi: 10.1126/science.3010459. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Malenka RC, Nicoll RA. Role of intercellular interactions in heterosynaptic long-term depression. Nature. 1996;380:446–450. doi: 10.1038/380446a0. [DOI] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J Neurophysiol. 2006;96:1383–1392. doi: 10.1152/jn.00449.2006. [DOI] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Volterra A, Steinhauser C. Glial modulation of synaptic transmission in the hippocampus. Glia. 2004;47:249–257. doi: 10.1002/glia.20080. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Abraham WC. The involvement of L-type calcium channels in heterosynaptic long-term depression in the hippocampus. Neurosci Lett. 1991;130:128–132. doi: 10.1016/0304-3940(91)90244-n. [DOI] [PubMed] [Google Scholar]
- Wigström H, Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 1985;125:159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- Winder DG, Ritch PS, Gereau RW 4th, Conn PJ. Novel glial-neuronal signalling by coactivation of metabotropic glutamate and β-adrenergic receptors in rat hippocampus. J Physiol. 1996;494:743–755. doi: 10.1113/jphysiol.1996.sp021529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]